Frizzled-2 plays an important role in maintaining normal hepatic cell functionality. This study aimed to investigate the role of inhibition of Frizzled-2 in protecting rat liver BRL-3A cells from Hypoxia/Reoxygenation (H/R). In vitro H/R hepatic cell model was established by culturing BRL-3A cells under H/R condition. Frizzled-2 siRNA was transfected into BRL-3A cells to inhibit Frizzled-2 signaling. Wnt5a and Frizzled-2 were significantly increased in BRL-3A cells upon H/R treatment. H/R treatment induced cell cytotoxicity, the early apoptosis rate and the intracellular Ca2+ level in BRL-3A cells while silencing frizzled-2 gene decreased the H/R induced cell cytotoxicity, apoptosis and intracellular Ca2+ level. In vivo mice study further showed the up-regulation of Frizzled-2/Wnt 5 pathway and cleaved Caspase-3 expression in liver tissues under ischemia and reperfusion injury (IRI). In summary, inhibition of Frizzled-2 by its siRNA may protects BRL-3A cells by attenuating the H/R induced cell cytotoxicity and apoptosis.
Frizzled-2 desempeña un papel importante en el mantenimiento de la funcionalidad normal de los hepatocitos. Este estudio tiene como objetivo analizar el papel de la inhibición de Frizzled-2 en la protección de los hepatocitos BRL-3A de rata de la hipoxia/reoxigenación (H/R). El modelo de hepatocitos H/R in vitro se demostró con el cultivo de células BRL-3A en condiciones de H/R. El ARNip de Frizzled-2 se transinfectó en células BRL-3A para inhibir la señalización de Frizzled-2. Wnt5a y Frizzled-2 aumentaron considerablemente en las células BRL-3A tras el tratamiento con H/R. El tratamiento con H/R provocó citotoxicidad celular, una tasa de apoptosis temprana y el nivel de Ca2+ intracelular en células BRL-3A mientras que el gen frizzled-2 silenciado redujo la citotoxicidad celular inducida por H/R, la apoptosis y el nivel de Ca2+ intracelular. El estudio in vivo con ratones mostró, además, la regulación al alza de la vía de Frizzled-2/Wnt 5 y la expresión de caspasa 3 escindida en tejidos hepáticos con lesión por isquemia y reperfusión (LIR). En resumen, la inhibición de Frizzled-2 por su ARNip puede proteger a las células BRL-3A al atenuar la citotoxicidad celular y la apoptosis inducida por H/R.
Hepatic ischemia–reperfusion injury (HIRI) is a major cause of morbidity and mortality in liver resection and transplantation surgery.1,2 HIRI of the liver results in microvascular changes and acute inflammation, ultimately leading to cell death.2 HIRI induced cell death includes apoptosis, autophagy, necrosis and necroptosis in hepatocytes and non-parenchymal cells.3,4 Restoration of blood flow is necessary to restore cellular function, but paradoxically reperfusion can initiate a cascade of pathways that cause further cellular injury after prolonged ischemia. Understanding the molecular events of liver ischemia–reperfusion injury helps develop strategies to counterattack this injury and reduce acute complications in hepatic resection and transplantation.
Wnt signaling pathway is selectively activated in hepatic development, regeneration and hepatocellular carcinoma (HCC), in a canonical or a non-canonical pathway.5–9 Wnt signaling is mediated by its receptors, Frizzled family proteins, which contain seven trans-membranes in humans and mice.9 Canonical Wnt signaling is mediated by Frizzled-1, and regulates the nuclear accumulation of β-catenin.10 Non-canonical Wnt signaling is β-catenin-independent, and includes pathways, such as mitogen-activated protein kinase,11 NFκB and c-Jun N terminal kinase (JNK),12 ROCK/Rho kinase,13 protein kinase C (PKC) and Ca2+/calmodulin-dependent protein kinase II (CaMKII).14 Frizzled-2 binds Wnt5a to activate the non-canonical Wnt pathway.15 Activation of Wnt5a/Frizzled-2 signaling has been shown to turn off β-catenin during hepatic development and liver regeneration after partial hepatectomy.16 Wnt5a loss leads to enhanced β-catenin signaling in hepatocellular carcinoma.7,17 Wnt5a/Frizzled-2 signaling pathway was activated in response to H/R in H9C2 cells,18 indicating its important role in in vivo myocardial cells post reperfusion. However, the Frizzled-2 signaling pathway has not been understood yet in the hepatic development under pathophysiological HIRI conditions.
In this study, we examined the effects of Frizzled-2 on the H/R treated rat normal liver BRL-3A cells, an in vitro cell model established to mimic the pathophysiological HIRI. The Wnt5a/Frizzled-2 signaling was suppressed by silencing frizzled-2 gene expression using the siRNA approach. Inhibition of Frizzled-2 significantly attenuated the H/R induced cytotoxicity, apoptosis and intracellular Ca2+ in BRL-3A cells. Our data suggest the inhibition of Wnt5a/Frizzled-2 signaling pathway may represent a novel and promising approach to protect liver cells against HIRI-related disorders.
Materials and methodsCell culture and Hypoxia/Reoxygenation (H/R) modelThis project was approved by the Ethical committee guide of our hospital, Zhujiang Hospital, and Southern Medical University for the care and the use of laboratory animals (No. ZJYY-2016-GDEK-001). BRL-3A cell (derived from rat liver cells) was purchased from ATCC (American Type Culture Collection, Manassas, VA, USA) and maintained in HyClone™ Dulbecco's modified Eagle's medium (DMEM) with 4.5g/L glucose supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% penicillin/streptomycin (v/v) in a humidified air atmosphere with a 5% CO2 at 37°C. All cell culture reagents were purchased from Thermo Scientific (Waltham, MA, USA). To develop cells under Hypoxia/Reoxygenation (H/R), BRL-3A cells in culture medium with low serum media (0.5% FBS) were exposed to hypoxia for 2h in a humidified incubation chamber at 37°C and flushed with a gas mixture of 95% N2 and 5% CO2, followed by incubation in fresh medium with 95% air and 5% CO2 for reoxygenation. The control cells were kept in normoxic conditions. After reoxygenation for16h, cells were harvested for RNA or protein analysis.
Transfection with small interfering RNA (siRNA) targeting frizzled-2 geneFor transfection, BRL-3A cells were plated in 6-well plate at a density of 105 cells per well or in 100mm dishes with 106 cells in growth medium without antibiotics. After cell growth reached 50–60% confluence, transfection was performed using Lipofectamine 2000 from Invitrogen (ThermoFisher Scientific, MA, USA). Cells were incubated in Opti-MEM I Reduced Serum Medium from Invitrogen with or without frizzled-2 siRNA–Lipofectamine 2000 complexes (25, 50, or 100nM) for 6h and then replaced with fresh DMEM for 48h. The transfection efficiency of siRNA was monitored by observing the fluorescence of cells transfected with FAM labeled siRNA negative control (siRNA-NC) after 6h. Then cells were subjected to H/R treatment and then harvested for other experiments. Three different non-overlapping siRNA duplexes and FAM labeled siRNA negative control were designed and purchased from Sigma-Aldrich (Guangzhou, China). The sequences were as follows: firizzled-2 siRNA #25: 5′-GUGCUGUGCUGCGCUUCUAdTdT-3′; firizzled-2 siRNA #33: 5′-CUAUCUCAGCUAUAAGUUUdTdT-3′; firizzled-2 siRNA #34: 5′-GCUGUACUAUACUCUUCAUdTdT-3′ and negative control: 5′-UUCUCCGAACGUGUCACGUdTdT-3′.
RNA isolation and quantitative real-time PCRTotal RNA was isolated from BRL-3A cells s using Trizol from Invitrogen (ThermoFisher Scientific, MA, USA) according to the manufacturer's protocols. Quantitative Real-Time PCR (qRT-PCR) was carried out using an ABI 7500 Real-time PCR System (Applied Biosystems, Foster, CA, USA). SYBR Green PCR SuperMix was used as a double-stranded DNA-specific dye according to the manufacturer's instructions (ThermoFisher Scientific, MA USA). Primers were designed for a single qRT-PCR thermal profile (95°C for 1min, and 40 cycles of 95°C for 15s and 55°C for 1min for frizzled-2 and 95°C for 1min, and 40 cycles of 95°C for 15s and 60°C for 1min for Wnt5a and β-actin). The expression levels of examined transcripts were normalized to β-actin. Primer sequences for frizzled-2, Wnt5a and β-actin were listed below: frizzled-2-forward: 5′-TCGTTTTGCCCGTCTCT-3′, frizzled-2-reverse: 5′-TAGCGGAATCGCTGCAT-3′; Wnt5a-forward: 5′-CGTGGCTATGACCAGTTTAAG-3′; Wnt5a-reverse: 5′-CCACAATCTCCGTGCACTT-3′; β-actin-forward: 5′-AGGGAAATCGTGCGTGACAT-3′; β-actin-reverse: 5′-GAACCGCTCATTGCCGATAG-3′.
Protein lysate preparation and Western blotCultured BRL-3A cells were lysed in ice-cold Radioimmunoprecipitation assay (RIPA) Lysis and Extraction Buffer (Beyotime Biotechnology, Shanghai, China) and total protein samples were prepared in extraction buffer (10mM Tris, 100mM NaCl, 1mM EDTA, 1mM EGTA, 1mM NaF, 20mM Na4P2O7, 2mM Na3VO4, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton-100, 10% glycerol and 1mM PMSF) containing 1× Cocktail of Proteinase inhibitors and Phosphatase inhibitors (ThermoFisher Scientific, MA, USA). Protein concentration was measured using BCA protein assay kit (Kaiji Biotechnology, Nanjing, China). A 20μg of protein for each sample was loaded into each well and resolved by SDS-10% PAGE and transferred onto PVDF membranes via electroblotting (Millipore, Billerica, MA, USA). After blocking 1h in 1× TBST containing 5% bovine serum albumin (BSA), membranes were washed three times in 1× TBST and probed with the following primary antibodies at 4°C overnight: rabbit polyclonal to Frizzled-2 (1:1000; Abcam, Cambridge, MA, USA), rabbit polyclonal to Wnt5a (1:2000; Abcam, Cambridge, MA, USA) and goat polyclonal antibody to human GAPDH (1:1000; Santa Cruz, California, CA, USA). The blots were then incubated with goat anti-rabbit IgG peroxidase-conjugated secondary antibody (1:6000; Calbiochem, Merck KgaA, Darmstadt, Germany) and visualized by enhanced SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, USA). GAPDH was used as the internal loading control. For quantification, Image-Pro Plus 6.0 software (Media Cybernetics Inc., Silver Spring, MD, USA) was used to measure the integrated optical density (IOD) of the bands. The relative level of protein expression was expressed as a ratio to GAPDH. Experiments were repeated three times for each experimental condition.
LDH cytotoxicity assayThe cytotoxicity of BRL-3A cells was determined by Lactate Dehydrogenase (LDH) released from the damaged cells. Cells were seeded in triplicate in 6-well plates and incubated overnight. Then cells were subject to transfection of siRNA of frizzled-2 or control siRNA for 24h, respectively then followed by H/R treatment. Cell lysates were harvested for LDH assay according to the manufactory protocol from Pierce Biotechnology (Rockford, IL, USA). The absorbance was measured at 490nm and 680nm. To determine LDH activity, subtract the 680nm absorbance value as background from the 490nm absorbance before calculation of % cytotoxicity. Experiments were repeated three times independently.
Apoptosis assay by flow cytometryTo assess cell apoptosis, an Annexin V-fluorescein isothiocyanate (V-FITC) staining assay from Invitrogen (ThermoFisher Scientific, MA, USA) was performed according to the manufacturer's instructions. Briefly, the BRL-3A cells were detached by 0.25% Trypsin and collected for staining with Annexin V-FITC and PI for 15min at room temperature (18–24°C). The cells were then washed twice with PBS, and the fluorescence was analyzed using a FACSCalibur flow cytometry system and CellQuest software (BD Biosciences, New Jersey, USA).
Fluorescence of Ca2+ and laser scanning confocal microscopyFor calcium detection, cells grown in the 6-well plates were incubated with the intracellular Ca2+ indicator Fluo 8-AM at 37°C for 30min according to the manufacturer's protocol from Invitrogen (ThermoFisher, MA, USA). The fluorescence intensity was monitored at 490nm/515nm (Ex/Em). Fluorescent images were acquired by a LSM510 confocal laser scanning microscope (Carl-Zeiss, Jena, Germany) and analyzed using Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA). The average fluorescence density of cell preparations from each group was compared among the groups. Values of integrated optical densities were expressed as the mean±SEM of at least three different experiments.
In vivo animal study and surgeries for hepatic ischemia and reperfusion injury (IRI)Total 12 male C57BL/6 mice at 8- to 10-week old were bred and housed at State Key Laboratory of Biological Artificial Liver in Zhujiang Hospital. For surgery for hepatic ischemia and reperfusion injury (IRI), 6 mice were anesthetized with isoflurane and a small midline was vertically incised on the abdominal wall to access the liver and portal triad. The blood supply to left and central hepatic lobes was occluded via vascular clips on the hepatic artery and portal vein for 90min, followed by a 6h period of reperfusion before sacrifice. For IRI sham operations, 6 mice were also anesthetized and the abdominal wall was vertically incised at midline and left open but covered with saline-soaked gauze for 30min before the incision was closed. Mice were placed on a warming pad during and after surgery to maintain a body temperature of 37°C. At sacrifice, liver tissues were collected and stored at −80°C for further analysis. All procedures were approved by the Institutional Animal Care and Use Committee and followed at guide of our hospital, Zhujiang Hospital, and Southern Medical University for the care and the use of laboratory animals.
Statistical analysisAll data are presented as the mean±standard error of the mean (SEM). Student's t-test and one-way analysis of variance (ANOVA) were used to examine the overall statistical differences using SPSS software (Version 24.0, Chicago, IL, USA). Differences between the groups were considered statistically significant at a p<0.05.
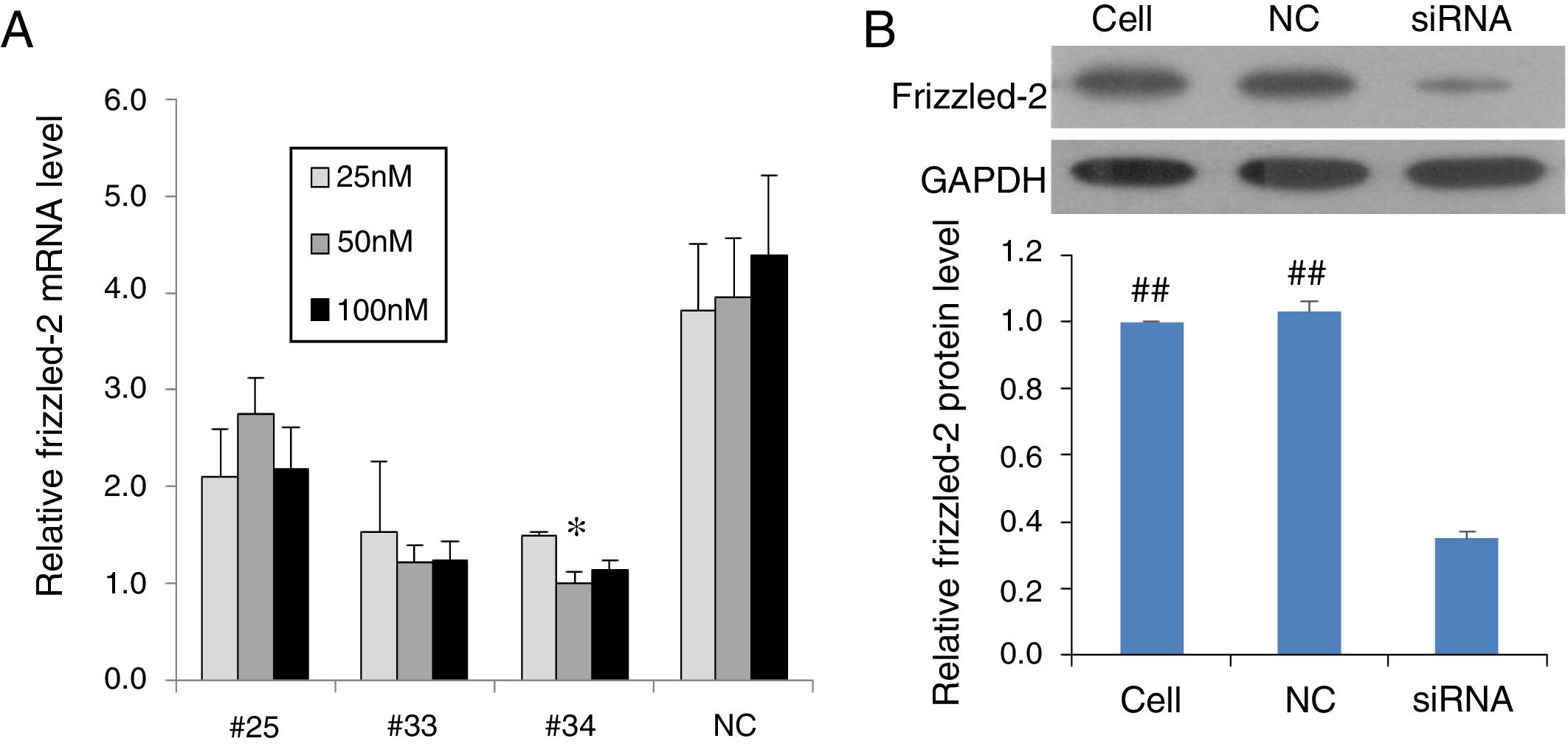
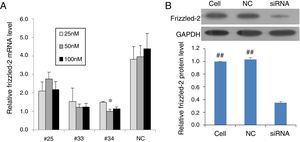
ResultsInhibition of frizzled-2 gene by siRNA in rat normal hepatic cell line BRL-3A cellsTo validate the frizzled-2 siRNA silencing efficiency, the frizzled-2 gene expression was detected in BRL-3A cells using qRT-PCR after siRNA transfection for 24h. The frizzled-2 mRNA levels were inhibited by 30.44%, 69.22% and 74.79% by transfection of siRNA-#25, -#33 and -#34 at 50nM, respectively, compared to the negative control siRNA (Fig. 1A). A significant decrease in Frizzle protein expression was also shown in the group with transfection of siRNA-#34 at 50nM by Western blot compared to mock control Cell group or negative control (NC) group (Fig. 1B). With the highest efficiency (74.79%) in silencing frizzled-2 expression in BRL-3A cells, transfection of siRNA-#34 at 50nM was chosen to knockdown frizzled-2 gene expression in the subsequent experiments.
Validation of siRNA silencing frizzled-2 gene expression. (A) Different siRNAs (#25, #33 and #34) targeting frizzled-2 mRNA at different regions and non-targeting siRNA serving as negative control (NC) were transfected at final doses of 25, 50 and 100nM for 24h in BRL-3 cells. Relative expression levels of frizzled-2 were analyzed qRT-PCR and normalized to β-actin. * denoted the group with the frizzled-2 siRNA (#34) at 50nM achieved maximally silencing efficiency among groups. (B) Transfection of frizzled-2 siRNA (#34) and negative control siRNA (NC) was performed at 50nM in BRL-3 cells for 48h. Cell group was set up as mock control. Frizzled-2 protein level was analyzed by Western blot. Relative expression of Frizzled-2 was normalized to GAPDH. Data were presented as means±SEM from three independent experiments. ##p<0.001, Cell or NC groups vs. siRNA group.
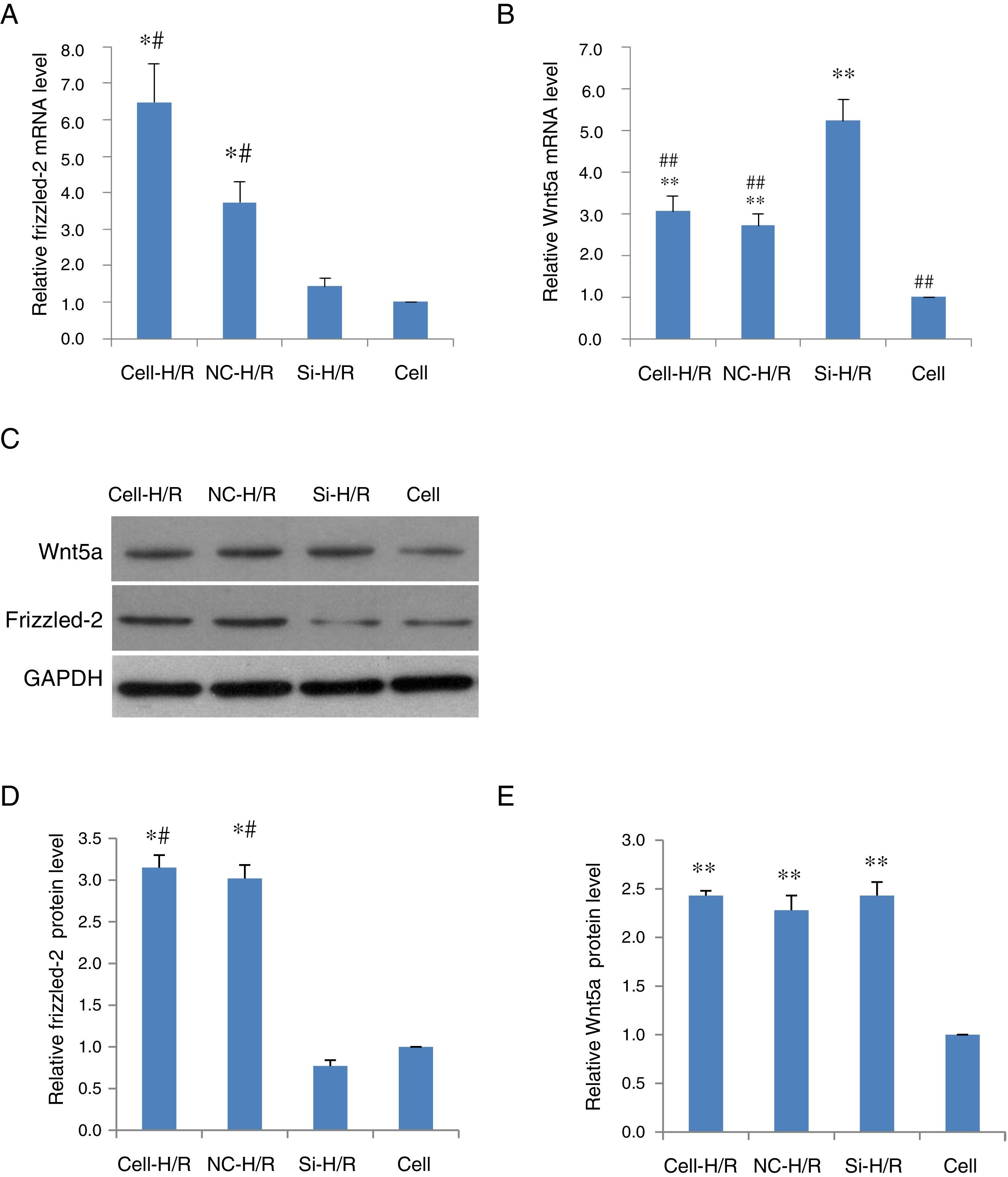
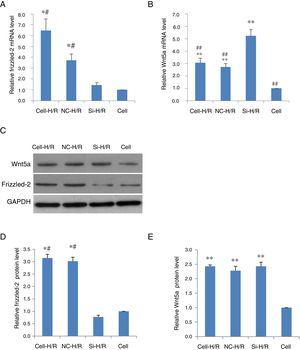
To determine whether Frizzled-2/Wnt5a pathway was responded to the H/R treatment in BRL-3A cells, the expression of Frizzled-2 and Wnt5a were examined in BRL-3A cells with or without H/R (Cell-H/R or Cell) by qRT-PCR and Western blot. Significant increases in the expression of both Frizzled-2 and Wnt5a were detected at mRNA (Fig. 2A and B) and protein levels in BRL-3A cells after H/R treatment (Fig. 2D–E). The H/R induced frizzled-2 mRNA as well as its protein levels were diminished by frizzled-2 siRNA transfection in BRL-3A cells (Fig. 2A, C and D), whereas Wnt5a gene expression was induced (Fig. 2B, C and E).
H/R treatment induced the expression of Frizzled-2 and Wnt5a in BRL-3A cells. (A) Relative Frizzled-2 gene expression. (B) Relative Wnt5a gene expression; (C) representative photographs of protein expression of Frizzled-2, Wnt5a and GAPDH; (D) relative protein level of Frizzled-2; (E) relative protein level of Wnt5a. The relative protein expression of Frizzled-2 and Wnt5a was normalized to GAPDH. Data were presented as means±SEM from three independent experiments. *p<0.05 and **p<0.001 when comparing to Cell group, and #p<0.05 and ##p<0.001 when comparing to Si-H/R group.
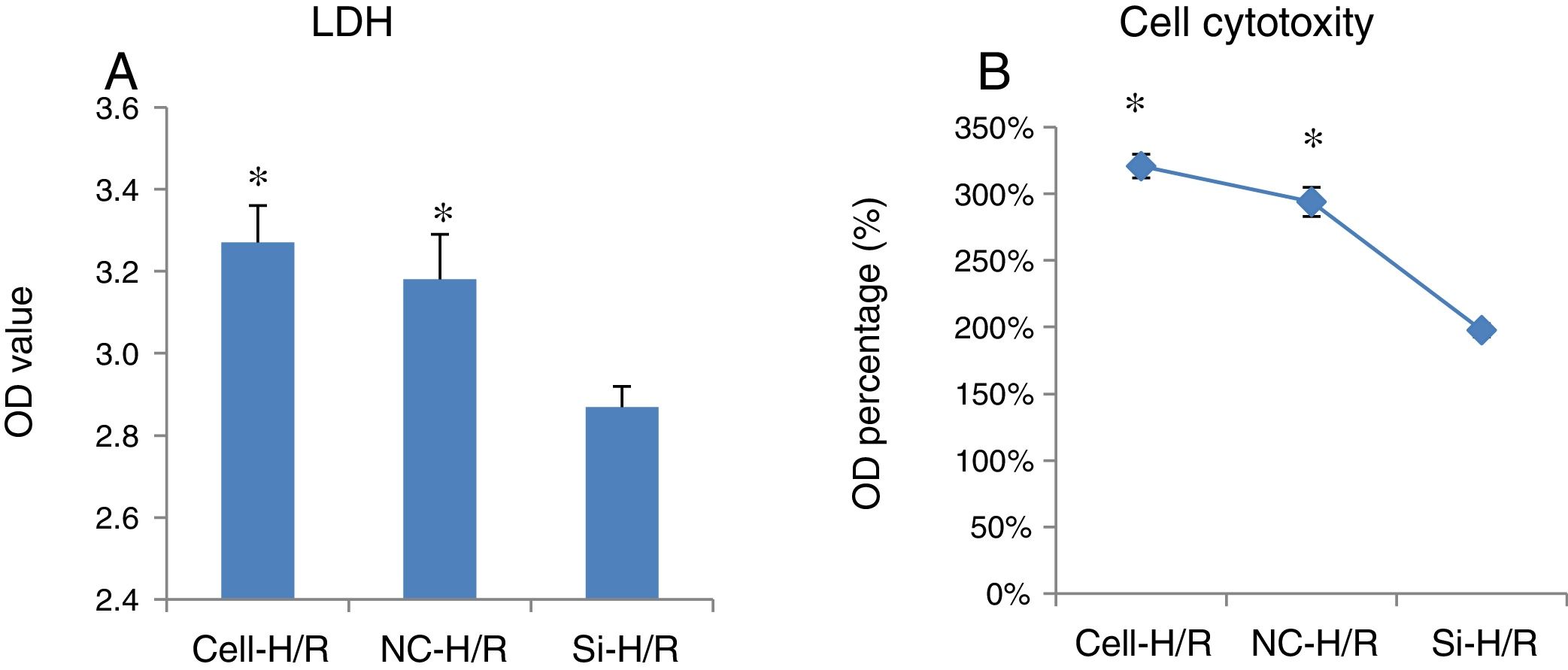
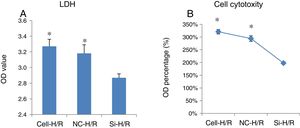
To examine the effect of H/R treatment on cell cytotoxicity in BRL-3A cells, the activity of LDH released from the dead cells was measured. LDH assay showed transfection of frizzled-2 siRNA significantly inhibited the LDH activity by reducing the OD value from 3.27±0.09 to 2.87±0.05 (p<0.05) (Fig. 3A and B), suggesting silencing frizzled-2 gene expression enables to protect hepatic cells injury caused by H/R.
The effect of transfection of frizzled-2 siRNA on cell cytotoxicity in BRL-3A cells. (A) LDH assay showed transfection of frizzled-2 siRNA significantly inhibited the LDH activity from 3.27±0.09 to 2.87±0.05 (p<0.05) at OD values; (B) LDH activities were shown as percentage of OD values. *p<0.05 Cell or NC groups vs. Si-H/R group.
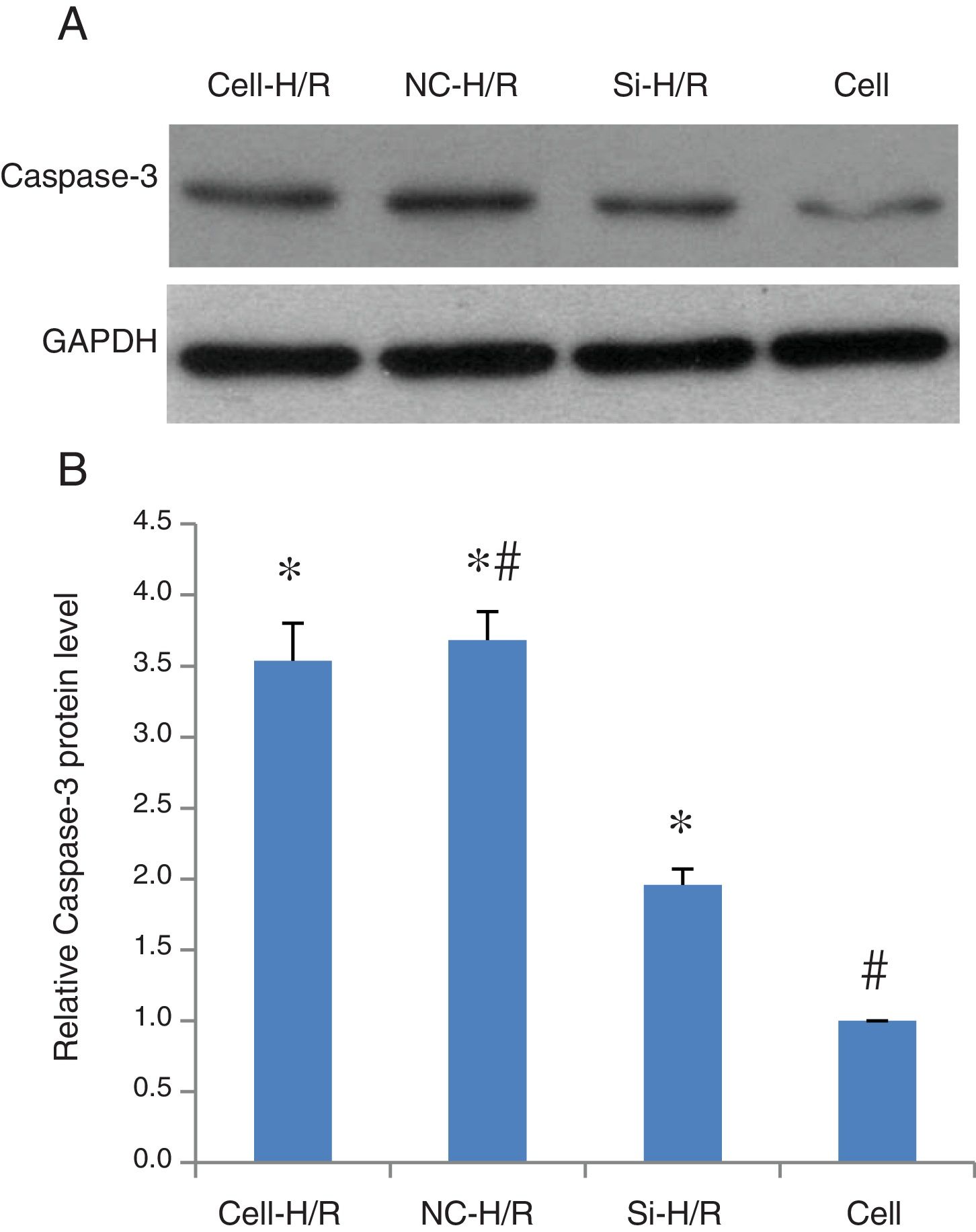
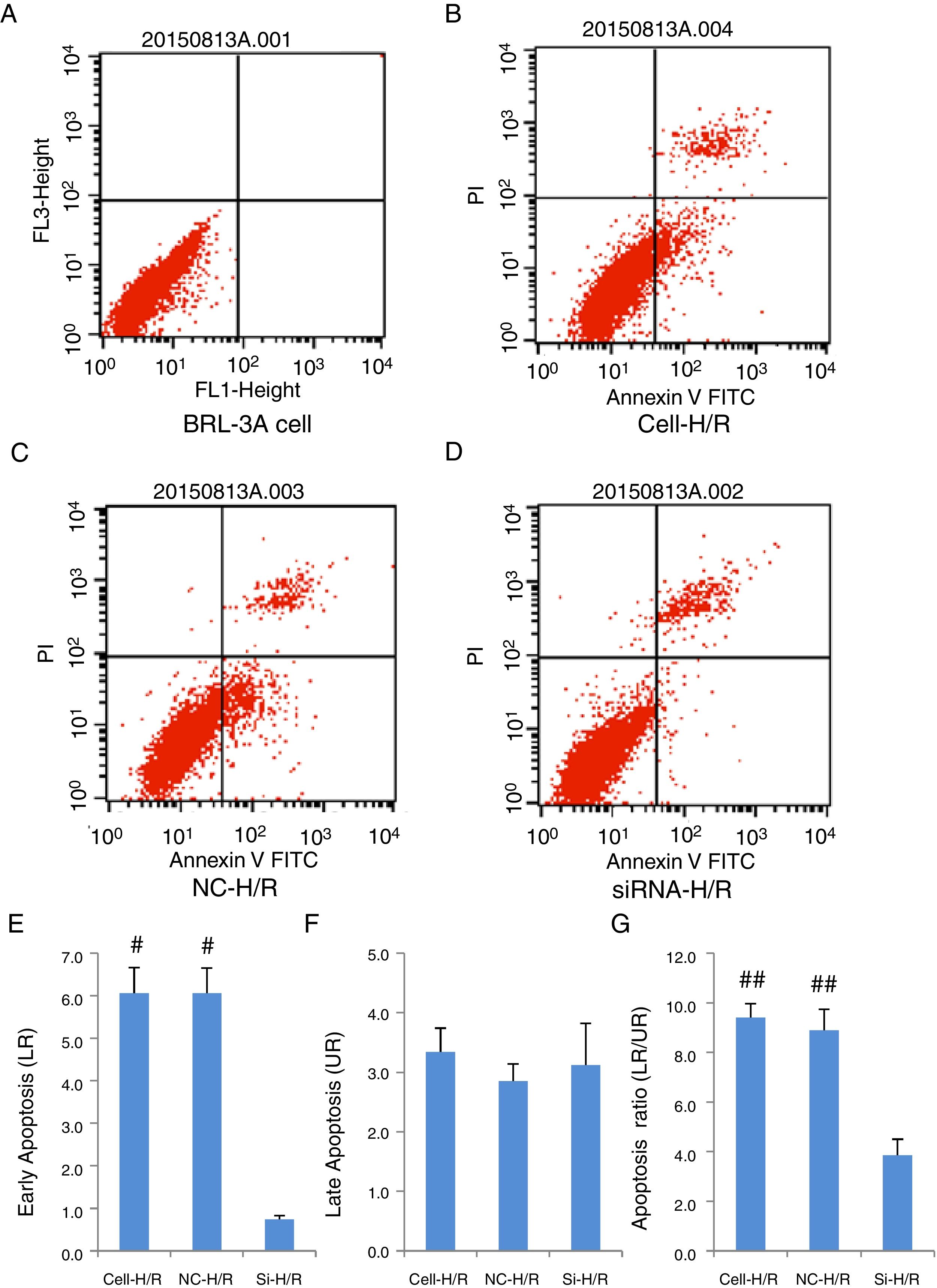
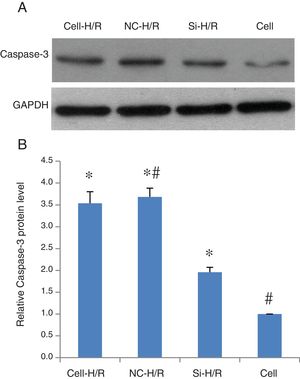
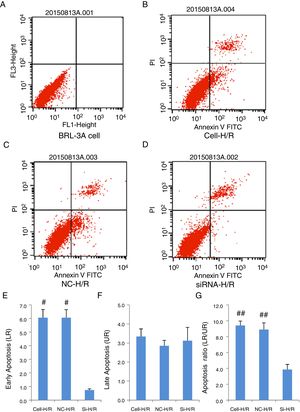
As a critical protein involved in cell apoptotic process, Caspase-3 protein level was used to monitor cell apoptosis during H/R. Western blot analysis showed Caspase-3 level was significantly increased in BRL-3A cells after H/R treatment (Fig. 4A) and this increase was suppressed by the frizzled-2 siRNA transfection. The fold change of Caspase-3 protein level was further presented by the bar graph (Fig. 4B). The early apoptosis rates were further determined by Annexin-V-FITC using flow cytometry assay. Total cell apoptosis was significantly induced by the H/R treatment in BRL-3A cells (Fig. 5A and B). Compared to the negative control group, transfection of frizzled-2 siRNA decreased early apoptosis in BRL-3A cells during H/R (0.74±0.09 vs. 6.06±0.59, p<0.001; Fig. 5C–E). Similar levels of cell late apoptosis rates (3.12±0.70 vs. 3.34±0.40, p>0.05; Fig. 5C, D and F) but a significant decrease in total cell apoptosis (3.86±0.64 vs. 9.41±0.56, p<0.001; Fig. 5C, D and G) were shown in cell group with the transfection of frizzled-2 siRNA during H/R treatment compared to the negative control group.
Caspase-3 protein expression was induced by H/R and suppressed by transfection of frizzled-2 siRNA. (A) Representative photographs of protein expression of Caspase-3 and GAPDH; (B) relative Caspase-3 protein level was normalized to GAPDH; data were presented as means±SEM from three independent experiments. *p<0.05 when comparing to Cell group, and #p<0.05 when comparing to Si-H/R group.
Transfection of frizzled-2 siRNA inhibited the H/R induced apoptosis in BRL-3A cells by Flow Cytometry using Annexin-V-FITC method. (A) BRL-3A cells alone; (B) BRL-3A cells with H/R treatment (Cell-H/R); (C) H/R treatment of BRL-3A cells with transfection of negative control siRNA (NC-H/R); (D) H/R treatment of BRL-3A cells with transfection of frizzled-2 siRNA (Si-H/R); (E) quantitative analysis of the early cell apoptosis; (F) quantitative analysis of the late cell apoptosis; (G) quantitative analysis of the early cell apoptosis. Data were presented as means±SEM from three independent experiments. *p<0.05 and **p<0.001 when comparing to Cell group, and #p<0.05 and ##p<0.001 when comparing to Si-H/R group.
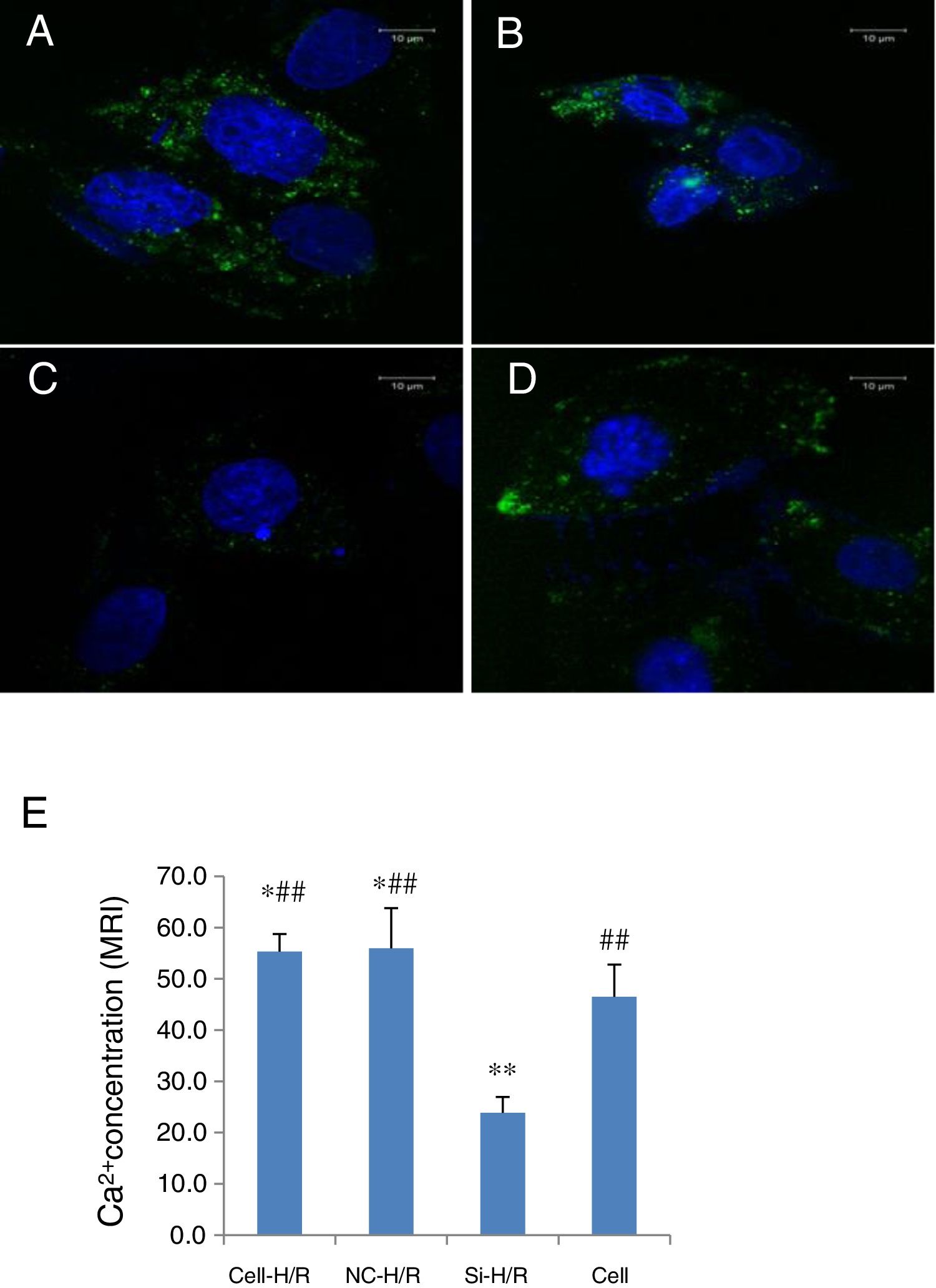
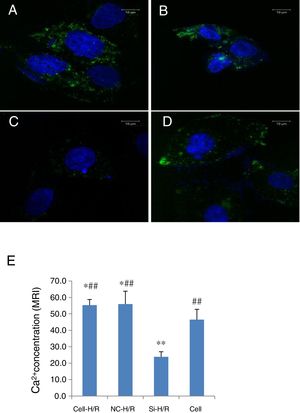
As Ca2+ overload is usually involved in the HIRI process, we detected the intracellular Ca2+ concentration in BRL-3A cells with H/R treatment through the mean fluorescence intensity (MFI) of Furo 8-AM using confocal microscopy. The intracellular Ca2+ level was clearly increased in the BRL-3A cells with H/R treatment compared to the normal cells (MFI: 55.34±3.42 vs. 46.49±6.3, p<0.5, Fig. 6A, D and E). To test whether inhibition of Wnt5a/Frizzled-2 pathway regulates the intracellular Ca2+ level, transfection of frizzled-2 siRNA was performed to examine the intracellular Ca2+ level. The Ca2+ concentration was significantly suppressed in the H/R treated BRL-3A cells with transfection of frizzled-2 siRNA compared to the normal cells (MFI 23.71±7.68 vs. 46.49±6.30, p<0.05; Fig. 6C–E).
Transfection of frizzled-2 siRNA inhibited the H/R-induced intracellular Ca2+ concentration in BRL-3A cells. Fluorescence was shown in green color (528nm) to monitor the intracellular Ca2+ in BRL-3A cells with H/R treatment by Fluro 8-AM using confocal microscopy. (A) BRL-3A cells with H/R treatment (Cell-H/R); (B) H/R treatment of BRL-3A cells with transfection of negative control siRNA; (C) H/R treatment of BRL-3A cells with transfection of frizzled-2 siRNA; (D) control cells without H/R treatment; (E) bar graph showed the quantitative analysis of mean fluorescence intensity (MFI) of the intracellular Ca2+ concentration in different cell groups. Blue color denoted the nuclear stained with DAPI. Data were presented as means±SEM from three independent experiments. *p<0.05 and **p<0.001 when comparing to Cell group, and #p<0.05 and ##p<0.001 when comparing to Si-H/R group.
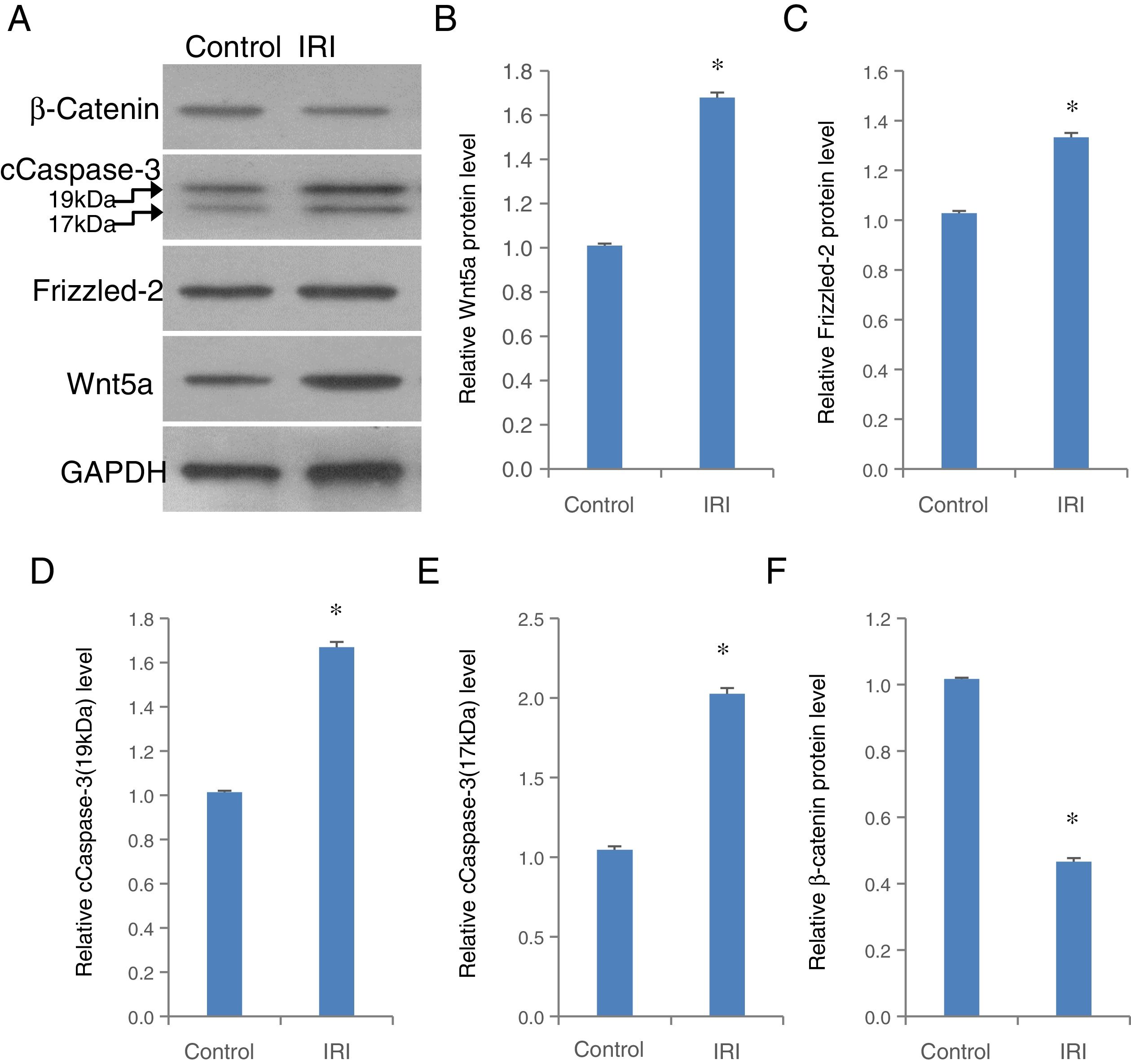
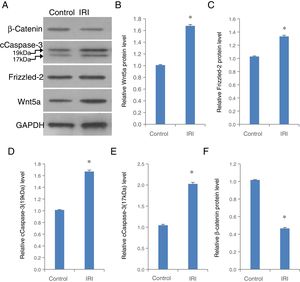
To validate the role of Frizzled-2/Wnt5 pathway mediating H/R in a hepatocyte-like cell line, in vivo animal study was further performed in a clinically relevant setting of hepatic IRI in mice (Fig. 7). The expression of Wnt5a, Frizzled-2, and cleaved Caspase-3 at 17kDa and 19kDa was individually up-regulated significantly accompanied by a significant decrease in β-catenin in liver tissues from mice with IRI compared to the control group (Fig. 7A–F).
The up-regulation of Wnt5a, Frizzled-2, cleaved Caspase-3 (cCaspase-3) accompanied by a decrease in β-catenin was shown in liver tissues from mice with IRI. (A) Representative photographs of protein expression of Frizzled-2, Wnt5a, cCaspase-3 (17kDa and 19kDa), β-catenin and GAPDH; (B) relative protein level of Wnt5a; (C) relative protein level of Frizzled-2; (D) relative cCaspase-3 (19kDa); (E) relative cCaspase-3 (17kDa); (F) relative β-catenin gene expression. Bar graphs showing the relative protein expression of Wnt5a, Frizzled-2, cCaspase-3 (17kDa and 19kDa) and β-catenin were individually normalized to GAPDH and presented as means±SEM from six mice from group IRI or control group with sham operations. *p<0.05 when comparing to control group.
As a clinical condition, HIRI may lead to cellular injury and organ dysfunction and is mediated mainly through different mechanisms, which include Wnt/β-catenin signaling pathway and Ca2+ overload. To address the potential hepatocyte functionality in response to HIRI, we first established the in vitro H/R hepatic cell model using rat normal liver BRL-3A cells to mimic in vivo hepatic cells under HIRI condition, which allows for in vitro cellular functional and mechanistic studies. In this study, we investigated the effects of H/R treatment on the expression of Frizzled-2/Wnt5a pathway. Frizzled-2 and Wnt5a gene and protein expression were significantly increased in BRL-3A cells by H/R treatment. We further demonstrated H/R treatment induced cell cytotoxicity, apoptosis and the intracellular Ca2+ level in BRL-3A cells. This induction could be suppressed by transfection of frizzled-2 siRNA, suggesting suppression of Frizzled-2/Wnt5a signaling played important roles in protection of hepatic cells against HIRI.
The ischemic injury could be mediated through various possible mechanisms. The Wnt signaling pathway is well established in liver cell biology and plays a critical role as a molecular regulator in hepatic development, regeneration and carcinogenesis.8 Wnt5a/Frizzled-2 has been shown to inhibit liver regeneration process by suppressing β-catenin signaling in hepatocytes.16 Frizzled proteins can stimulate different Wnts at the cell surface and discriminate among different Wnt ligands,2,19 which determines the specific downstream pathway activated in the cells. Pharmacologic activation of canonical β-catenin dependent Wnt signaling protects against hepatic ischemia/reperfusion injury through inducing proliferation and reducing apoptosis.20 Noncanonical Wnt5a strongly inhibited canonical Wnt signaling.7 In hepatocellular carcinoma, high levels of Frizzled-2 expression induced EMT and enhanced cell migration and invasiveness, while transfection of frizzled-2 siRNA resulted in a significant reduction in cell migration and invasion but not proliferation in HCC cell lines.21 Canonical and noncanonical Wnt pathways have complementary roles in HCC, where the canonical signaling contributes to tumor initiation, and noncanonical signaling to tumor progression.7 In the present study, siRNA silencing the frizzled-2 gene expression led to a decrease in cell cytotoxicity and apoptosis, indicating the activation of Wnt5a/Frizzled-2 might enhance the cell death after HIRI whereas inhibition of Frizzled-2 may protect liver cell against injury and help to maintain hepatic cell integrity.
During reperfusion injury, both apoptosis and necrosis pathways are present in cell death.3 Several proapoptotic proteins are activated during the reperfusion phase, including the proteases Caspase-3 and Caspase-8, which finally leads to the destruction of nuclear DNA, resulting in cell death.1 Caspase-3 is one of the mediators in apoptotic pathway which can be enhanced by Ca2+ accumulation post-injury. In this study, our data demonstrated the H/R induced cell death and apoptosis in BRL-3A cells. In addition to morphological criteria, induction of Caspase-3 activity provided corroborative evidence for hepatocyte apoptosis. Transfection of frizzled-2 siRNA blocked the H/R-induced LDH and Caspase-3 levels, which emphasized the role of Frizzled-2 in apoptotic cell death in the hepatic H/R. The liver cell membrane structure, the endoplasmic reticulum and sarcoplasmic reticulum are damaged in response to HIRI, which may lead to the abnormal function of the calcium pump and subsequent elevation of the intracellular Ca2+ concentration. Activation of Wnt5a pathway has been shown to trigger the intracytoplasmic release of Ca2+ and the activation of subsequent Ca2+-related signaling.22 HIRI-induced elevations in intracellular Ca2+ can also lead to the pathological activation of Ca2+/calmodulin-dependent protein kinases (CaMKs),4 eventually contributing to cell death and organ dysfunction following ischemia. We demonstrated H/R treatment induced the intracellular Ca2+ level in BRL-3A cells and this induction was attenuated after siRNA silencing frizzled-2 gene expression, which therefore may rescue calcium level and efficiently mitigate the defects of HIRI.
Using in vivo animal study, the expression of Wnt5a and Frizzled-2 was up-regulated significantly in liver tissues from mice with IRI accompanied by a significant decrease in β-catenin compared to the control mice with sham operations, indicating the Wnt5a/Frizzled-2 pathway is involved in mediating hepatic IRI. The increase of cleaved Caspase-3 in liver tissues suggested the involvement of Caspase-3 dependent apoptosis on hepatic IRI.
In summary, we showed Wnt5a/Frizzled-2 pathway was involved in maintaining cell cytotoxicity and apoptosis in BRL-3A cells with H/R treatment as well as in liver tissues from mice with IRI. Frizzled-2 mediated Wnt/Ca2+ signaling pathway via Ca2+ overload during H/R. Our data provided an understanding of the role of Wnt5a/Frizzled-2 signaling in the hepatic cell growth and apoptosis under hepatic IRI, indicating Frizzled-2 may be a molecular therapeutic target in the management of a complex clinical physiopathological HIRI condition.
Conflict of interestThe authors declare no conflicts of interest.
This research was supported by the Science and Technology Planning Project of Guangdong Province (2015B020229002), the National Natural Science Foundation of China (81470875) and the Natural Science Foundation of Guangdong Province (2014A030312013). We are grateful to Dr. David Fu for assisting in the preparation of this manuscript.