Acute hepatitis A is usually a self-limited viral disease but can be severe and even fatal in special groups of patients including those with chronic liver disease and recipients of liver transplantation. To take appropriate preventive measures, it is important to determine the immune status against the hepatitis A virus in patients at risk of grave clinical outcomes following infection. To assess the need for immunization against hepatitis A, we aimed to determine the immune status against hepatitis A in a population of liver transplant recipients. We also investigated the association between hepatitis A immune status and demographic factors such as age and sex, underlying liver disease, source of drinking water, geographical area of residence and socioeconomic status.
MethodsThis cross-sectional study was performed on 242 recipients of allogenic liver transplants at Abu Ali Sina Organ Transplant Hospital in Shiraz, Iran, between January 2017 and April 2017. The level of immunity was assessed using hepatitis A antibody detection kits.
ResultsThe rate of immunity against hepatitis A was detected as 88.8% in our study population. In the multivariable logistic regression model, younger age (OR=1.175, P<0.001) and higher education level (OR=2.142, P=0.040) were the main determinants of non-immune status. However, hepatitis A immunity was independent of gender, monthly family income, water supply source, residential area and underlying liver disorder.
ConclusionAlthough a significant proportion of liver transplant recipients in this study showed evidence of natural immunity to hepatitis A, a considerable proportion of younger patients and those with a higher level of education were non-immune. The results of this study signify the importance of screening for hepatitis A immunity in this at-risk population of patients and the need for vaccinating non-immune patients.
La hepatitis A aguda suele ser una enfermedad viral autolimitada, pero puede ser grave e incluso mortal en grupos especiales de pacientes, incluidos aquellos con enfermedad hepática crónica y los receptores de un trasplante de hígado. Para tomar las medidas preventivas adecuadas, es importante determinar el estado inmunológico frente al virus de la hepatitis A en pacientes con riesgo de sufrir resultados clínicos graves después de la infección. Para evaluar la necesidad de inmunización contra la hepatitis A, nuestro objetivo fue determinar el estado inmunológico contra la hepatitis A en una población de receptores de trasplante de hígado. También investigamos la asociación entre el estado inmunológico de la hepatitis A y factores demográficos como la edad y el sexo, la enfermedad hepática subyacente, la fuente de agua potable, el área geográfica de residencia y el nivel socioeconómico.
Métodoseste estudio transversal se realizó en 242 receptores de trasplantes de hígado alogénicos en el hospital de trasplantes de órganos “Abu Ali Sina” en Shiraz, Irán, entre enero de 2017 y abril de 2017. El nivel de inmunidad se evaluó mediante kits de detección de anticuerpos contra la hepatitis A.
ResultadosLa tasa de inmunidad contra la hepatitis A se detectó como 88,8% en nuestra población de estudio. En el modelo de regresión logística multivariable, la edad más joven (OR=1,175, p<0,001) y el nivel de educación superior (OR=2,142, p=0,040) fueron los principales determinantes del estado no inmunitario. Sin embargo, la inmunidad contra la hepatitis A fue independiente del sexo, el ingreso familiar mensual, la fuente de suministro de agua, el área residencial y la enfermedad hepática subyacente.
ConclusiónAunque una proporción significativa de los receptores de trasplante de hígado en este estudio mostró evidencia de inmunidad natural a la hepatitis A, una proporción considerable de pacientes más jóvenes y aquellos con un mayor nivel de educación no eran inmunes. Los resultados de este estudio demuestran la importancia del cribado de la inmunidad contra la hepatitis A en esta población de pacientes en riesgo y la necesidad de vacunar a los pacientes no inmunes.
Hepatitis A infection is a self-limited acute viral infection of the liver with an oral-fecal route of transmission. At-risk populations include individuals from endemic regions (India, Africa, and the Middle-East), infants and children, people living in poorly sanitized conditions, and men who have sex with men (MSM).1 The hepatitis A virus (HAV) has a worldwide distribution with over 1.4 million cases of infection being reported annually.2 However, more recent data suggest a declining rate of infection and a gradual change in epidemiological characteristics.3 Clinical manifestations can vary from a lack of symptoms with only abnormal liver biochemistry to presentations with general symptoms such as nausea, vomiting, and jaundice.4 In its most severe form, acute hepatitis A can lead to liver failure requiring transplantation, which is rare and occurs in less than 1% of cases.5 Mortality from acute HAV infection is seen mainly in older patients and subjects with chronic liver disease.4,6 The initial antibody response to the HAV infection consists of developing immunoglobulin (Ig) M, IgA, and IgG antibodies. However, the IgG antibody is the major class of antibodies detected during the recovery phase.7 Anti-HAV IgG frequently remains detectable for several decades after acute infection, providing lifelong immunity against reinfection.7–9 Acute and chronic liver diseases in their most severe and end-stage forms are the main indications for liver transplantation. The post-liver transplant survival rate has significantly increased in recent years due to the development of advanced surgical techniques besides improvements in post-transplant medical care and the successful management of postoperative complications,10–12 with the one-year post-liver transplantation survival rate exceeding 85%.13 To prevent graft rejection, there is a need for prolonged immunosuppressive therapy following transplantation, with greater intensity being required in the first few months; this renders recipients of solid organ transplants vulnerable to different types of infections including viral infections. It should also be noted that infections like hepatitis A and hepatitis E may mimic features of acute cellular rejection in liver transplant recipients.5,14 Furthermore, following transplantation, the immunosuppressed status of the recipients may alter the kinetics and persistence of protective antibodies against different infections including hepatitis A regardless of whether the antibodies developed from natural infection or through vaccination. Theoretically, there could be a risk of Anti-HAV IgG antibody loss by strong anti-rejection regimens administered following liver transplantation as these immune-suppressing drugs, including high-dose systemic steroids and mycophenolate mofetil, may decimate some pools of memory B-cells.15,16 Thus, these patients may benefit from immunization against HAV under appropriate situations.17 In most developing countries, a significant number of liver transplant candidates remain unvaccinated for HAV before transplantation because there is the conventional belief of universal natural and life-long immunity against the virus.18 However, vaccination against hepatitis A for non-immune patients in both the pre- and post-liver transplantation phase may fulfill an important role in disease prevention and is recommended by the related guidelines.19 Therefore, determining the HAV immune status of liver transplant recipients is essential as it allows the identification of non-immune patients at risk of infection, thereby facilitating appropriate preventive management.
The present study was performed to determine the immune status against hepatitis A in a population of unvaccinated liver transplant recipients from a large liver transplant center in Iran, which is considered an endemic region for hepatitis A infection. We also attempted to identify factors associated with natural immunity to hepatitis A in this population of patients.
Materials and methodsPatients and methodsThis cross-sectional study was performed on 261 patients above six years of age who underwent liver transplantation at Abu Ali Sina Organ Transplant Hospital in Shiraz, Iran. Patients were transplanted between January 2014 and December 2016. Immunity to HAV was checked in the post-transplantation follow-up visits between January and April 2017, with a mean follow-up time of 24±10 months.
Approval for the study was obtained from the Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1397.483) and all participants gave written informed consent before participating. Subjects who wished to discontinue taking part in the study at any time were excluded. Baseline data were extracted from the transplantation unit database. Furthermore, information about variables including age, gender, the underlying liver disease leading to transplantation, MELD/PELD scores, socioeconomic status (residential area, ownership of residential place, and monthly income), education level, and source of drinking water (tap water, bottled water, or well water) was gathered through a questionnaire.
For laboratory testing, 5ml of the venous blood was taken through venipuncture from each participant under sterile conditions. Centrifugation was done and the sera were isolated and stored at −20°C. Immunity against hepatitis A was assessed by checking the total HAV antibody level using the DIA.PRO Diagnostic Bioprobes Srl Total HAV Ab kit (Milano, Italy).
Statistical analysisFor statistical analysis, Statistical Package for Social Sciences (SPSS) version 22.0 (IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp) was used. Results were presented as mean±standard deviation (SD) for quantitative variables and were summarized as absolute frequencies and percentages for categorical variables. The normality of data distribution was analyzed using the Kolmogorov–Smirnov test. Categorical variables were compared using the chi-squared test or Fisher's exact test, while quantitative variables were compared with the t-test or Mann–Whitney U test. Due to the scarcity of responses, the standard deviation was overestimated. Hence, to evaluate the moderated effect of various factors on hepatitis A immunity, the Fierth method was used instead of the usual logistic regression method. Odds ratios (ORs) were calculated to investigate the power of communication. The enter method was applied in the multivariable model. P-values of 0.05 or less were considered statistically significant.
ResultsOf 261 patients initially assessed for immunity against hepatitis A, 19 failed to fill the study questionnaire and were thus excluded from the study. Among 242 cases who completed the study course, 151 (62.39%) were male and 91 (37.61%) were female. The mean MELD and PELD scores were 19±2.5 and 11.25±5.5 respectively. The patients’ age range was from 8 to 74 years with a mean and SD of 43.6±14.1 years. The most common causes of transplantation were Crigler–Najjar syndrome and Wilson's disease in the pediatric group and hepatitis B and primary sclerosing cholangitis among the adult patients. The patients who received liver transplantation for hepatocellular carcinoma were non-cirrhotic. Immunity against hepatitis A was detected in 215 (88.84%) patients. The mean age differed significantly between patients immune and non-immune to HAV (46.1±12.5 years and 24.4±10.8 years, respectively; P<0.001). The subjects were divided into three age groups: 0–18 years (n=10), 19–30 years (n=45), and over 30 years of age (n=187); immunity against hepatitis A was investigated separately in each group. The rate of immunity against hepatitis A in the first, second, and third age groups was 40%, 60%, and 98%, respectively. The minimum immunity rate was seen in patients below 18 years of age, with the immunity rate against hepatitis A increasing with age (Fig. 1).
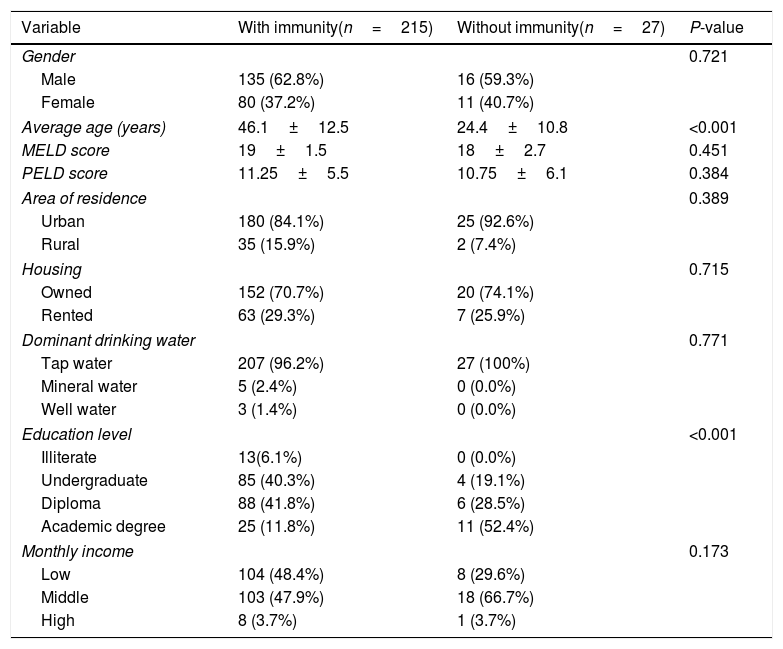
As shown in Table 1, the immunity rate was univariately independent of patient gender (P=0.823), area of residence (urban: 87.8%, rural: 94.4%; P=0.389), ownership of residential place (P=0.715), monthly income (P=0.173), and source of drinking water (P=0.771). However, the rate of immunity showed an inverse relationship with the level of education (illiterate: 100%; undergraduate: 95.5%; diploma: 93.6%; academic degree: 69.4%; P<0.001). The immunity rate was significantly different among different indications of liver transplantation (P<0.001), with the lowest immunity rate (33.3%) being found among the three patients with Crigler–Najjar syndrome, followed by an immunity rate of 54.5% among 11 patients who had Wilson's disease (Fig. 2). Crigler–Najjar syndrome and Wilson's disease were common indications of liver transplantation in our pediatric population of patients but not among the adults.
Comparing baseline characteristics between the patients with and without immunity against hepatitis A infection after liver transplantation.
| Variable | With immunity(n=215) | Without immunity(n=27) | P-value |
|---|---|---|---|
| Gender | 0.721 | ||
| Male | 135 (62.8%) | 16 (59.3%) | |
| Female | 80 (37.2%) | 11 (40.7%) | |
| Average age (years) | 46.1±12.5 | 24.4±10.8 | <0.001 |
| MELD score | 19±1.5 | 18±2.7 | 0.451 |
| PELD score | 11.25±5.5 | 10.75±6.1 | 0.384 |
| Area of residence | 0.389 | ||
| Urban | 180 (84.1%) | 25 (92.6%) | |
| Rural | 35 (15.9%) | 2 (7.4%) | |
| Housing | 0.715 | ||
| Owned | 152 (70.7%) | 20 (74.1%) | |
| Rented | 63 (29.3%) | 7 (25.9%) | |
| Dominant drinking water | 0.771 | ||
| Tap water | 207 (96.2%) | 27 (100%) | |
| Mineral water | 5 (2.4%) | 0 (0.0%) | |
| Well water | 3 (1.4%) | 0 (0.0%) | |
| Education level | <0.001 | ||
| Illiterate | 13(6.1%) | 0 (0.0%) | |
| Undergraduate | 85 (40.3%) | 4 (19.1%) | |
| Diploma | 88 (41.8%) | 6 (28.5%) | |
| Academic degree | 25 (11.8%) | 11 (52.4%) | |
| Monthly income | 0.173 | ||
| Low | 104 (48.4%) | 8 (29.6%) | |
| Middle | 103 (47.9%) | 18 (66.7%) | |
| High | 8 (3.7%) | 1 (3.7%) | |
The rate of immunity to hepatitis A in liver transplant recipients according to the type of underlying liver disease. PBC, primary biliary cholangitis; HCV, hepatitis C virus; HBV, hepatitis B virus; PSC, primary sclerosing cholangitis; NASH, non alcoholic steatohepatitis; AIH, autoimmune hepatitis; HAV, hepatitis A virus.
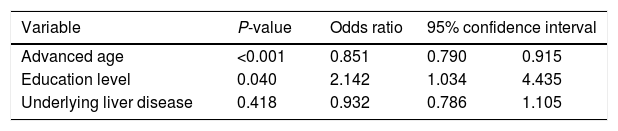
In the multivariable logistic regression model, younger age (OR=1.175, P<0.001) and higher education level (OR=2.142, P=0.040) represented the two main determinants of HAV non-immune status in our population of patients (Table 2).
Multivariable logistic regression model for predicting determinants of non-immunity against hepatitis A among liver transplant recipients.
| Variable | P-value | Odds ratio | 95% confidence interval | |
|---|---|---|---|---|
| Advanced age | <0.001 | 0.851 | 0.790 | 0.915 |
| Education level | 0.040 | 2.142 | 1.034 | 4.435 |
| Underlying liver disease | 0.418 | 0.932 | 0.786 | 1.105 |
Hosmer–Lemeshow goodness of fit test: chi-squared=91.183; P<0.001.
According to the universal survey conducted by the World Health Organization (WHO) in 2009, there has been a significant decline in HAV infection rates among children and young adults worldwide. This is while greater susceptibility to HAV infection is expected during adulthood if there is less exposure to the virus during childhood.20,21 As a developing country, Iran remains an intermediate-endemic area for HAV infection according to a WHO study that divided the whole globe into 21 divisions in terms of HAV epidemiology.20,22 However, based on the most recent local epidemiologic studies, there might be a move toward low endemicity with improving socioeconomic status and hygiene, at least in some parts of the country.23 In high-endemic areas, mass vaccination is not recommended as most individuals develop natural immunity to hepatitis A secondary to asymptomatic childhood exposure. For countries transitioning from high to intermediate endemicity, a considerable percentage of the adult population possesses no history of exposure to the virus and remain non-immune.24 In such countries, the inclusion of the hepatitis A vaccine in large-scale vaccination programs seems to be reasonable and cost-effective for disease prevention beyond childhood.24
Hepatitis A vaccination is also recommended in high-risk individuals including patients with chronic liver disease and recipients of organ transplantations. In patients with chronic liver disease, the incidence of hepatitis A infection does not rise, though these patients are at risk of a complicated clinical course (fulminant hepatitis and even death) upon exposure.25 It has been proven that the immunologic response to vaccination against HAV decreases with the progression of chronic liver disease. In compensated liver disease, the immune response to vaccination is comparable to that of healthy individuals. However, the rate of response declines with the progression of liver disease, and response rates as low as 26% have been reported with advanced liver disease in comparison with 99% in the normal population.26 The seroprevalence of hepatitis A has been evaluated in normal populations; however, data on patients undergoing liver transplantation is limited, especially in the early pre-operation period. It is still unclear whether high pre-transplant serum HAV IgG levels predict protection against hepatitis A infection after transplantation.7,9 Currently, pre-transplant vaccination for the prevention of post-transplant hepatitis A infection is recommended due to its high safety and efficacy.27,28 However, the vaccine-related longevity of protection in such patients has not exclusively been studied. In a study from the United States done in 2000, Arslan et al. reported HAV IgG loss rates of 18% and 29% among their patients one and two years after transplantation, respectively.17
Reviewing the literature shows that immunity against hepatitis A infection is associated with several factors such as age, water supply source, level of sanitation, and even ethnic background. In the United States, the seroprevalence of the hepatitis A antibody is higher among males than females, among older patients than the younger, and among Hispanic population than African Americans and whites.29,30 In our study, the rate of immunity against HAV among recipients of liver transplantation was around 88%. Vaccination against hepatitis A is not routinely done in Iran and the vaccine is not administered to cirrhotic patients and liver transplant candidates. Hence, this immunity rate of 88% represents the natural immunity in our population of patients. As expected, the immunity rate against hepatitis A was variable among different age groups. In the population of pediatric liver recipients, 40% were immune to hepatitis A, while among young adults aged between 19 and 30 years, the immunity rate was 60%. This shows that 60% of pediatric and 40% of young adult liver transplant recipients aged below 30 years are susceptible to hepatitis A infection. This is very important as this susceptible population becomes immunosuppressed following the administration of post-liver transplant medications. High rates of susceptibility to infection are perhaps even more alarming in adult recipients as a more severe clinical disease is expected following acute hepatitis A. In a recent nationwide study by Bagheri Lankarani et al., the hepatitis A immunity rate in Iran was shown to be 82.6% by the age of 29 years, which is higher than that of our patients within the same age group. In their nationwide study, they showed an immune rate of 41.1% in children by the age of 15 years, which is comparable to our result of 40% immunity in children under the age of 18 years.31 In another study by Izadi et al. on Iranian soldiers aged 18–34 years, an immunity rate of 80.3% was reported.32
In this study, we also showed that the rate of immunity increases with age, which is expected due to the increased chance of exposure to the virus over time. However, no significant difference was observed in the rate of immunity to hepatitis A between male and female patients; this is while some studies describe a higher immunity rate among men. In a study from the United States, the rate of immunity to hepatitis A was about 20% more among men than women.17 As another determinant for the rate of immunity against hepatitis A, those with lower education levels showed increased rates of immunity against HAV, representing a higher rate of previous exposure to the virus in this group of patients. Merat et al. presented a similar finding from their study on HAV immune status in three large cities of Iran. They showed a significant correlation between a higher level of paternal education and the non-immune status against hepatitis A in their pediatric population of patients.20
Multiple similar studies have been conducted on the subject of hepatitis A immune status of recipients of liver transplants. In a study from Spain in 2008 by Aoufi et al., the rate of immunity against HAV was reported to be 93.3% among cirrhotic patients being evaluated for liver transplantation.33 In another study from the same center in 2012 by Gutiérrez Domingo et al. on a larger population of candidates for liver transplantation, a similar rate of immunity was reported (91.8% immunity; 8.2% non-immunity). In that study, patients in the non-immune group shared the characteristics of being younger, non-diabetic, non-alcoholic, and negative for hepatitis B markers.34 Both study results represented lower HAV immunity rates in younger patients.
From our study, it was concluded that, as expected, the rate of immunity in younger recipients of liver transplants is significantly lower relative to older patients. The rate of non-immunity in our total patient population was detected as 11.16%. However, the rate of non-immunity against hepatitis A was found to be as high as 61.1% among patients younger than 22 years of age. These findings show that a significant proportion of young liver transplant recipients in our center are at increased risk of HAV infection due to a lack of natural immunity against the virus. This indicates the importance of preventive measures, particularly hepatitis A vaccination, in this population of patients as non-immune individuals possess a higher risk of serious complications following post-transplantation hepatitis A infection. For the best results, the advice on HAV vaccination could be generalized to the pre-transplantation period and even to patients with compensated cirrhosis before immediate transplantation becomes necessary.
The main limitation of our study was the fact that it was conducted in a single center. It should be noted, however, that our center is the largest and leading liver transplant center in the country, accepting a high volume of liver transplant candidates from the whole country. Furthermore, our center dominates among a limited number of centers that perform pediatric liver transplantation. Another limitation is the lack of information about the pre-transplant HAV immune status of our patients. It is also important to note that the underlying liver disease leading to transplantation may differ from other countries given the lack of alcohol-related liver disease in our patient population.
ConclusionThis study found a considerable rate of non-immunity to hepatitis A among younger recipients of liver transplants in our center. Appropriate testing to measure HAV immune status is indicated in these patients, preferably in the pre-transplantation period; vaccination against hepatitis A should be considered for non-immune candidates.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by a grant from Shiraz University of Medical Sciences (Grant No. 14649).