Several cases of chronic infection by hepatitis E virus (HEV) in immunocompromised patients have been described recently. Patients with inflammatory bowel disease (IBD) are frequently immunocompromised because of the disease itself or due to therapy. Our aims were to determine HEV seroprevalence in patients with IBD and to detect possible chronic forms.
MethodsWe prospectively selected a random sample of 87 patients from our local IBD clinic database at the Gastroenterology Service, Hospital Ramón y Cajal, in Madrid, Spain. Patients completed an oral epidemiologic interview. Anti-HEV IgG and IgM antibodies and HEV-RNA were determined. Medical records were reviewed, focusing on drug exposure.
ResultsWe included 87 patients, with a mean age of 44.7 years (SD 16) and a mean of 10.4 years (SD 8.4) with IBD. Fifty-seven percent were diagnosed with Crohn's disease, 41.4% with ulcerative colitis and 1.1% with unclassified IBD. A total of 41.4% had received systemic glucocorticoids for more than 3 months, 32.2% had been treated with thiopurines, 16.1% with biological drugs, and 3.4% with methotrexate. Anti HEV-IgM was determined in 75 patients and IgG in 80, and were positive in 2.7% and 1.3%, respectively. HEV-RNA was analyzed in a random subset of 46 patients, and all determinations were negative. Therefore, no case of chronic HEV infection was detected.
ConclusionsWe found a low HEV seroprevalence of just 1.14% in patients with IBD, similar to that in the general population. This could be due to the lower degree of immunosuppression in this group, or to different dietary habits.
Recientemente se han descrito varios casos de infección crónica por el virus de la hepatitis E (VHE) en pacientes inmunodeprimidos. Los pacientes con enfermedad inflamatoria intestinal (EII) suelen estar inmunodeprimidos debido a la enfermedad en sí o debido a los tratamientos recibidos. Nuestro objetivo fue determinar la seroprevalencia de VHE en pacientes con EII y detectar posibles formas crónicas.
MétodosAnalizamos de forma retrospectiva una muestra aleatorea de 87 pacientes de nuestra base de datos de la consulta de EII en el Servicio de Gastroenterología del Hospital Ramón y Cajal, en Madrid, España. Los pacientes respondieron una encuesta epidemiológica oral y se determinaron anticuerpos IgG e IgM frente al VHE, así como RNA de VHE. Se revisaron las historias médicas, haciendo especial hincapié en los tratamientos recibidos.
ResultadosIncluímos 87 pacientes con una edad media de 44,7 años (D.E.16) y una media de 10,4 (D.E. 8,4) años de enfermedad. El 57% tenían una enfermedad de Crohn, 41,4% colitis ulcerosa y 1,1% colitis indeterminada. El 41,4% de ellos habían recibido corticoides sistémicos durante más de 3 meses, el 32,3% habían sido tratados con tiopurinas, el 16,1% con fármacos biológicos y el 3,4% con metotrexato. Se determinó la IgM frente a VHE en 75 pacientes y la IgG en 80, resultando positivos en 2,7% y 1,3% respectivamente. El RNA de VHE se analizó en un subgrupo aleatorio de 46 pacientes, y todas las determinaciones fueron negativas, así que no se detectó ningún caso de infección crónica por VHE.
ConclusionesEncontramos una baja seroprevalencia de tan sólo 1,14% en los pacientes con EII, dato similar al de la población general. Esto podría explicarse por un menor grado de inmunosupresión en este grupo, o a diferentes hábitos dietéticos.
Immunocompromised hosts are at an increased risk of infections, both severe and non-severe. Immunocompromised state can be achieved, either intentionally or as a side effect, through the use of a therapeutic plan. Patients with Inflammatory Bowel Disease (IBD) are frequently treated with drugs such as glucocorticosteroids, thiopurines, and anti-TNF agents or other biologicals, all of which are affected by this problem. Some of these infections are clinically evident, whereas the increased incidence of others has to be studied specifically through epidemiological or serological surveys.
Hepatitis E is an emerging disease, caused by the hepatitis E virus (HEV), a small icosahedral, round, nonenveloped virus, with a single-stranded RNA, classified into the Hepeviridae family, genus Hepevirus.1 Four genotypes have been described, with a specific geographical distribution. In developing countries (India, Southeast and Central Asia, the Middle East, Africa and Mexico)2 genotypes 1 and 2 predominate, appearing in epidemics and clearly transmitted from human to human by a fecal-oral route.3 In developed countries, infection can also be caused by genotypes 1 and 2, after importation from developing countries, or alternatively be due to autochthonous genotypes 3 and 4,4–6 probably in more than 90% of cases.7 Hepatitis E is a zoonosis that has been identified in domestic and wild porcines, chickens, rats, rabbits, deer, mongoose, cattle and sheep, among other.8 For this reason, eating raw meat, or having a close contact with animals (hunters, farmers, having pets, living in the countryside…) are risk factors for HEV infection.
The incidence of HEV infection is difficult to estimate clinically, because most cases are paucisymptomatic. Seroprevalence in developed countries is variable: 10.72–19.96% in Spanish healthy blood donors,9 16% in English healthy blood donors10 and 21% in the general population of the USA.11 This wide variety is not only explained by geographic factors, but also by the low concordance between the different tests employed,12 and by the diminished diagnostic power of anti HEV-IgG in non-endemic countries.13
Different serological markers of HEV infection are available. Anti HEV IgM, appears in the acute phase of the infection and lasts for 4–5 months,14 whereas IgG, another very early marker, rises very slightly later or simultaneously to IgM response. In endemic areas, these markers are good indicators of current infection and past exposure to HEV respectively. However, this is not applicable in non-endemic countries. Viral RNA is the most important virological marker, especially in immunocompromised patients. When it is detected for more than 6 months, it gives the definition of chronic infection, whereas if it lasts for less than 6 months, it is considered as an acute infection.
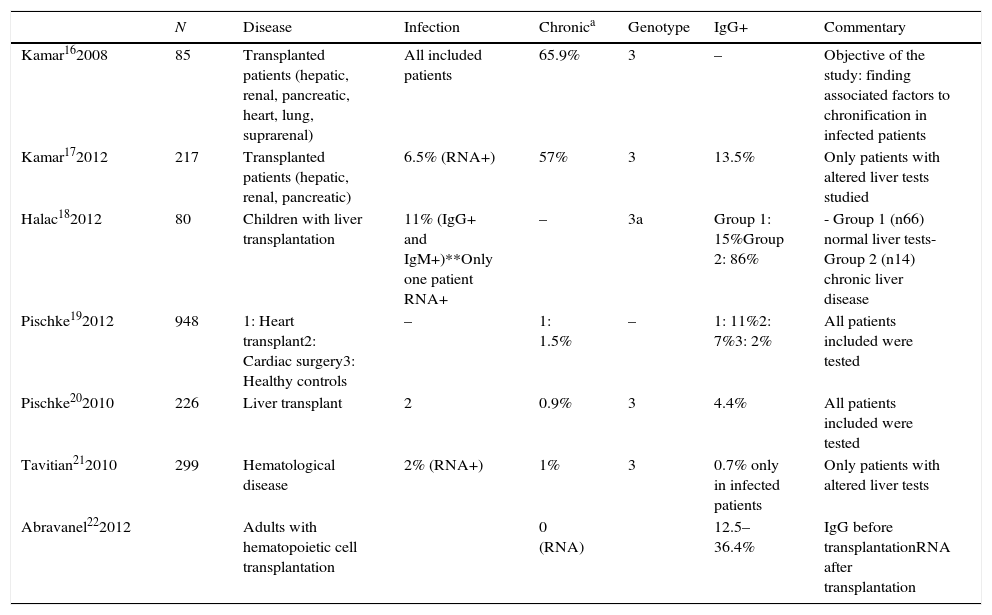
Traditionally, HEV has been considered a pathogen responsible of acute infection. It is usually asymptomatic, although infection can result in jaundice, tiredness, nausea, and vomiting. In cirrhotic patients and pregnant women, HEV infection can cause a fulminant hepatic failure.15 However, in the last years, several cases of chronic infection in immunocompromised patients have been described (including patients with transplanted organs, hematological malignancies or HIV). Table 1 shows a compilation of studies including more than 10 patients and describing cases of chronic HEV hepatitis.16–22 Most authors agree that HEV seroprevalence is higher in immunocompromised patients than in healthy controls. Seroprevalence varies from 5% to 86%. This wide range is due to the different criteria chosen for patient selection: some studies included only patients with altered liver tests, while others selected patients with clinical liver disease. The low seroprevalence communicated by some authors21 is due to their choice of viral RNA as only marker for diagnosis of HEV infection.
Published studies of HEV seroprevalence.
| N | Disease | Infection | Chronica | Genotype | IgG+ | Commentary | |
|---|---|---|---|---|---|---|---|
| Kamar162008 | 85 | Transplanted patients (hepatic, renal, pancreatic, heart, lung, suprarenal) | All included patients | 65.9% | 3 | – | Objective of the study: finding associated factors to chronification in infected patients |
| Kamar172012 | 217 | Transplanted patients (hepatic, renal, pancreatic) | 6.5% (RNA+) | 57% | 3 | 13.5% | Only patients with altered liver tests studied |
| Halac182012 | 80 | Children with liver transplantation | 11% (IgG+ and IgM+)**Only one patient RNA+ | – | 3a | Group 1: 15%Group 2: 86% | - Group 1 (n66) normal liver tests- Group 2 (n14) chronic liver disease |
| Pischke192012 | 948 | 1: Heart transplant2: Cardiac surgery3: Healthy controls | – | 1: 1.5% | – | 1: 11%2: 7%3: 2% | All patients included were tested |
| Pischke202010 | 226 | Liver transplant | 2 | 0.9% | 3 | 4.4% | All patients included were tested |
| Tavitian212010 | 299 | Hematological disease | 2% (RNA+) | 1% | 3 | 0.7% only in infected patients | Only patients with altered liver tests |
| Abravanel222012 | Adults with hematopoietic cell transplantation | 0 (RNA) | 12.5–36.4% | IgG before transplantationRNA after transplantation |
Patients with IBD are frequently immunocompromised, not only because of their disease, but also due to treatments received. However, HEV prevalence in this population has not been studied yet. Our aim was to determine HEV seroprevalence and to detect the possible existence of chronic forms in patients with IBD.
Patients and methodsWe prospectively selected a random sample of 87 patients from the database used in the IBD Clinic of the Gastroenterology Service, Hospital “Ramón y Cajal”, in Madrid, Spain. This means 5.8% of 1050 patients included in this database. The random selection was intentionally applied in order to avoid an infra- or over-representation of certain forms of disease. Patients were contacted, informed and asked to participate. After giving their informed consent, they completed an oral interview on lifestyle and dietary habits, including possible risk factors for transmission of HEV, such as international travel to developing countries, contact with animals, living in a rural environment, surgical interventions, professional exposure, blood transfusions, or ingestion of potentially risky food (raw, partially cooked).
A blood sample was drawn and medical files were reviewed, analyzing disease evolution and treatments received, focusing on drugs with direct effect on immune system, such as systemic glucocorticosteroids, thiopurines, biological treatments (infliximab, adalimumab) and metothrexate. Anti-HEV-IgG antibodies were detected in serum by commercial enzyme immunoassay (EIA) (HEV Ab, DiaPro Diagnostic Bioprobes, Milan, Italy), following the manufacturer's instructions. All samples positive to IgG and additionally, 74 samples negative to IgG were studied further for the presence of anti-HEV IgM antibodies (HEV IgM, DiaPro Diagnostic Bioprobes, Milan, Italy). A result was considered positive by both tests when the ratio of the sample optical density and the cut-off value was higher than 1. Positive results by EIA were confirmed by Western blot analysis (RecomBlot HEV IgG/IgM; Mikrogen, Martinsried, Germany). In addition, HEV-RNA was determined by reverse transcriptase (RT)-nested PCR amplification in all serum samples with IgM or IgG anti-HEV (three patients) and in 43 aleatorised IgG and IgM negative serum samples. HEV-RNA was not determined in all the patients due to economic reasons.
For analysis, we employed SPSS 20.00. Quantitative variables were expressed in means and standard deviation (S.D.), and qualitative variables in percentages and absolute numbers.
Ethical considerationsThe study protocol was approved by the local Ethic Committee. All the patients received complete information about the aims and procedures of the study, and gave their written consent to be included in it.
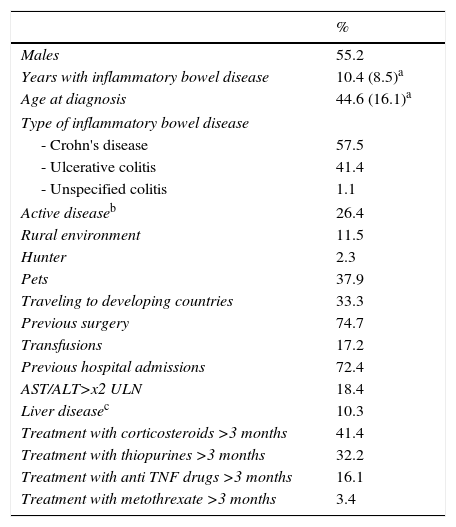
ResultsDemographicsWe included 87 patients (48 men), with a mean age of 44.7 years (S.D. 16) and 10.4 (D.E. 8.4) years with IBD. According to the type of disease, 57.5% were diagnosed with Crohn's disease, 41.4% with ulcerative colitis and 1.1% with unclassified IBD. Disease was active in 26.4% of patients. Factors and markers of risk are shown in Table 2. Focusing on previous drug exposure, 41.4% had received systemic glucocorticosteroids for more than 3 months, 32.2% had been treated with thiopurines, 16.1% with biological drugs and 3.4% with methotrexate.
Demographical characteristics of patients recluted.
| % | |
|---|---|
| Males | 55.2 |
| Years with inflammatory bowel disease | 10.4 (8.5)a |
| Age at diagnosis | 44.6 (16.1)a |
| Type of inflammatory bowel disease | |
| - Crohn's disease | 57.5 |
| - Ulcerative colitis | 41.4 |
| - Unspecified colitis | 1.1 |
| Active diseaseb | 26.4 |
| Rural environment | 11.5 |
| Hunter | 2.3 |
| Pets | 37.9 |
| Traveling to developing countries | 33.3 |
| Previous surgery | 74.7 |
| Transfusions | 17.2 |
| Previous hospital admissions | 72.4 |
| AST/ALT>x2 ULN | 18.4 |
| Liver diseasec | 10.3 |
| Treatment with corticosteroids >3 months | 41.4 |
| Treatment with thiopurines >3 months | 32.2 |
| Treatment with anti TNF drugs >3 months | 16.1 |
| Treatment with metothrexate >3 months | 3.4 |
ULN: upper limit of normal values.
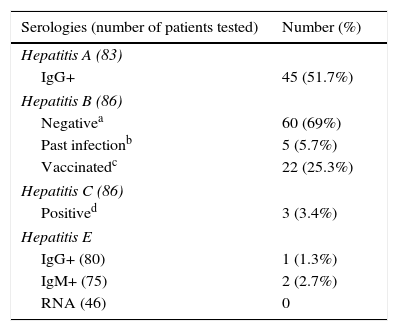
Results of the antibody determinations against HEV, HAV, HBV, and HCV are shown in Table 3.
Antibodies against HAV were determined in 83 patients: IgG was positive in 45 patients (51.7%). HBV serologies were tested in 86 patients: 25.3% were vaccinated, 5.7% had serological markers of past hepatitis B infection with HBs antigen and the rest of them were negative. Anti HCV antibodies were positive in 3 out of 86 (3.4%) patients.
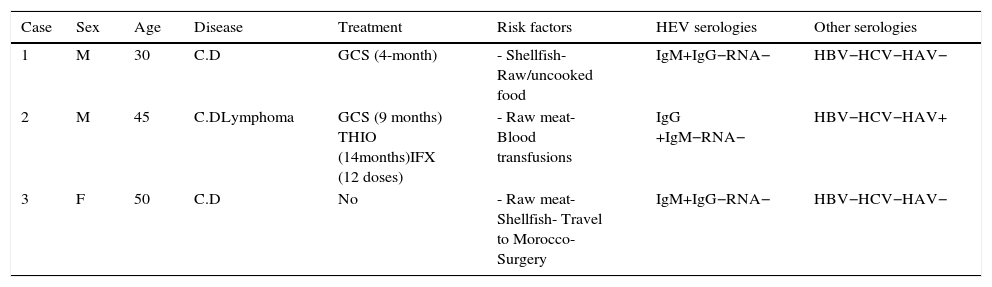
Five patients did not have blood drawn for determination of HEV serologies, despite having answered the oral interview. IgM was indeterminate in seven patients and IgG in two patients, being positive in 2.7% and 1.3%, respectively, HEV-RNA was negative in all determinations (46 patients), so no case of chronic infection by HEV was detected. We have recently done a retest on patients who were positive to IgM, and none of them have now IgM and IgG. This could be explained by two possibilities: they could have been false positive results or there could have been a clearance of IgG after such a long time between the first and the second test (3 years approximately). A more detailed account of cases with positive IgG or IgM against HEV is shown in Table 4.
Cases with positive IgG or IgM against HEV.
| Case | Sex | Age | Disease | Treatment | Risk factors | HEV serologies | Other serologies |
|---|---|---|---|---|---|---|---|
| 1 | M | 30 | C.D | GCS (4-month) | - Shellfish- Raw/uncooked food | IgM+IgG−RNA− | HBV−HCV−HAV− |
| 2 | M | 45 | C.DLymphoma | GCS (9 months) THIO (14months)IFX (12 doses) | - Raw meat- Blood transfusions | IgG +IgM−RNA− | HBV−HCV−HAV+ |
| 3 | F | 50 | C.D | No | - Raw meat- Shellfish- Travel to Morocco- Surgery | IgM+IgG−RNA− | HBV−HCV−HAV− |
M: male; F: female; C.D: Crohn's disease; GCS: glucocorticosteroids; THIO: thiopurines; IFX: infliximab; HEV: hepatitis E virus; HBV: hepatitis B virus; HCV: hepatitis C virus.
Patients with IBD represent an immunosuppressed subpopulation, at a higher risk for infections. This has clinical importance; for instance, CMV infection has been described as a clinical course modifier23 and certain treatments have been found to worsen the course of HBV infection.24
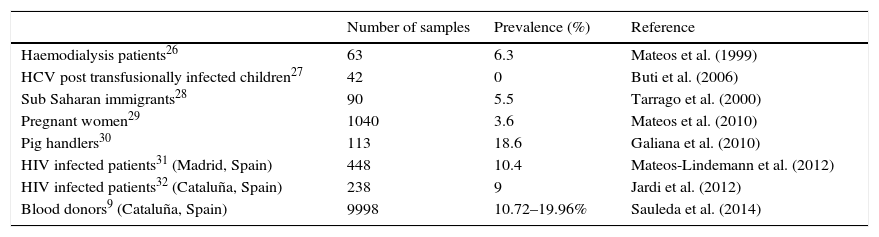
Published surveys suggest a higher seroprevalence and trend to chronification of HEV in immunosuppressed populations; therefore, a similar situation could be expected in IBD patients. However, in the population studied (which can be considered representative of the whole group of IBD patients, due to its random selection), a low seroprevalence of just 1.14%, was found, well below the corresponding figure found in Spanish healthy blood donors in Spain, namely 10.72–19.96%.9 This seroprevalence is even much lower than expected if it is compared with other Spanish risk groups (Table 5). All the studies in the table have used the same serological tests, so results are comparable.
Prevalence of anti-HEV IgG antibodies among different groups of population in Spain.
| Number of samples | Prevalence (%) | Reference | |
|---|---|---|---|
| Haemodialysis patients26 | 63 | 6.3 | Mateos et al. (1999) |
| HCV post transfusionally infected children27 | 42 | 0 | Buti et al. (2006) |
| Sub Saharan immigrants28 | 90 | 5.5 | Tarrago et al. (2000) |
| Pregnant women29 | 1040 | 3.6 | Mateos et al. (2010) |
| Pig handlers30 | 113 | 18.6 | Galiana et al. (2010) |
| HIV infected patients31 (Madrid, Spain) | 448 | 10.4 | Mateos-Lindemann et al. (2012) |
| HIV infected patients32 (Cataluña, Spain) | 238 | 9 | Jardi et al. (2012) |
| Blood donors9 (Cataluña, Spain) | 9998 | 10.72–19.96% | Sauleda et al. (2014) |
In our study, patients were in different moments according to their treatments: some of them were receiving immunosuppressive therapy at the moment of the study and others had been treated before. Both groups were considered, because immunosuppressive therapy is important, not only due to its effects on immune system, but also as an indicator of illness severity.
It must be noted that the degree of immunosupression in this group is not the same magnitude as in transplanted patients. Kamar et al.16 showed that independent risk factors for HEV infection in transplanted patients were platelet count and use of tacrolimus, a drug not used in our patient set. Other studies performed in different patient populations, such as oncohematologic patients, showed a rate of HEV infection similar to the one described by us.22 Clearly, there must be other yet unknown factors that play an important role in the pathogenesis of this infection. Some of the mechanisms of transmission in industrialized countries are related to dietary and life habits, which are difficult to assess. These habits may differ in IBD patients may, and thus result in a lower rate of infection in this subgroup.
The main limitation present in our study that may interfere with the results is the small sample size, which can result in low potency for detecting a real seroprevalence, justifying the lower percentage than the general population. Besides, the most reliable marker for diagnosis of HEV infection is RNA-PCR, because immunodepressed patients create antibodies later and in a smaller quantity than healthy people. Anti-HEV IgM has a specificity of 78–98% and a sensitivity of 72–98% in the detection of HEV infection.25 Because of Budget limitations, viral RNA was determined in a random subsample of our series, so this could have interfered our results. However, it is remarkable that none of these patients had a positive result for RNA-PCR.
In summary, we cannot conclude that patients with IBD have a higher rate of HEV infection than the general population. On the contrary, our data show that the prevalence may be lower than in other population groups such as blood donors. Confirmatory studies with a larger number of patients could be useful to determine the real rate of HEV infection in this population.
Conflicts of interestNone declared.
Source of fundingNone.