This study was aimed to determine the relationship of alcohol-metabolizing enzymes ADH2, ADH3, and ALDH2 polymorphisms with the susceptibility to alcoholic chronic pancreatitis (ACP).
MethodsMeta-analyses that evaluated the association of ADH2, ADH3, and ALDH2 variations with ACP were performed.
ResultsEight case–control studies were selected for analysis. The overall data revealed a significant association of ADH2 polymorphism (OR=1.56, 95% CI=1.42–1.72, P=0.000 for dominant model; OR=1.63, 95% CI=1.55–1.71, P=0.000 for homozygote comparison model; OR=1.11, 95% CI=1.01–1.22, P=0.030 for allelic contrast model), ADH3 polymorphism (OR=0.95, 95% CI=0.86–1.06, P=0.389 for dominant; OR=0.64, 95% CI=0.44–0.93, P=0.020 for homozygote comparison; and OR=0.87, 95% CI=0.77–0.99, P=0.039 for allelic contrast model) and ALDH2 polymorphism (OR=0.57, 95% CI=0.40–0.81, P=0.002 for dominant; OR=0.50, 95% CI=0.23–1.08, P=0.079 for homozygote comparison; and OR=0.58, 95% CI=0.41–0.84, P=0.003 for allelic contrast model) with ACP risk. The subgroup analyses suggested that the variant ADH2*2/*2+*1/*2, ADH2*2/*2 genotype and ADH2*2 allele significantly increased ACP risk among Asian individuals; the variant ADH3*2/*2 genotype and ADH3*2 allele significantly decreased ACP risk among non-Asian individuals; and the variant ALDH2*2/*2+*1/*2 genotype and ALDH2*2 allele significantly decreased ACP risk among Asians.
ConclusionsADH2, ADH3 and ALDH2 polymorphisms may be susceptibility facts of ACP, and it may be ethnic and race-dependent.
El objetivo de este estudio fue determinar la relación entre los polimorfismos de las enzimas metabolizantes del alcohol ADH2, ADH3 y ALDH2, y la susceptibilidad a la pancreatitis crónica alcohólica (PCA).
MétodosSe realizó un metaanálisis con estudios que habían evaluado la asociación de las variantes ADH2, ADH3 y ALDH2 con la PCA.
ResultadosSe seleccionaron para el análisis ocho estudios de casos con controles. Los datos globales mostraron una asociación significativa con el riesgo de PCA de los polimorfismos ADH2 (OR=1,56; IC del 95%=1,42–1,72; p=0,000 para el modelo dominante; OR=1,63; IC del 95%=1,55–1,71; p=0,000 para el modelo de comparación homocigótico y OR=1,11; IC 95%=1,1–1,22, P=0,030 para el modelo de contraste alélico), ADH3 (OR=0,95; IC 95%=0,86–1,06; p=0,389 para el modelo dominante; OR=0.64, 95% CI=0,44–0,93, P=0,020, para la comparación homocigótica; y OR=0,87, IC 95%=0,77–0,99; p=0,039 para el modelo de contraste alélico) y para el polimorfismo ALDH2 (OR=0.57, IC 95%=0,40–0,81, p=0,002 para el modelo dominante; OR=0,50; IC 95%=0,23–1,08; p=0,079 para la comparación homocigótica; y OR=0,58 IC 95%=0,41–0,84; p=0,003 para el modelo de contraste alélico). El análisis de los subgrupos reveló que las variantes ADH2*2/*2+*1/*2, ADH2*2/*2 y alelo ADH2*2 reducen de forma significativa el riesgo de PCA en los individuos asiáticos; el genotipo ADH3*2/*2 y alelo ADH3*2 disminuyen significativamente el riesgo de PCA en individuos no asiáticos; y la variante ALDH2*2/*2+*1/*2 y alelo ALDH2*2 disminuyen significativamente el riesgo de PCA entre asiáticos.
Conclusioneslos polimorfismos ADH2, ADH3 y ALDH2 pueden ser factores de susceptibilidad a la PCA, y pueden depender de la etnia y la raza.
Chronic pancreatitis is a progressive inflammatory disease that finally leads to damage of exocrine and endocrine functions of the pancreas.1,2 Among the different risk factors for chronic pancreatitis alcohol abuse is the most prevalent.
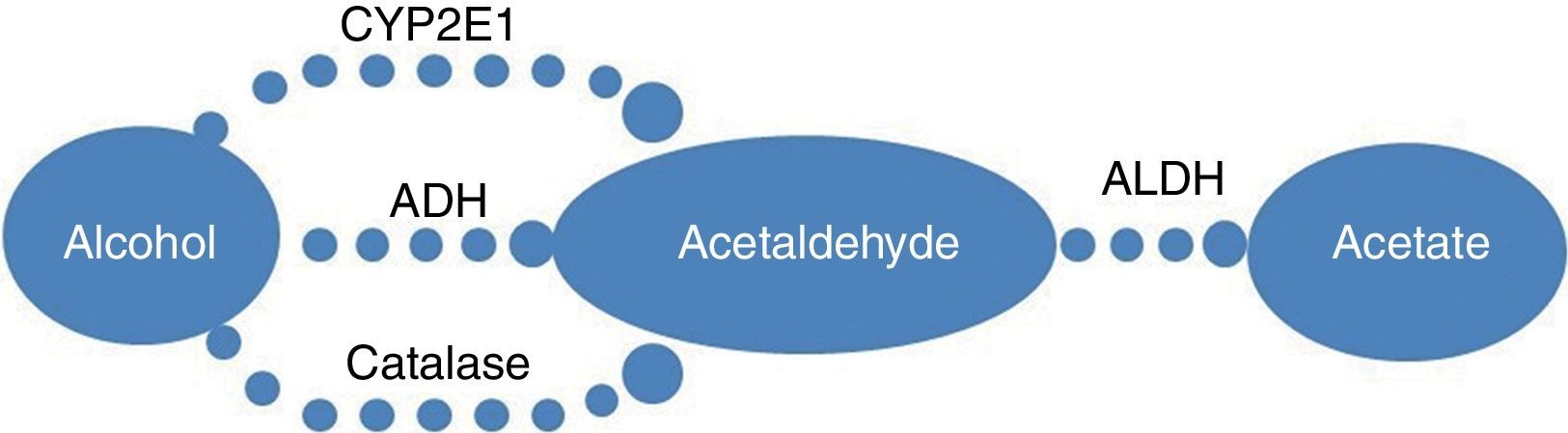
The metabolism pathway of alcohol is shown in Fig. 1. Alcohol dehydrogenase such as ADH2 (ADH1B) and ADH3 (ADH1C), and aldehyde dehydrogenase 2 (ALDH2) played central roles in the initial stages of alcohol metabolism.
The metabolism pathway of alcohol. The primary pathway for alcohol metabolism involves in alcohol dehydrogenase (ADH), cytochrome P450 2E1 (CYP2E1) and catalase, enzymes that catalyze the conversion of alcohol to acetaldehyde. Acetaldehyde is then oxidized by aldehyde dehydrogenase (ALDH) to acetate.
It is believed that the individual differences in the susceptibility to alcoholic chronic pancreatitis are likely to be the genetically determined differences in alcohol-metabolizing enzymes with different metabolizing rate. So many studies have been conducted on the possible associations between polymorphisms of alcohol-metabolizing enzymes and alcoholic chronic pancreatitis risk. But, the results are matters in dispute. Whether genetic variations of these enzymes can elevate the alcoholic chronic pancreatitis risk remains uncertain.
Furthermore, the presence of polymorphic iso-enzymes ADH2, ADH3, and ALDH2 variations is different from ethnic groups.3–6 ADH2*2 allele occurs in 0–10% of white population, 0–5.7% among Europeans, and 60–80% in Asians.7–9 In Caucasians, the rate of ADH3*1 is 50–60%, while >90% in Asians.4,5
Therefore, in this study, we conducted a quantitative meta-analysis with subgroup analysis on ethnicity that included published data up to August 2013, involving in three main alcohol-metabolizing enzymes ADH2, ADH3, and ALDH2. This coverage may increase the statistical power to determine accurately the associations of ADH2, ADH3 and ALDH2 polymorphisms with alcoholic chronic pancreatitis risk.
Materials and methodsLiterature search strategyWe carried out searches in Medline, Sciencedirect, and Chinese National Knowledge Infrastructure (CNKI) without a language limitation, covering all papers published up to August 2013. The following keywords were used: alcohol dehydrogenase 2 (or alcohol dehydrogenase 1B), ADH2 (or ADH1B), alcohol dehydrogenase 3 (or alcohol dehydrogenase 1C), ADH3 (or ADH1C), aldehyde dehydrogenase 2, ALDH2, alcohol, alcoholic, chronic pancreatitis, polymorphism and genotype. All searched studies were retrieved and the bibliographies were further checked for other relevant publication. Review articles and the bibliographies of other relevant studies identified were hand searched to identify additional eligible studies.
Inclusion criteriaThe following criteria were used for the literature selection. First, the study should concern the association of alcoholic chronic pancreatitis and genotype polymorphisms of ADH2, ADH3 and ALDH2. Second, the study must be observational (case–control or cohort). Third, the studies must indicate the sample size, odds ratios (ORs), and their 95% confidence intervals (CIs), as well as the genetic distribution or the information that can help infer the results. After rigorous searching, we reviewed all papers based on the above criteria for further analysis.
Data extractionTwo of the authors (Zhong and Cao) carefully extracted Data independently from all eligible publications in accordance with the inclusion criteria above. For conflicting evaluations, an agreement was reached after a discussion. When a consensus cannot be reached, another author was to be consulted to resolve the dispute, and then a final decision was made based on a majority of votes. Where authors had published more than one article using the same case series, most recent publication was selected. Pooled data of these control groups (including healthy alcoholics, non-alcoholic healthy volunteers and blood donors) were considered as a control group as some included articles using healthy controls with or without drinking, some used healthy controls whose history of alcoholism were unknown, and some involved in several separate control groups. The extracted data were summarized into a database.
Statistical analysesThe ORs of ADH2, ADH3, and ALDH2 polymorphisms and alcoholic chronic pancreatitis risk were estimated for each study. The pooled ORs were determined for a dominant model (*2/*2+*1/*2 vs. *1/*1), a homozygote comparison model (*2/*2 vs. *1/*1) and an allelic contrast model (*2 allele vs. *1 allele). To detect any possible sample size bias, the OR and its 95% CI for each study were plotted against the number of participants. The I2 value was used as an index for the heterogeneity test and a chi-squared-based Q-test was also performed to assess heterogeneity. If the P value for the Q-test was more than 0.1, ORs were pooled according to the fixed-effect model (Mantel–Haenszel); otherwise, the random-effect model (DerSimonian and Laird) was used. The significance of the pooled ORs was determined by the Z-test. Publication bias was assessed by visual inspection of funnel plots, in which the standard error of log (OR) of each study was plotted against its log (OR). An asymmetric plot indicated possible publication bias. The symmetry of the funnel plot was further evaluated by Egger's linear regression test. Statistical analysis was performed using the program Stata 12.0 (StataCorp LP).
ResultsStudy characteristicsRelevant publications were searched and preliminarily reviewed. Sixty-four publications were identified, in which 46 irrelevant papers were excluded. Two original articles that used controls from other paper were excluded.10,11 Two studies12,13 on the connection between alcohol-metabolizing enzyme (ADH2, ADH3 and ALDH2) and alcoholic acute pancreatitis were excluded. Three reviews were also excluded.14,15 Four studies16–19 used the same case series respectively, so the most recent publication was included;19 lastly, eight case–control studies were included in the meta-analyses.2,19–25
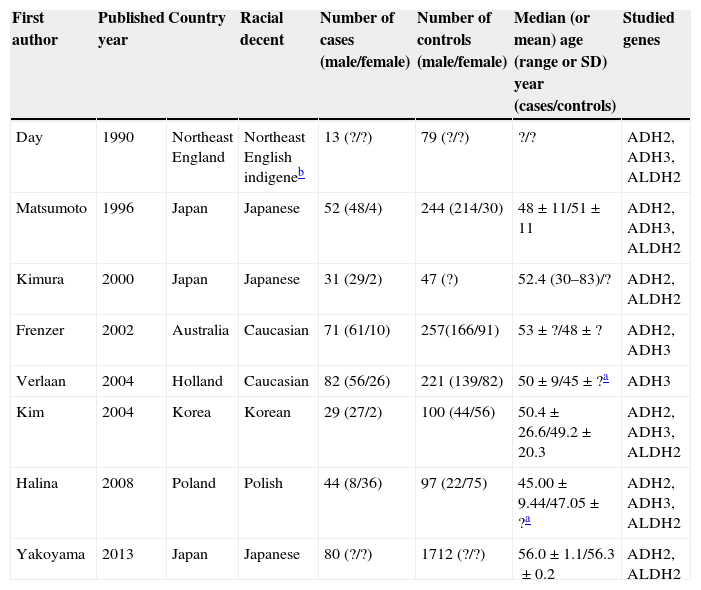
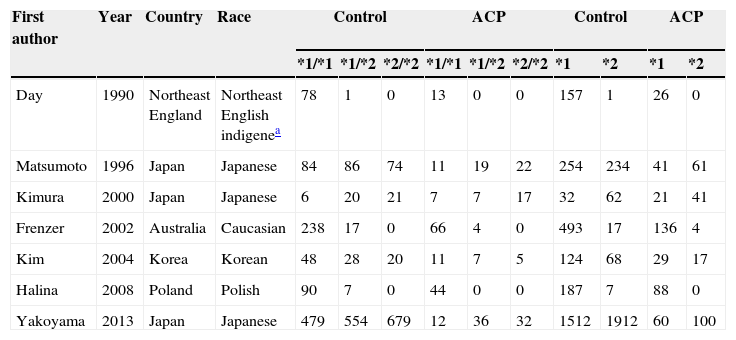
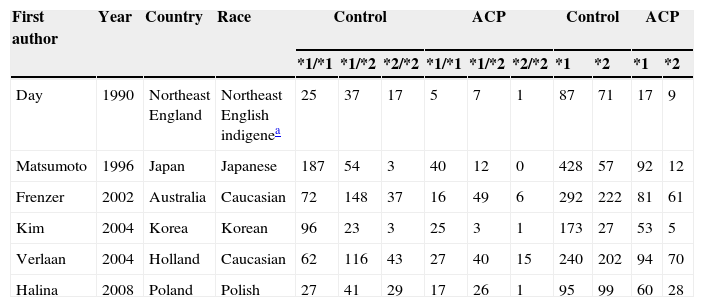
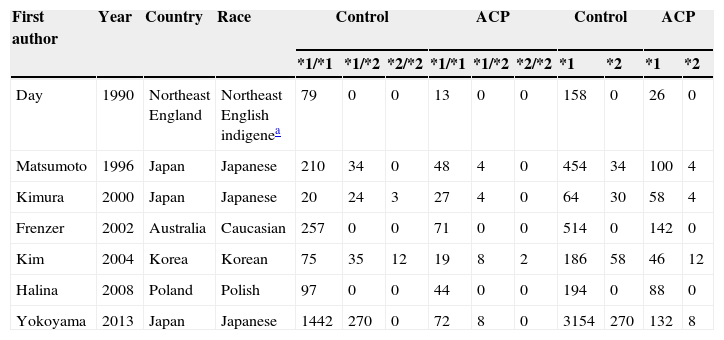
Seven publications were written in English,2,19,21–25 and one in Korean.20 The relevant information is listed in Table 1. The first author, published year, the number and characteristics of cases and controls, and studied genes for each study, and other necessary information are presented. The selected articles included 4 groups of Asian20,22,24,25 and 4 groups of non-Asian.2,19,21,23 Six articles mentioned gene polymorphism of ADH2,19–24 six articles of ADH32,19–21,23,24 and seven articles polymorphism of ALDH2.12,19–25 The distributions of the ADH2, ADH3 and ALDH2 genotypes are presented in Tables 2–4, respectively.
Characteristics of studies included in the meta-analysis.
| First author | Published year | Country | Racial decent | Number of cases (male/female) | Number of controls (male/female) | Median (or mean) age (range or SD) year (cases/controls) | Studied genes |
|---|---|---|---|---|---|---|---|
| Day | 1990 | Northeast England | Northeast English indigeneb | 13 (?/?) | 79 (?/?) | ?/? | ADH2, ADH3, ALDH2 |
| Matsumoto | 1996 | Japan | Japanese | 52 (48/4) | 244 (214/30) | 48±11/51±11 | ADH2, ADH3, ALDH2 |
| Kimura | 2000 | Japan | Japanese | 31 (29/2) | 47 (?) | 52.4 (30–83)/? | ADH2, ALDH2 |
| Frenzer | 2002 | Australia | Caucasian | 71 (61/10) | 257(166/91) | 53±?/48±? | ADH2, ADH3 |
| Verlaan | 2004 | Holland | Caucasian | 82 (56/26) | 221 (139/82) | 50±9/45±?a | ADH3 |
| Kim | 2004 | Korea | Korean | 29 (27/2) | 100 (44/56) | 50.4±26.6/49.2±20.3 | ADH2, ADH3, ALDH2 |
| Halina | 2008 | Poland | Polish | 44 (8/36) | 97 (22/75) | 45.00±9.44/47.05±?a | ADH2, ADH3, ALDH2 |
| Yakoyama | 2013 | Japan | Japanese | 80 (?/?) | 1712 (?/?) | 56.0±1.1/56.3±0.2 | ADH2, ALDH2 |
Distribution of ADH2 genotype among alcoholic chronic pancreatitis cases and controls included in the meta-analysis.
| First author | Year | Country | Race | Control | ACP | Control | ACP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| *1/*1 | *1/*2 | *2/*2 | *1/*1 | *1/*2 | *2/*2 | *1 | *2 | *1 | *2 | ||||
| Day | 1990 | Northeast England | Northeast English indigenea | 78 | 1 | 0 | 13 | 0 | 0 | 157 | 1 | 26 | 0 |
| Matsumoto | 1996 | Japan | Japanese | 84 | 86 | 74 | 11 | 19 | 22 | 254 | 234 | 41 | 61 |
| Kimura | 2000 | Japan | Japanese | 6 | 20 | 21 | 7 | 7 | 17 | 32 | 62 | 21 | 41 |
| Frenzer | 2002 | Australia | Caucasian | 238 | 17 | 0 | 66 | 4 | 0 | 493 | 17 | 136 | 4 |
| Kim | 2004 | Korea | Korean | 48 | 28 | 20 | 11 | 7 | 5 | 124 | 68 | 29 | 17 |
| Halina | 2008 | Poland | Polish | 90 | 7 | 0 | 44 | 0 | 0 | 187 | 7 | 88 | 0 |
| Yakoyama | 2013 | Japan | Japanese | 479 | 554 | 679 | 12 | 36 | 32 | 1512 | 1912 | 60 | 100 |
Notes: ADH2, alcohol dehydrogenase 2; ACP, alcoholic chronic pancreatitis.
Distribution of ADH3 genotype among alcoholic chronic pancreatitis cases and controls included in the meta-analysis.
| First author | Year | Country | Race | Control | ACP | Control | ACP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| *1/*1 | *1/*2 | *2/*2 | *1/*1 | *1/*2 | *2/*2 | *1 | *2 | *1 | *2 | ||||
| Day | 1990 | Northeast England | Northeast English indigenea | 25 | 37 | 17 | 5 | 7 | 1 | 87 | 71 | 17 | 9 |
| Matsumoto | 1996 | Japan | Japanese | 187 | 54 | 3 | 40 | 12 | 0 | 428 | 57 | 92 | 12 |
| Frenzer | 2002 | Australia | Caucasian | 72 | 148 | 37 | 16 | 49 | 6 | 292 | 222 | 81 | 61 |
| Kim | 2004 | Korea | Korean | 96 | 23 | 3 | 25 | 3 | 1 | 173 | 27 | 53 | 5 |
| Verlaan | 2004 | Holland | Caucasian | 62 | 116 | 43 | 27 | 40 | 15 | 240 | 202 | 94 | 70 |
| Halina | 2008 | Poland | Polish | 27 | 41 | 29 | 17 | 26 | 1 | 95 | 99 | 60 | 28 |
Notes: ADH3, alcohol dehydrogenase 3; ACP, alcoholic chronic pancreatitis.
Distribution of ALDH2 genotype among alcoholic chronic pancreatitis cases and controls included in the meta-analysis.
| First author | Year | Country | Race | Control | ACP | Control | ACP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| *1/*1 | *1/*2 | *2/*2 | *1/*1 | *1/*2 | *2/*2 | *1 | *2 | *1 | *2 | ||||
| Day | 1990 | Northeast England | Northeast English indigenea | 79 | 0 | 0 | 13 | 0 | 0 | 158 | 0 | 26 | 0 |
| Matsumoto | 1996 | Japan | Japanese | 210 | 34 | 0 | 48 | 4 | 0 | 454 | 34 | 100 | 4 |
| Kimura | 2000 | Japan | Japanese | 20 | 24 | 3 | 27 | 4 | 0 | 64 | 30 | 58 | 4 |
| Frenzer | 2002 | Australia | Caucasian | 257 | 0 | 0 | 71 | 0 | 0 | 514 | 0 | 142 | 0 |
| Kim | 2004 | Korea | Korean | 75 | 35 | 12 | 19 | 8 | 2 | 186 | 58 | 46 | 12 |
| Halina | 2008 | Poland | Polish | 97 | 0 | 0 | 44 | 0 | 0 | 194 | 0 | 88 | 0 |
| Yokoyama | 2013 | Japan | Japanese | 1442 | 270 | 0 | 72 | 8 | 0 | 3154 | 270 | 132 | 8 |
Notes: ALDH2, aldehyde dehydrogenase 2; ACP, alcoholic chronic pancreatitis.
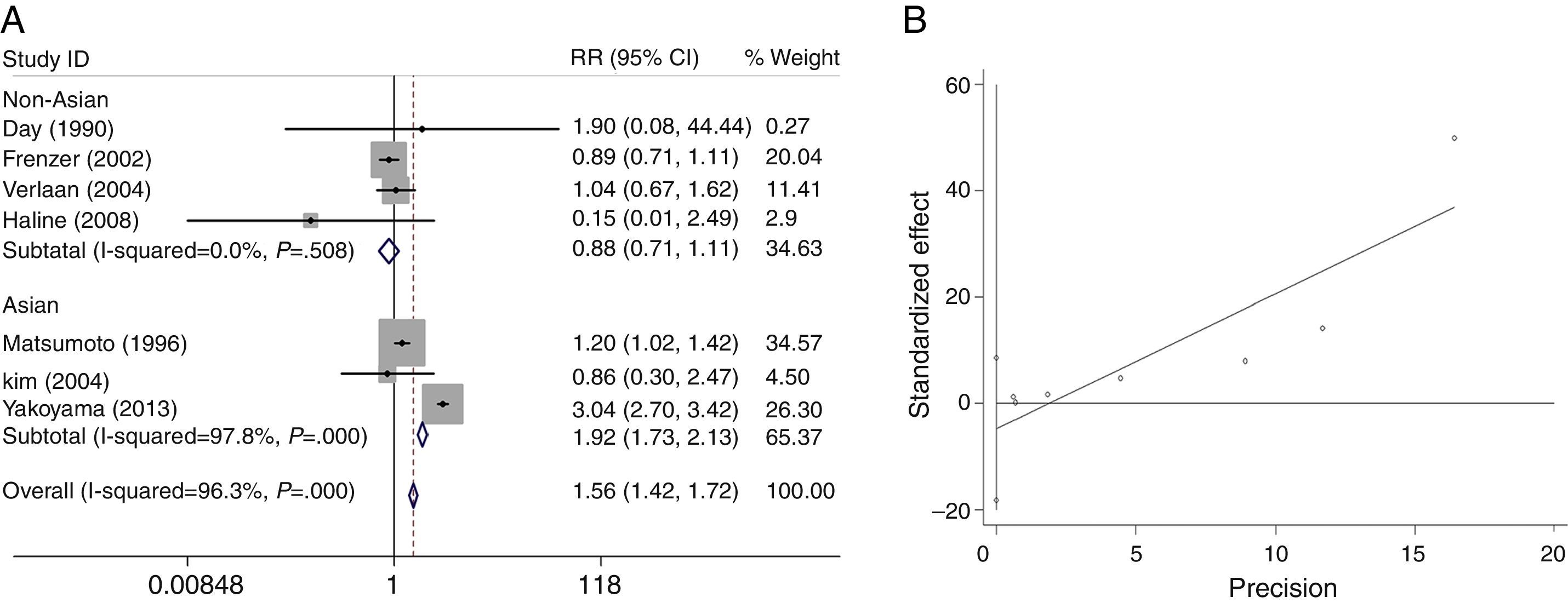
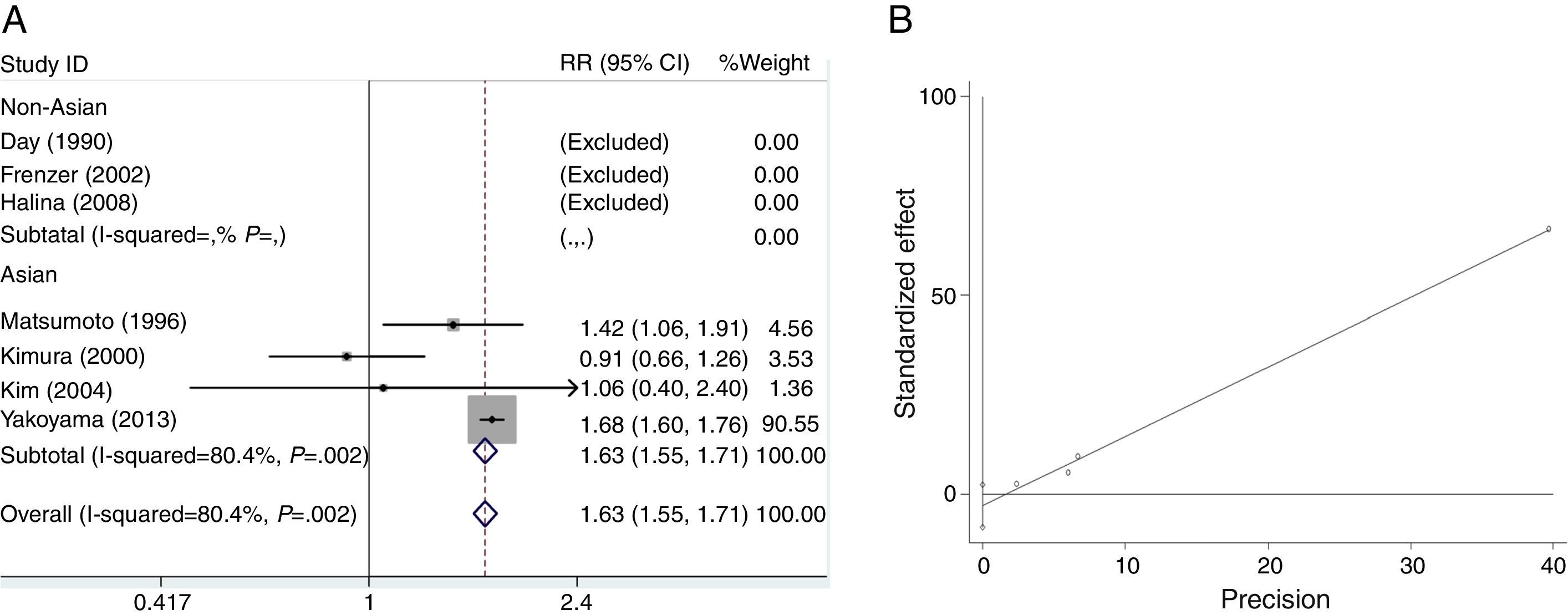
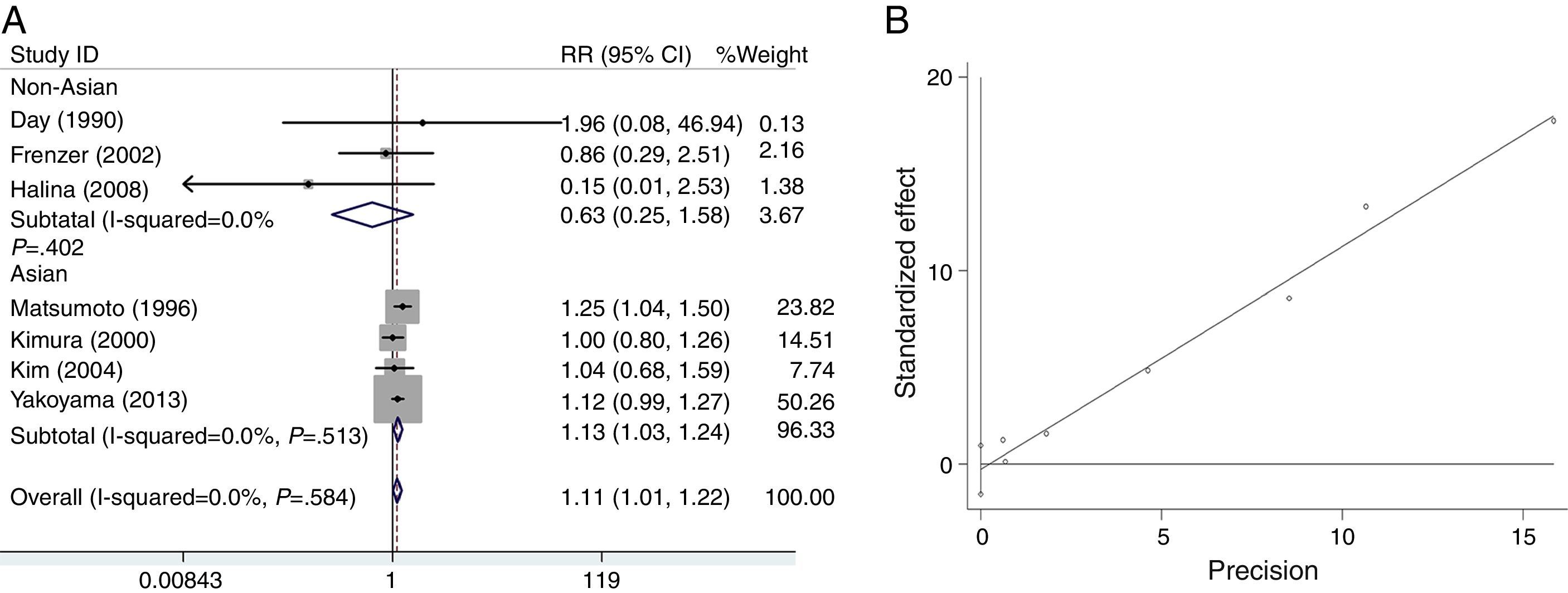
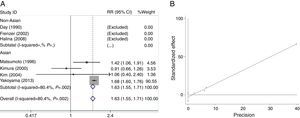
We analyzed the heterogeneity for the dominant (*2/*2+*1/*2 vs. *1/*1), a homozygote comparison model (*2/*2 vs. *1/*1) and allelic contrast (*2 vs. *1) models of ADH2 gene, respectively. Significant heterogeneities were found in *2/*2+*1/*2 vs. *1/*1 (I2=96.3%; P=0.000 for Q-test) (Fig. 2A) and *2/*2 vs. *1/*1 model (I2=80.4%; P=0.002 for Q-test) (Fig. 3A). Meanwhile, there were significant heterogeneities in Asian group for *2/*2+*1/*2 vs. *1/*1 (I2=97.8%; P=0.000 for Q-test) (Fig. 2A) and *2/*2 vs. *1/*1 (I2=80.4%; P=0.002 for Q-test) (Fig. 3A). Furthermore, no marked heterogeneities were found in *2 vs. *1 model (Fig. 4A).
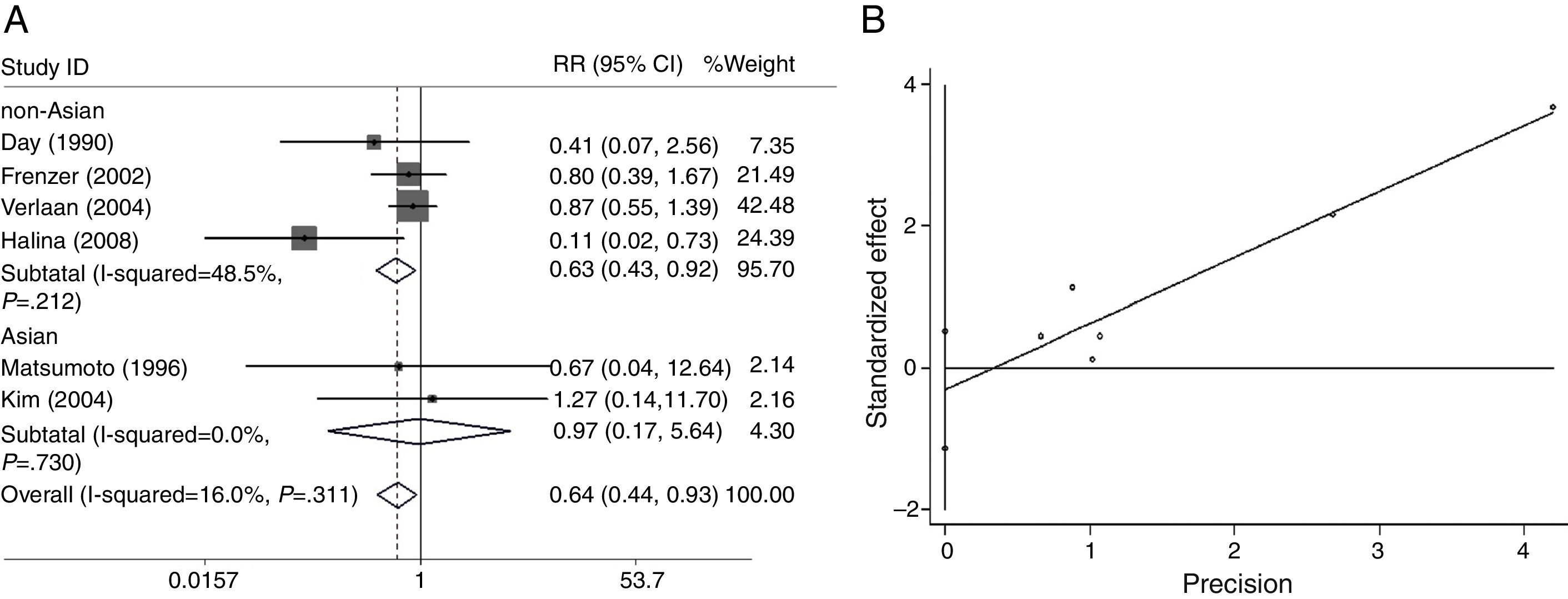
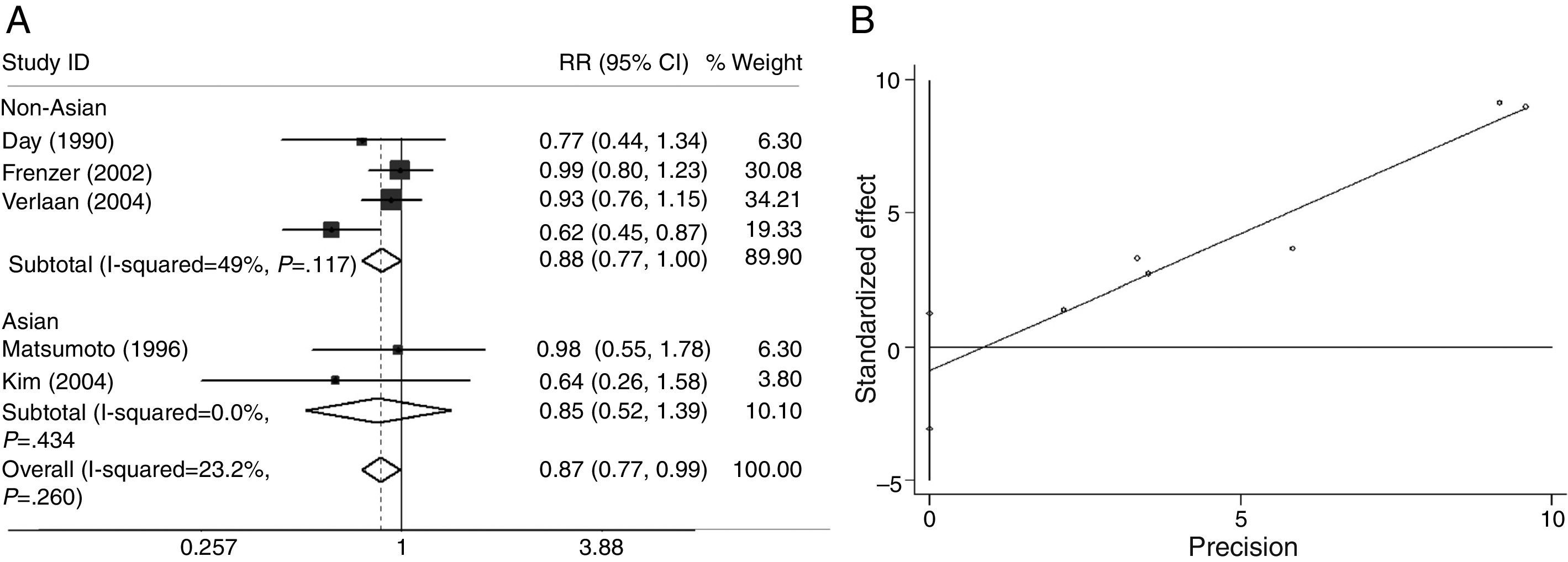
As shown in Figs. 5A and 6A, we analyzed the heterogeneity for the dominant (*2/*2+*1/*2 vs. *1/*1, data not shown), a homozygote comparison model (*2/*2 vs. *1/*1, Fig. 5A) and allelic contrast (*2 vs. *1, Fig. 6A) models of ADH3 gene, respectively. No marked heterogeneities for the overall data were found in three models (*2/*2+*1/*2 vs. *1/*1: I2=0.0%; P=0.542 for Q-test; *2/*2 vs. *1/*1: I2=16.0%; P=0.311 for Q-test; *2 vs. *1: I2=23.2%; P=0.260 for Q-test). Meanwhile, no heterogeneity was observed in the subgroup analyses (data not shown).
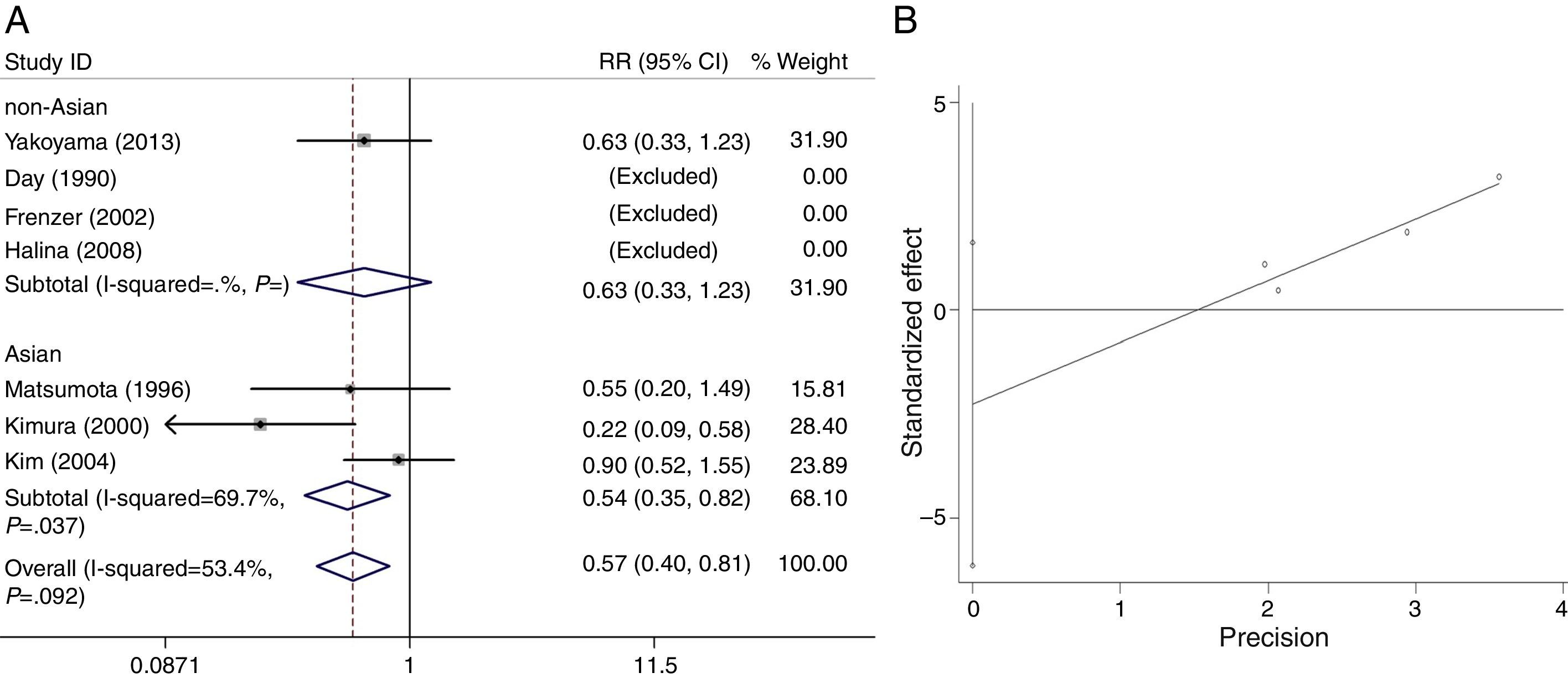
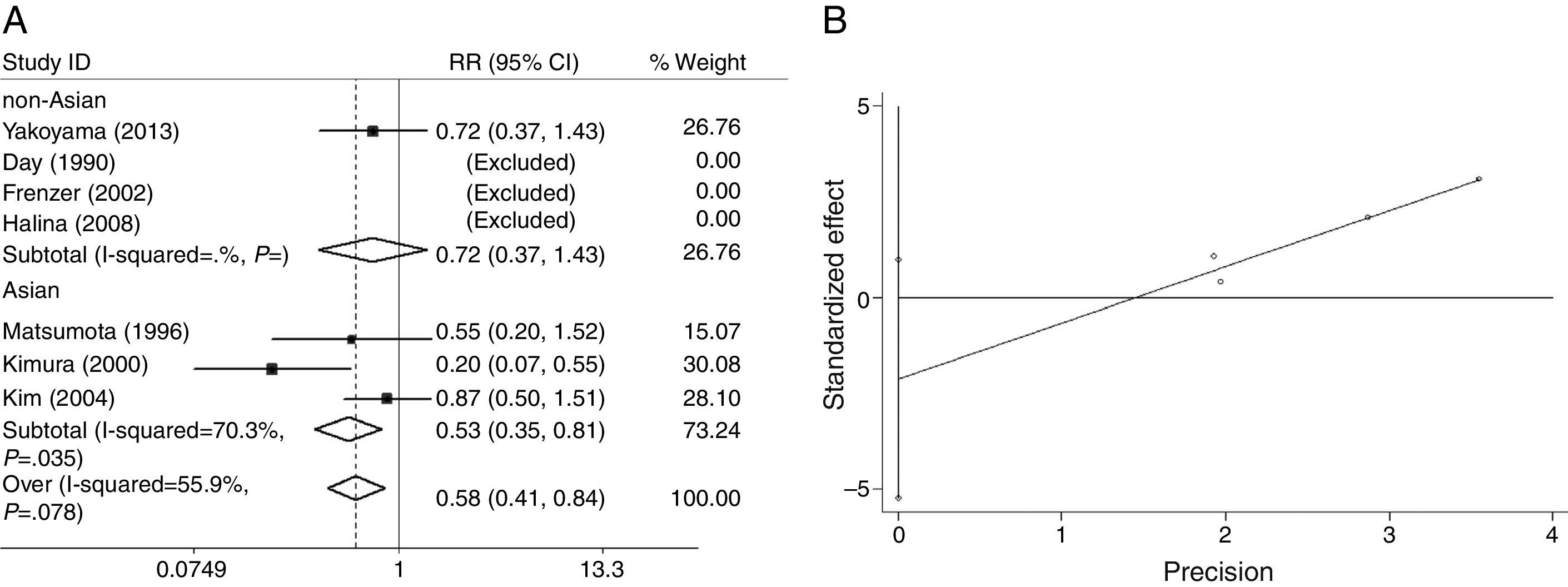
The heterogeneity for the dominant (*2/*2+*1/*2 vs. *1/*1, Fig. 7A), a homozygote comparison (*2/*2 vs. *1/*1) and allelic contrast (*2 vs. *1, Fig. 8A) models of ALDH2 gene were also analyzed, respectively. No marked heterogeneities for the overall data were found in three models (*2/*2+*1/*2 vs. *1/*1: I2=53.4%; P=0.092 for Q-test; *2/*2 vs. *1/*1: I2=0.0%; P=0.569 for Q-test; *2 vs. *1: I2=55.9%; P=0.078 for Q-test). However, in Asian groups, the results showed statistically conspicuous heterogeneity in dominant model (I2=69.7%, P=0.037, Fig. 7A) and allelic contrast model (I2=70.3%, P=0.035, Fig. 8A).
For the overall data including 313 cases and 2530 controls, results of the meta-analysis for ADH2 showed that the pooled ORs for the dominant, homozygote comparison, and allelic contrast models were 1.56 (95% CI=1.42–1.72, P=0.000), 1.63 (95% CI=1.55–1.71, P=0.000), and 1.11 (95% CI=1.01–1.22, P=0.030), respectively (Figs. 2A, 3A and 4A). These results suggested that ADH2 variations may increase alcoholic chronic pancreatitis susceptibility. Meanwhile, we further performed subgroup analysis on ethnicity and similar results were found in Asians (OR=1.92, 95% CI=1.73–2.13, P=0.000 for dominant model; OR=1.63, 95% CI=1.55–1.71, P=0.000 for homozygote comparison model; OR=1.13, 95% CI=1.03–1.24; P=0.011 for allelic contrast model); and non-Asians (OR=0.88, 95% CI=0.71–1.11, P=0.290 for dominant model, data not shown; OR=0.63, 95% CI=0.25–1.58; P=0.322 for allelic contrast model) (Figs. 2A, 3A and 4A). Analysis was not suitable for homozygote comparison in non-Asians because all the non-Asian studies were excluded.
The main results of the meta-analysis for ADH3 are listed in Figs. 5A and 6A. For the overall data including 291 cases and 1020 controls, the pooled ORs for the dominant, homozygote comparison, and allelic contrast models were 0.57 (95% CI=0.40–0.81, P=0.389) (data not shown), 0.64 (95% CI=0.44–0.93, P=0.020), and 0.87 (95% CI=0.77–0.99, P=0.039), respectively. A significant decrease in alcoholic chronic pancreatitis risk was found in individuals with ADH3 *2/*2 genotype (OR=0.64, 95% CI=0.44–0.93, P=0.020, Fig. 5A) or *2 allele (OR=0.87, 95% CI=0.77–0.99, P=0.039, Fig. 6A). Subgroup analysis on ethnicity showed that in the non-Asian subgroups, a significant decrease in alcoholic chronic pancreatitis risk was found in homozygote comparison model (OR=0.63, 95% CI=0.43–0.92, P=0.017, Fig. 5A) and allelic contrast model (OR=0.88, 95% CI=0.77–1.00, P=0.049, Fig. 6A), while not in dominant model (OR=0.97, 95% CI=0.87–1.07, P=0.500, data not shown) and all three models of Asians (OR=0.87, 95% CI=0.54–1.71, P=0.581 for dominant model, data not shown; OR=0.97, 95% CI=0.17–5.64, P=0.972 for homozygote comparison, Fig. 5A; OR=0.85, 95% CI=0.52–1.39; P=0.525 for allelic contrast, Fig. 6A).
The main results of the meta-analysis for ALDH2 are listed in Figs. 7A and 8A. For the overall data including 320 cases and 2556 controls, the pooled ORs for the dominant, homozygote comparison, and allelic contrast models were 0.57 (95% CI=0.40–0.81, P=0.002) (Fig. 7A), 0.50 (95% CI=0.23–1.08, P=0.079) (data not shown), and 0.58 (95% CI=0.41–0.84, P=0.003, Fig. 8A), respectively. A significant decrease in alcoholic chronic pancreatitis risk was found in individuals with ALDH2 *2/*2+*1/*2 genotypes or *2 allele. Subgroup analysis on ethnicity showed that in the Asians, a significant decrease in alcoholic chronic pancreatitis risk was found in dominant model (OR=0.54, 95% CI=0.35–0.82, P=0.004, Fig. 7A) and allelic contrast model (OR=0.53, 95% CI=0.35–0.81, P=0.004, Fig. 8A), while not in homozygote comparison model (OR=0.50, 95% CI=0.23–1.08, P=0.079, data not shown), and two models of non-Asians (OR=0.63, 95% CI=0.33–1.23, P=0.179 for dominant model, Fig. 7A; OR=0.72, 95% CI=0.37–1.43; P=0.355 for allelic contrast, Fig. 8A) (analysis was not suitable for homozygote comparison in non-Asians because all the studies were excluded).
Sensitivity analysis and bias diagnosticsWhen the effect models were changed, the significance of the overall data for the three models of ADH2, ADH3 and ALDH2 was not statistically altered (data not shown).
Funnel plots were created to assess possible publication biases (Figs. 2B–8B). The results of funnel plots and Egger's tests indicated the absence of publication biases for ADH2 (dominant: t=1.35, P=0.176; homozygote comparison: t=−0.68, P=0.497; and allelic contrast: t=−0.19, P=0.851) (Figs. 2B, 3B and 4B); ADH3 (dominant: t=−1.40, P=0.234, data not shown; homozygote comparison: t=−1.05, P=0.353, Fig. 5B; and allelic contrast: t=−1.15, P=0.312, Fig. 6B); and ALDH2 (dominant: t=−2.50, P=0.130, Fig. 7B; homozygote comparison: t=−0.85, P=0.552, data not shown; and allelic contrast: t=−2.95, P=0.099, Fig. 8B).
DiscussionOur study showed that ADH2 significantly increased alcoholic chronic pancreatitis risk in ADH2*1/*2+*2/*2, ADH2*2/*2 and ADH2*2 allele. This may be because of different susceptibilities of alcoholism affected by ADH2 polymorphism;26 the less-active form of ADH2 encoded by ADH2*1/*1 increases the risk of alcoholism compared with the super-active forms of ADH2 encoded by the ADH2*2 allele. Moreover, significantly ethnic differences existed between ADH2 polymorphism and alcoholic pancreatitis risk such that markedly increased risks were found among Asian individuals, but not in non-Asians. ADH2*2/*2 occurs rarely among white populations or Europeans.8 In the present meta-analysis, among the obtained three studies of non-Asians, the rates of ADH2*2/*2 frequency in both control and alcoholic chronic pancreatitis were 0%; thus, analysis was not suitable for homozygote comparison in non-Asians.
Our results of ADH3 suggested that significantly decreased alcoholic chronic pancreatitis risk was found in ADH3*2/*2 genotype and ADH3*2 allele but not in ADH3 dominant model. Furthermore, in the subgroup analysis on ethnicity, significantly decreased alcoholic pancreatitis risk was found among non-Asians with ADH3*2/*2 genotype and ADH3*2 allele; but not in Asians. These results may be owing to chance because the limited number of included studies of Asian groups and small sample sizes may give rise to insufficient statistical power to assess a minor effect. Therefore, the results should be interpreted with judgment. Further investigations with more studies and larger sample sizes regarding Asians are needed to clear the possible effects of ADH3 ethnic variations on alcoholic chronic pancreatitis risk.
It is reported that functional polymorphism of ALDH2 gene can alter its ethanol-metabolizing activity in pancreatitis.27 The ALDH2*1*2 and *2*2 genotypes are known as protective factors of alcoholism, in pure and alcoholism comorbid with anxiety and depression. In the current study, alcoholic chronic pancreatitis risk significantly decreased in ALDH2*2/*2+*1/*2 genotype and ALDH2*2 allele. But only decreased risk trend was found in ALDH2*2/*2 genotype. Meanwhile, in the subgroup analysis on ethnicity, significantly decreased alcoholic chronic pancreatitis risk was found among Asians with ALDH2*2/*2+*1/*2 genotype and ALDH2*2 allele; but not in non-Asians. There were some reasons for these results. Firstly, the frequency of ALDH2*2/*2 rarely occurs in white population.6,28 In our data, the rates of ALDH2*2/*2 frequency in both control and alcoholic chronic pancreatitis of non-Asian groups were 0%; therefore, analysis was not suitable for homozygote comparison in non-Asians. Secondly, only three studies were included and the sample sizes may be a little small, so only increased risk trend was found in ALDH2*2/*2 genotype.
Although the latest studies for ADH2, ADH3 and ALDH2 polymorphisms and alcoholic chronic pancreatitis were included in this meta-analysis, several limitations are presented. First, only published articles were included. Therefore, publication bias may have occurred, though funnel plots did not indicate any significant publication biases. Second, our results were based on unadjusted evaluations of ORs without adjustment for individual's age and sex, race, due to a lack of original data. Third, obvious heterogeneities were found in analyses for two models of ADH2 and ALDH2, and some underlying heterogeneity may also exist owing to individuals’ age, sex difference and variation in sample sizes, study designs and genotyping methods. Fourth, mixed control groups were used in this study, whose risks of developing pancreatitis may be different regardless of genotype; this may devalue the results of this study. Subgroup analysis on control groups is required, but this meta-analysis did not include sufficient studies to permit such an analysis. Fifth, the very low number of studies available may devalue this meta-analysis, especially the conclusion about ethnic differences (sometimes derived from only one or two studies). In the metabolism of alcohol, it is metabolized to acetaldehyde by ADH and by ALDH2 to acetate. Perhaps both metabolism of alcohol and aldehyde affect the risk of chronic pancreatitis. Thus, large and well-designed studies are needed to confirm these results.
In conclusions, the overall data revealed a significant association of ADH2, ADH3 and ALDH2 polymorphism with alcoholic chronic pancreatitis risk. The subgroup analyses suggested that the variant ADH3*2/*2 genotype and ADH3*2 allele may modify alcoholic chronic pancreatitis susceptibility among non-Asian individuals; and the variant ADH2*2/*2+*1/*2, ADH2*2/*2 genotype, ADH2*2 allele and ALDH2*2/*2+*1/*2 genotype, ALDH2*2 allele may modify alcoholic chronic pancreatitis susceptibility among Asians.
Conflict of interestThe authors declare no conflict of interest.
This work was supported by Scientific Research Program for Undergraduates of Wuhan University (S2008259).