This research was conducted to obtain accurate information on the protective effects of Portulaca oleracea L. against hepatogastric diseases.
ResultsP. oleracea L. (Purslane) has traditionally been used for the treatment of hepatogastric diseases. However, the low number of research studies has shown that P. oleracea L. possesses protective effects against hepatotoxic agents. The safety of P. oleracea L. has been demonstrated in several clinical trials.
ConclusionModern pharmacological studies have indicated the gastroprotective and hepatoprotective effects of P. oleracea L. by using in vivo and in vitro models. However, due to lack of information of its effects in humans, more studies should be conducted to confirm the efficacy of P. oleracea L. in humans.
Se realizó esta investigación para disponer de información precisa sobre los efectos protectores de Portulaca oleracea L. frente a enfermedades hepatogástricas.
ResultadosPortulaca oleracea L. (Purslane) se ha utilizado tradicionalmente para el tratamiento de enfermedades hepatogástricas. Sin embargo, las investigaciones limitadas han demostrado que Portulaca oleracea L. posee efectos protectores frente a sustancias hepatotóxicas. Varios ensayos clínicos han demostrado la seguridad de Portulaca oleracea L.
ConclusiónEstudios farmacológicos modernos han revelado los efectos gastroprotectores y hepatoprotectores de Portulaca oleracea L. mediante el uso de modelos in vivo e in vitro. Sin embargo, debido a la falta de información sobre sus efectos en el ser humano, se deben realizar más estudios para confirmar la eficacia de Portulaca oleracea L. en el ser humano.
Portulaca oleracea L. (Portulaceae, common name purslane) is an annual herbaceous plant with reddish stems and alternate leaves from family Portulacaceae that is classified as a C4 plant.1 The plant is identified as one of the effective medicinal plants and named “Global Panacea” by the World Health Organization.1 Numerous pharmacological effects such as antioxidant, anti-inflammatory, and immunomodulatory activities have been found for P. oleracea L.2–6 It is also used as a vegetable, widely sold in shops in China, United Arab Emirates, and Oman.7P. oleracea L. presents variable chemical constituents, mainly belong to flavonoid, alkaloid, terpenoid, organic acid, and other natural compounds such as terpenoids, fatty acids, polysaccharides, sterols, vitamins, proteins, and minerals.8–10 Flavonoids are the main active constituents of P. oleracea L.11–13 The concentration of flavonoids in different plant parts are different. The highest amount is found in the root followed by stem and the leaf.14 Kaempferol, apigenin, luteolin, myricetin, and quercetin are major flavonoids in P. oleracea L.15P. oleracea L. prevents neurodegenerative disorders, coronary artery diseases, diabetes and its complications, respiratory failures, hepatic diseases, gastrointestinal diseases, and renal failures.16–18 Thus, the present study has been designed to provide an update review of the gastro-hepatoprotective effects of P. oleracea L.
MethodsOnline literature resources have been checked using different search engines such as PubMed, Scopus, and Google Scholar to identify articles, editorials, and reviews about the gastroprotective and hepatoprotective effects of P. oleracea L. Hepatoprotective, P. oleracea L., gastroprotective, inflammation, and oxidative stress were selected as key words.
Gastroprotective effectsThe aqueous and ethanolic extracts of P. oleracea L. (0.8–1.77g/kg) were inhibited gastric lesions induced by ethanol in mice. The findings have indicated that administration of extracts reduced the gastric acidity in pylorus-ligated mice.19 The gastroprotective effects of P. oleracea L. has been studied in acute gastric ulcer induced by aspirin, ethanol, cold restraint stress, as well as pyloric ligation and chronic ulcers induced by acetic acid in rats. P. oleracea L. (50–150mg/kg, PO) presented the significant protective effects against gastric ulcer. The study suggested that P. oleracea L. prevents the oxidative damage of gastric mucosa via increasing superoxide dismutase (SOD) and catalase (CAT) activities as well as decreasing lipid peroxidation. Additionally, P. oleracea L. decreased the acid and pepsin secretions and increased the synthesis of mucus.20
Hepatoprotective effectsProtective effects of P. oleracea L. against hepatocellular carcinomaHepatocellular carcinoma (HCC) is the most common primary liver malignancy and also a leading cause of cancer-related death worldwide.21 The inflammatory cytokines and reactive oxygen species (ROS) play main roles in the initiation and progression of liver carcinogenesis.22–25 The anticancer effect of seed extracts of P. oleracea L. on the human hepatocellular carcinoma cells (HepG2) has been investigated.26 The results showed that P. oleracea L. decreases the cell viability of HepG2 in a dose dependent manner. The findings also indicated that P. oleracea L. decreases the adhesion capacity of HepG2 cells. The study has indicated the anticancer activity of P. oleracea against HepG2 cells, which may be used for the development of a potential therapeutic anticancer drug.26 The effects of P. oleracea L. against N-nitrosodiethylamine- (NDEA-) induced hepatocellular carcinomas (HCC) has also been investigated in mice. The results indicated that P. oleracea L. decreases the activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in liver and serum. P. oleracea L. reduced the contents of interleukin-6 (IL-6), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and malondialdehyde (MDA) as well as increased the activity of SOD in serum. P. oleracea L. attenuated the hepatic pathological changes. Treatment with P. oleracea L. inhibited the phosphorylations of phosphatidylinositol 3 kinase (PI3K), protein kinase B (Akt), mammalian target of rapamycin (mTOR), nuclear factor-kappa B (NF-κB), inhibitor of NF-κBα (IκBα) as well as up-regulated the expressions of NF-E2-related factor 2 (Nrf2) and heme oxygenase- (HO-) 1. The study suggested that P. oleracea L. has protective effects against NDEA-induced hepatocellular carcinoma by anti-inflammatory and antioxidative activities via the PI3K/Akt/mTOR and Nrf2/HO-1/NF-κB pathways.27
Protective effects of P. oleracea L. against bile duct ligation (BDL)-induced hepatic fibrosisHepatic fibrosis is a major characteristic of many chronic liver diseases. The antioxidant effects of P. oleracea L. hydro-ethanolic extract against bile duct ligation-induced hepatic fibrosis have been investigated. Bile duct ligation increased liver enzymes, total bilirubin (TB), and TNF-α in serum along with the MDA level in liver tissues as well as decreased hepatic antioxidant defense system in rats. However, P. oleracea L. administration ameliorated all these alterations induced by BDL. The present study suggested that P. oleracea L. presents protective effects against cholestasis-induced liver fibrosis via inhibiting of oxidative stress and decreasing the expression of profibrogenic cytokines, collagenolytic activity as well as activation of hepatic stellate cells.28
Protective effects of Portulaca oleracea L. against hepatotoxic agentsCarbon tetrachlorideCarbon tetrachloride (CCl4) induces liver damage in animal models.29 Oxidative stress plays a main role in the progression of hepatic damage induced by CCl4. Additionally, the hepatotoxicity of CCl4 may be mediated by the indirect activation of Kupffer cells/macrophages and neutrophils.7 Kupffer cells induce the hepatic inflammation responses by generating TNF-α and other cytotoxic cytokines.30 CCl4 treatment increases inflammatory cascade by inducing the NF-κB pathway in the liver.31 Natural antioxidants are effective factors for preventing oxidative stress related liver diseases.32 The protective effect of P. oleracea L. ethanolic extract against carbon tetrachloride (CCl4)-induced hepatic toxicity has been investigated in rats. CCl4 increased serum activities of liver enzymes (ALT, AST, ALP, GGT) and decreased SOD level. CCl4 treatment induced histological alterations in the liver tissue. P. oleracea L. extract treatment ameliorated these alterations in CCl4-treated rats.21 The protective effect of the P. oleracea L. aqueous extract in combination with lycopene against CCl4 induced-hepatotoxicity has also been investigated in rats. Findings indicated that P. oleracea L. aqueous extract in combination with lycopene enhanced the hepatoprotective effect against CCl4 by ameliorating the levels of serum liver enzymes.33 The protective effects of ethanol extract of P. oleracea L. against CCl4-induced acute liver damage in mice have also been studied. The findings has indicated that P. oleracea L. treatment decreases the serum AST level and also the liver histological damage in mice. Nuclear translocation of p65 was enhanced in liver tissues of mice treated with P. oleracea. Furthermore, P. oleracea L. increased the NF-κB luciferase reporter gene activity and up regulated the level of phosphorylation of p65, but had no effects on mice liver SOD activity and MDA level in cultured hepatic cells. This study suggested that P. oleracea ameliorated CCl4 induced mice liver damage by enhancement of NF-κB activity.34
CisplatinCisplatin (Cis), an anti-tumor drug, induces hepatotoxicity through induction of oxidative stress and inflammation.35,36 The protective role of aqueous extract of aerial parts of P. oleracea L. against cisplatin-induced hepatotoxicity in chick embryonic liver has been investigated. Cisplatin exposure increased ALT, AST, ALP, LDH and MDA levels and also decreased SOD, catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR), glutathione-S-transferase (GST) and reduced glutathione (GSH) in the rat liver tissue. It was found that P. oleracea L. prevents cisplatin-induced hepatotoxicity by ameliorating oxidative stress in rat liver.37
AcetaminophenAcetaminophen (APAP) is an analgesic and antipyretic drug and long term use or over dosage of APAP may cause liver injury. N-acetyl-p-ben-zoquinone (NAPQI), an intermediate metabolite of APAP, binds to GSH and impairs the oxidant–antioxidant balance in hepatocytes.38,39 It was reported that the ethanolic extracts of P. oleracea L. prevented APAP-induced hepatotoxicity. The findings have indicated that P. oleracea L. reverse APAP induced hepatotoxicity by decreasing the ROS production in the liver of mice. In addition, P. oleracea L. administration decreased mice serum levels of IL-6 and TNFα and their mRNA expression in liver tissue. These findings have suggested that P. oleracea L. administration may be an effective against APAP-induced hepatotoxicity by ameliorating oxidative stress and inflammation responses.40
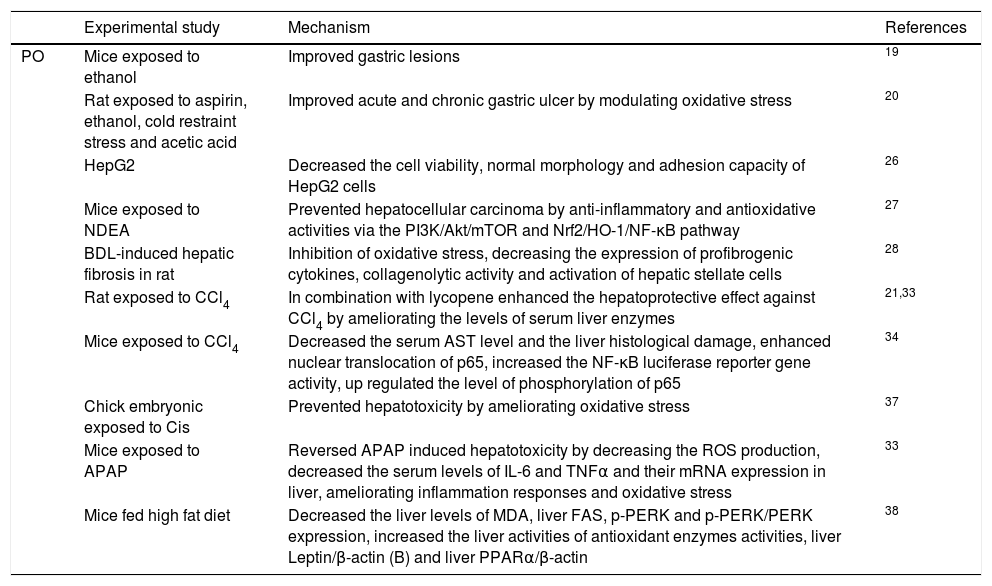
High fat dietA high-fat diet is a major environmental factor that disturbed lipoprotein metabolism and is significantly associated with an increased prevalence of metabolic disorders.1,41 A high-fat/cholesterol diet increased oxidative stress in body contents.42 Natural antioxidants have been used to treat various metabolic diseases with no side effects.43 It was reported that P. oleracea L. prevents high-fat-diet-induced oxidative damage in mice liver. High-fat diet increased blood and liver lipid peroxidation level, and also decreased antioxidant enzymes activities in mice. Aqueous extract of P. oleracea L. decreased the levels of blood and liver MDA and increased the activities of blood and liver antioxidant enzymes activities in high fat mice. Additionally, aqueous extract of P. oleracea L. increased liver Leptin/β-actin (B), and Liver PPARα/β-actin, and also decreased liver, spleen FAS mRNA, p-PERK and p-PERK/PERK protein expression levels. These findings indicated that aqueous extract of P. oleracea L. ameliorated high fat diet-induced oxidative damage by increasing blood and liver antioxidant enzyme activities, modulating Leptin/β-actin (B), and Liver PPARα/β-actin, decreasing liver, spleen FAS mRNA, p-PERK and p-PERK/PERK protein expression levels in mice.44 The hepatoprotective effects of P. oleracea L. are presented in Table 1.
The gastro-protective and hepato-protective effects of Portulaca oleracea L.
| Experimental study | Mechanism | References | |
|---|---|---|---|
| PO | Mice exposed to ethanol | Improved gastric lesions | 19 |
| Rat exposed to aspirin, ethanol, cold restraint stress and acetic acid | Improved acute and chronic gastric ulcer by modulating oxidative stress | 20 | |
| HepG2 | Decreased the cell viability, normal morphology and adhesion capacity of HepG2 cells | 26 | |
| Mice exposed to NDEA | Prevented hepatocellular carcinoma by anti-inflammatory and antioxidative activities via the PI3K/Akt/mTOR and Nrf2/HO-1/NF-κB pathway | 27 | |
| BDL-induced hepatic fibrosis in rat | Inhibition of oxidative stress, decreasing the expression of profibrogenic cytokines, collagenolytic activity and activation of hepatic stellate cells | 28 | |
| Rat exposed to CCl4 | In combination with lycopene enhanced the hepatoprotective effect against CCl4 by ameliorating the levels of serum liver enzymes | 21,33 | |
| Mice exposed to CCl4 | Decreased the serum AST level and the liver histological damage, enhanced nuclear translocation of p65, increased the NF-κB luciferase reporter gene activity, up regulated the level of phosphorylation of p65 | 34 | |
| Chick embryonic exposed to Cis | Prevented hepatotoxicity by ameliorating oxidative stress | 37 | |
| Mice exposed to APAP | Reversed APAP induced hepatotoxicity by decreasing the ROS production, decreased the serum levels of IL-6 and TNFα and their mRNA expression in liver, ameliorating inflammation responses and oxidative stress | 33 | |
| Mice fed high fat diet | Decreased the liver levels of MDA, liver FAS, p-PERK and p-PERK/PERK expression, increased the liver activities of antioxidant enzymes activities, liver Leptin/β-actin (B) and liver PPARα/β-actin | 38 |
Abbreviations – PO: Portulaca oleracea L., HepG2: hepatocellular carcinoma cells, NDEA: N-nitrosodiethylamine, PI3K: phosphatidylinositol 3 kinase, Akt: protein kinase B, mTOR: mammalian target of rapamycin, Nrf2: NF-E2-related factor 2, HO: heme oxygenase, NF-κB: nuclear factor-kappa B, BDL: bile duct ligation, CCl4: carbon tetrachloride, AST: aspartate aminotransferase, STZ: streptozotocin, CPOP: crude Portulaca oleracea L. polysaccharide, GTT:glucose tolerance test, FBG:fasting blood glucose, FINS: fasting serum insulin, ISI: insulin sensitivity index, TNF-α: tumor necrosis factor-α, IL-6: interleukin-6, MDA: methane dicarboxylic aldehyde,SOD: superoxygen dehydrogenises, Cis:Cisplatin, APAP: acetaminophen, FAS mRNA: fatty acid synthase mRNA, p-PERK: phospho-protein kinase RNA-like endoplasmic reticulum kinase, PPARα/β: peroxisome proliferator-activated receptor α/β.
In vitro studies did not indicate the cytotoxic effects of P. oleracea L.45 Acute toxicity studies on the methanolic extract of P. oleracea L. in mice have indicated that P. oleracea L. is moderately toxic with LD50 value of 1853mg/kg. In the histopathologic studies, the methanolic extract have indicated hepatic and renal toxicities.46 According to the findings, P. oleracea L. has a high therapeutic index. However, more studies should be done on chronic toxicity of P. oleracea L. Most clinical trials have not indicated any adverse effects of P. oleracea L. However, thyroiditis, skin rash, facial nerve palsy have been found in three patients.47
ConclusionIn vitro and in vivo studies on P. oleracea L. have found that it has several biological activities such as gastro-protective and hepato-protective effects. According to the results of several studies, P. oleracea L. also acts as an antidote in hepatotoxicity induced by CCl4, Cis, APAP, and high-fat. In addition, P. oleracea L. has indicated the protective effects against hepatocellular carcinoma and hepatic fibrosis. These findings can help to produce hypothesis for therapeutic effects of P. oleracea L. which have to be confirmed by clinical trials. Clinical studies have indicated that oral dose of 180mg/day of the P. oleracea L. extract is safe and effective in the treatment of hypertension and hyperglycemia. Additionally, administration of P. oleracea L. (1g, orally) improved safely the psychological condition in chronic schizophrenic patients. Different mechanisms such as inhibition of oxidative stress, inflammation, and apoptosis are involved in the hepato-protective and gastro-protective effects of P. oleracea L. However, many pharmacological aspects of P. oleracea L. are yet to be studied. Therefore, the mechanisms of actions, clinical effectiveness, pharmacokinetic properties, and proper dosages should need to be further investigated.
Conflict of interestThe authors declare no conflict of interests.