Topotecan is an anti-cancer chemotherapy drug with common side effects, including hepatotoxicity. In this study, we aim to investigate the mechanisms of topotecan-induced hepatocellular injury beyond conventional DNA damage.
Materials and methodsMethyl Thiazolyl Tetrazolium (MTT) assay was used to detect the inhibitory effect of topotecan on cell proliferation. Western blot was used to detect protein expression. Flow cytometry assay was performed to determine apoptosis rate under topotecan treatment. ASCT2 overexpression was addressed using adenovirus vector. qRT-PCR and western blot assay were used to detect the expression of ASCT2. Glutamine uptake, intracellular glutathione (GSH) and reactive oxygen species (ROS) level were detected by glutamine detection kit, GSH detection kit and ROS detection kit respectively.
ResultsMTT results showed that topotecan had an inhibitory effect on cell proliferation and induced apoptosis in both L02 and HepG2 cell lines. Topotecan inhibited the expression of glutamine transporter ASCT2 and the uptake of glutamine in both L02 and HepG2 cell lines. The uptake of glutamine and the GSH level was increased in both L02 and HepG2 cell lines after ASCT2 overexpression. The ROS level was inhibited by ASCT2 overexpression upon topotecan treatment in both L02 and HepG2 cell lines. Topotecan-induced hepatocellular apoptosis and proliferation inhibition were attenuated by ASCT2 overexpression in both L02 and HepG2 cell lines.
ConclusionTopotecan-induced hepatocytes death is dependent on ASCT2 down-regulation, which causes oxidative stress via inhibiting GSH production.
El topotecán es un fármaco quimioterapéutico antineoplásico con efectos secundarios frecuentes, incluida la hepatotoxicidad. En este estudio nos proponemos investigar los mecanismos de la lesión hepatocelular inducida por el topotecán más allá del daño convencional del ADN.
Materiales y métodosSe utilizó el ensayo de metil tiazolil tetrazolio (MTT) para detectar el efecto inhibitorio del topotecán sobre la proliferación celular. Se utilizó inmunoelectrotransferencia para detectar la expresión de las proteínas. Se realizó un ensayo de citometría de flujo para determinar la tasa de apoptosis con el tratamiento con topotecán. La sobreexpresión del ASCT2 se abordó utilizando un vector adenoviral. Se utilizaron la qRT-PCR y el ensayo de inmunoelectrotransferencia para detectar la expresión del ASCT2. La absorción de glutamina, el nivel de glutatión intracelular (GSH) y el nivel de especies reactivas del oxígeno (ERO) se detectaron mediante un equipo de detección de glutamina, un equipo de detección de GSH y un equipo de detección de ERO, respectivamente.
ResultadosLos resultados del ensayo de MTT mostraron que el topotecán tenía un efecto inhibitorio sobre la proliferación celular y que inducía la apoptosis en las estirpes celulares L02 y HepG2. El topotecán inhibió la expresión del transportador de glutamina ASCT2 y la absorción de glutamina en las estirpes celulares L02 y HepG2. La absorción de glutamina y el nivel de GSH aumentaron en las estirpes celulares L02 y HepG2 después de la sobreexpresión del ASCT2. El nivel de ERO fue inhibido por la sobreexpresión del ASCT2 tras el tratamiento con topotecán en las estirpes celulares L02 y HepG2. La apoptosis hepatocelular y la inhibición de la proliferación inducidas por el topotecán fueron atenuadas por la sobreexpresión del ASCT2 en las estirpes celulares L02 y HepG2.
ConclusiónLa muerte de hepatocitos inducida por el topotecán depende de la regulación descendente del ASCT2, que causa estrés oxidativo al inhibir la producción de GSH.
Topotecan is a DNA topoisomerase I (Topo I) inhibitor as a camptothecin derivative. It can prevent DNA replication and RNA synthesis via binding with Topo I-DNA complex and finally induces the death of cancer cells.1 However, as a widely used broad anti-cancer medicine, topotecan could bring hepatotoxicity when treats several tumor types in clinical use.2 In a phase I/II study of cyclophosphamide and topotecan in patients with high-risk malignancies undergoing autologous hematopoietic cell transplantation, two patients experienced reversible hepatotoxicity.3 In a study reported on a three-drug (topotecan, thiotepa, and carboplatin) myeloablative regimen designed to consolidate remission and to prevent central nervous system (CNS) relapse of high-risk neuroblastoma (NB) in 66 patients, Grade 2 hepatotoxicity led to truncating cytoreduction in two patients.4 Moreover, in another Phase I and pharmacokinetic study of topotecan performed in patient with refractory solid tumors, elevated AST and ALT was observed.5
Living cells need increased antioxidant to keep redox equilibrium when faces danger signaling caused excessive reactive oxygen species (ROS).6 Glutathione (GSH) is the most important antioxidant and plays the most important role in preventing ROS induced cell death.7 Glutamine is one of the three amino forming GSH. Thus, the glutamine level must influence GSH production in living cells. Alanine, serine, cysteine-preferring transporter 2 (ASCT2; SLC1A5) is a cell surface solute-carrying transporter that mediates uptake of neutral amino acids including glutamine. In recent years, ASCT2 has been demonstrated as the most important glutamine transporter in cancer cells.8–10 Inhibition ASCT2 could kill cancer cells and ASCT2 has already been raised as a drugable target of cancer treatment. Recently, topotecan was demonstrated to inhibit gastric cancer cell proliferation via suppressing ASCT2 expression.11 However, whether ASCT2 involves in topotecan-induced hepatocellular injury is still unclear. In our study, we aimed to investigate the role of ASCT2 in topotecan-induced hepatocellular injury.
Materials and methodsTested drugTopotecan was purchased from Dalian Meilun Biotechnology Co., LTD. CAS: 119413-54-6.
Cell linesThe human normal hepatocytes L02 cells and HepG2 hepatocellular carcinoma cells were purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). All cells were cultured in Dulbecco's modified Eagle's medium (Gibco, Grand Island, USA) with 10% fetal bovine serum (FBS; Gibco, Grand Island, USA), 1% penicillin-streptomycin and maintained at 37°C in a humidified incubator with 5% CO2.
ReagentsAnnexin V-FITC/PI Apoptosis Detection Kit (Containing 250μl Annexin V-FITC, 250μl PI Staining Solution and 25ml 1× Binding Buffer) was purchased from Vazyme Biotech (Nanjing, China). p-mTOR (Ser473), mTOR, p-p70S6k (Thr389), p70S6k primary antibodies were purchased from Cell Signaling Technol (USA), β-Actin, Caspase 3, Caspase 9, PARP primary antibodies were purchased from Proteintech Group (Wuhan, China). MTT was purchased from Beyotime (Nanjing, China).
Quantitative real-time PCR (qRT-PCR)L02 and HepG2 cells were treated with topotecan with different concentrations (0.01μM, 0.1μM, 1μM) for 24h. Total cellular RNA was isolated with the TRIzol Reagent (Vazyme, Nanjing, China) and reverse transcribed with the Revert Aid TM First Strand cDNA Synthesis Kit (Vazyme, Nanjing, China). The mRNA level was measured with the SYBR Green master mix (Vazyme, Nanjing, China). The amount of mRNA for each gene was standardized with the internal control (18s rRNA). Each treatment group was compared with the control group to show the relative mRNA level. The primer sequences for qRT-PCR are provided in Table 1.
Western blot analysisL02 and HepG2 cells were treated with topotecan with different concentrations (0.01μM, 0.1μM, 1μM) for 24h, cellular proteins were extracted. The BCA method was used to detect the protein concentration. SDS-PAGE was used to separate proteins and then transfer the protein to the NC membrane. After blocked by 5% non-fat milk for 1 hour, the membrane was incubated with indicated primary antibodies overnight at 4°C (All primary antibodies were diluted with 5% BSA with a ratio of 1:1000). After then the NC membrane was washed with TBST three times (10min each time). The membrane was incubated in HRP labeled secondary antibodies for 2h at room temperature (Secondary antibodies were diluted with 5% BSA with a ratio of 1:5000). After washing with TBST three times (10min each time), the NC membrane was visualized by using ECL coloring solution for 5min. The photos were captured by Image Lab 5.0 (Bio-Rad Laboratories, USA) and the density values were calculated by Image J (National Institutes of Health, USA).
Annexin V/PI double stainingAfter treatment with 0.01μM, 0.1μM, 1μM topotecan for 24h, L02 and HepG2 cells were digested with 0.25% trypsin without EDTA and were collected via centrifugation at a rotate speed of 500rpm/min. Apoptotic cells were identified by the Annexin V-FITC Apoptosis Detection kit (Vazyme, Nanjing, China) in accordance with the manufacturer's instructions. Cells were washed with fresh PBS buffer for 3 times. Took out around 5×105cells into another tube, then centrifuged it at a rotate speed of 500rpm/min for 5min. Then remove the supernatant carefully. Add 100μl 1× Binding Buffer and gently blow into the single-cell suspension. Then add 5μl Annexin V-FITC solution and 5μl PI Staining Solution for staining for 10min in dark. Flow cytometric analysis was performed immediately after supravital staining. Data acquisition and analysis were performed in a Becton Dickinson FACS-Calibur flow cytometer using the Cell Quest software (Franklin Lakes). For blocking reactive oxygen species (ROS), cells were treated with 5mM N-acetylcysteine (Beyotime, Nanjing, China).
Establishment of ASCT2 overexpression cellsL02 and HepG2 cells were seeded into small dishes at a confluence around 40–50%. Cells were treated with 100μl adenovirus suspension and 4μl polybrene solution for 48h, then changed fresh DMEM medium with 10% FBS. Fluorescence was observed under a microscope. ASCT2 expression efficiency was checked by Quantitative real-time PCR and Western Blot.
Cell proliferation assayControl and ASCT2 overexpression L02 cells or HepG2 cells were plated in 12-well culture plates. After the adherence of the cells, 3 wells of control and 3 wells of ASCT2 overexpression cells were treated with 0.1μM topotecan. Cell number was counted using automatic counter (Countstar) every 24h, which lasted for 72h.
Detection of glutamine uptakeL02 and HepG2 cells were seeded into 6-wells plates. After adherence, medium was replaced with 1.8ml fresh medium containing 10% FBS. Topotecan DMSO solution with high concentration was diluted into 0.1μM, 1μM, 10μM topotecan working solution with fresh medium containing 10% FBS. 200μl topotecan working solution (0.1μM, 1μM, 10μM) was added into wells to make topotecan concentration 0.01μM, 0.1μM, 1μM respectively. After 24h treatment, medium in each well was collected for glutamine detection and cells was digested for counting. Glutamine was detected with Glutamine and Glutamate Determination Kit (GLN1, Sigma Aldrich, St. Louis, MO). Total glutamine uptake was calculated according to glutamine concentration in DMEM medium. Total glutamine uptake dividing cell number was the glutamine uptake of every single cell.
GSH detectionGSH was detected using a GSH and GSSG Assay Kit (Beyotime, Nanjing, China). In brief, L02 and HepG2 cells were seeded into 6-wells plates. After adherence, L02 and HepG2 cells were treated with topotecan with various concentrations (0.01μM, 0.1μM, 1μM) for 24h. Then, cells were washed with PBS to remove medium. Cells were digested and collected. Then the samples were freeze-thawed rapidly twice by liquid nitrogen and 37°C water bath. Samples were placed at 4°C or in an ice bath for 5min. Centrifuged at 10,000×g at 4°C for 10min. Supernatant was used for the determination of GSH according to the manufacturer's protocol.
Determination of cellular reactive oxygen species (ROS)ROS was detected using Reactive Oxygen Species Assay Kit (Beyotime, Nanjing, China). In brief, L02 and HepG2 cells were seeded into 6-wells plates. After adherence, L02 and HepG2 cells were treated with topotecan with various concentrations (0.01μM, 0.1μM, 1μM) for 24h. DCFH-DA was diluted in serum-free medium at 1:1000 to a final concentration of 10mM. Removed the cell culture medium and added the DCFH-DA diluted in the appropriate volume. The added volume should be sufficient to cover the cells, and the diluted DCFH-DA should be no less than 1ml for one hole of the 6-wells plate. The cells were incubated in a 37°C cell incubator for 20min. Then, cells were washed with serum-free cell culture medium three times to fully remove the DCFH-DA that did not enter the cells. The ROS level was detected at 488nm excitation wavelength and 525nm emission wavelength with Varioskan LUX Multimode Microplate Reader (Thermo Scientific).
Statistical analysisAll results are expressed as the mean±SD. Student's t-test was performed to compare and detect significance differences between paired groups. Values of P<0.05 were accepted as statistically significant. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001. All experiments were repeated at least 3 times.
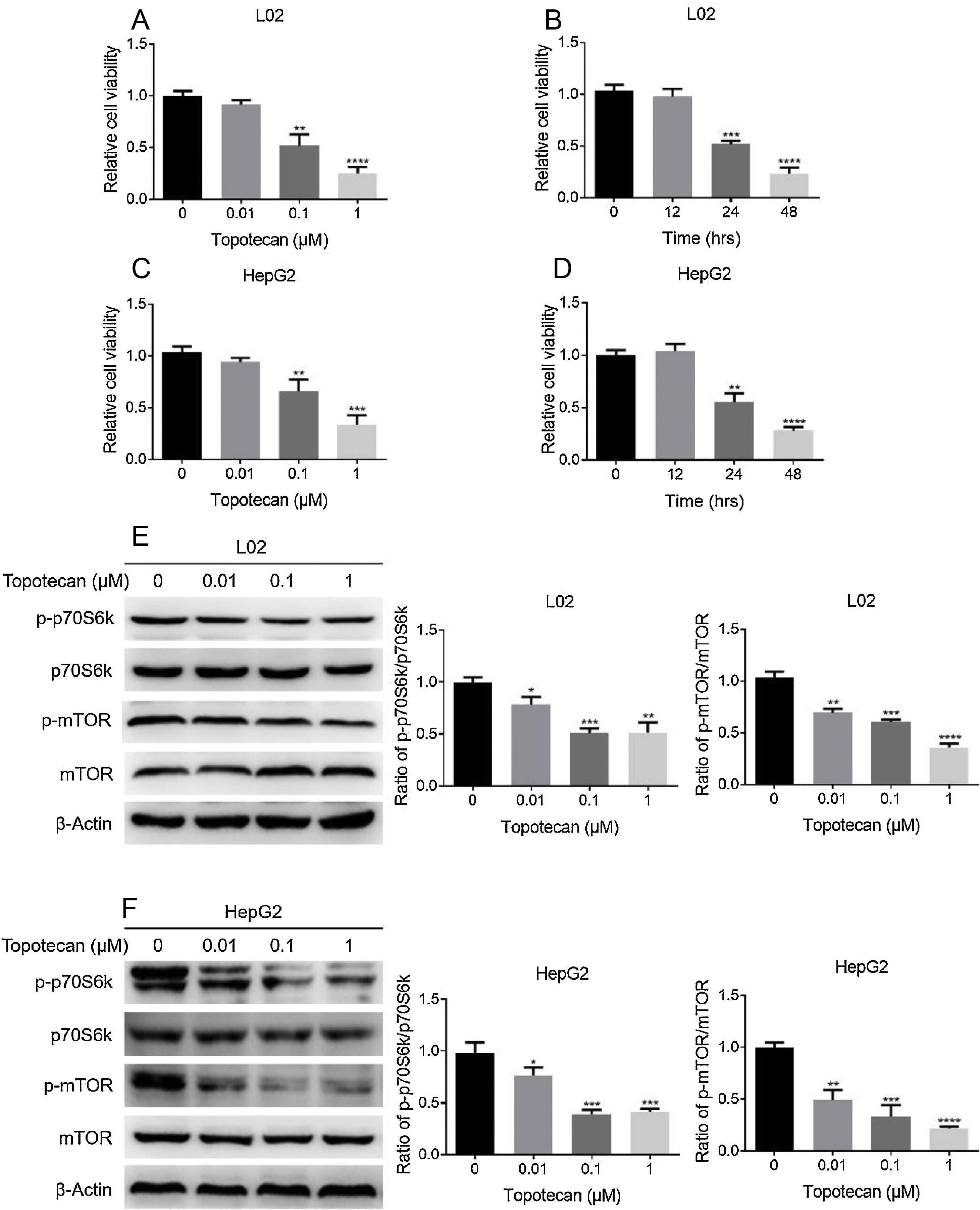
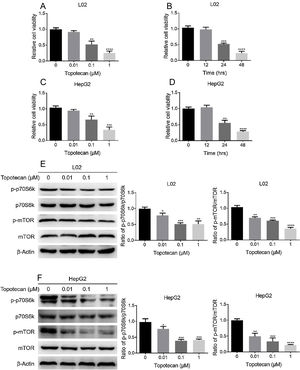
ResultsTopotecan inhibited proliferation of L02 and HepG2 cells.To investigate the toxic effect of topotecan, we treated L02 hepatocytes and HepG2 hepatocellular carcinoma cells with topotecan for 24h. As showed in Fig. 1A and C, topotecan suppressed the proliferation of L02 and HepG2 cells in a dose-dependent manner. The relative cell viabilities of 0.01μM, 0.1μM and 1μM topotecan in L02 cells were 0.9133±0.04509, 0.5167±0.1102, 0.2500±0.06245 respectively. The relative cell viabilities of 0.01μM, 0.1μM and 1μM topotecan in HepG2 cells were 0.9423±0.03859, 0.6597±0.1150, 0.3367±0.09067 respectively. Besides, topotecan also inhibited the growth of L02 and HepG2 cells in a time-dependent manner. When L02 and HepG2 cells were treated with 0.1μM topotecan for 12h, 24h, 48h, the relative cell viabilities of 0.1μM topotecan treatment for 12h, 24h and 48h were 0.9767±0.07506, 0.5167±0.03512, 0.2333±0.05859 in L02 cells (Fig. 1B) and 1.040±0.06557, 0.5533±0.08386, 0.2800±0.03606 in HepG2 cells (Fig. 1D). mTOR regulates the proliferation of eukaryotic cell.12 We next checked whether topotecan inhibited mTOR signaling pathway. As shown in Fig. 1E and F, topotecan inhibited the phosphorylation of mTOR and its downstream target p70S6k in both L02 and HepG2 cells, which indicated that topotecan inhibited mTOR signaling pathway. Taken together, these results show that topotecan indeed possess a powerful capacity to inhibit cell growth.
Topotecan inhibited proliferation of L02 and HepG2 cells. (A) and (C) L02 and HepG2 cells were treated with topotecan with different concentration for 24h. MTT assay was performed to detect the viability of L02 (A) and HepG2 (C) cells. (B) and (D) L02 and HepG2 cells were treated with topotecan (0.1μM) for 12h, 24h and 48h, MTT assay was performed to detected the viability of L02 (B) and HepG2 (D) cells. (E) and (F) L02 and HepG2 cells were treated with topotecan with different concentration for 24h. Protein samples were harvested and the expression of p-p70S6k, p70S6k, p-mTOR and mTOR in L02 (E) and HepG2 (F) cells was detected by western blot. β-Actin was used as a loading control. Protein amount was quantified using Image J. Data represent the mean±SD of three independent experiments. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
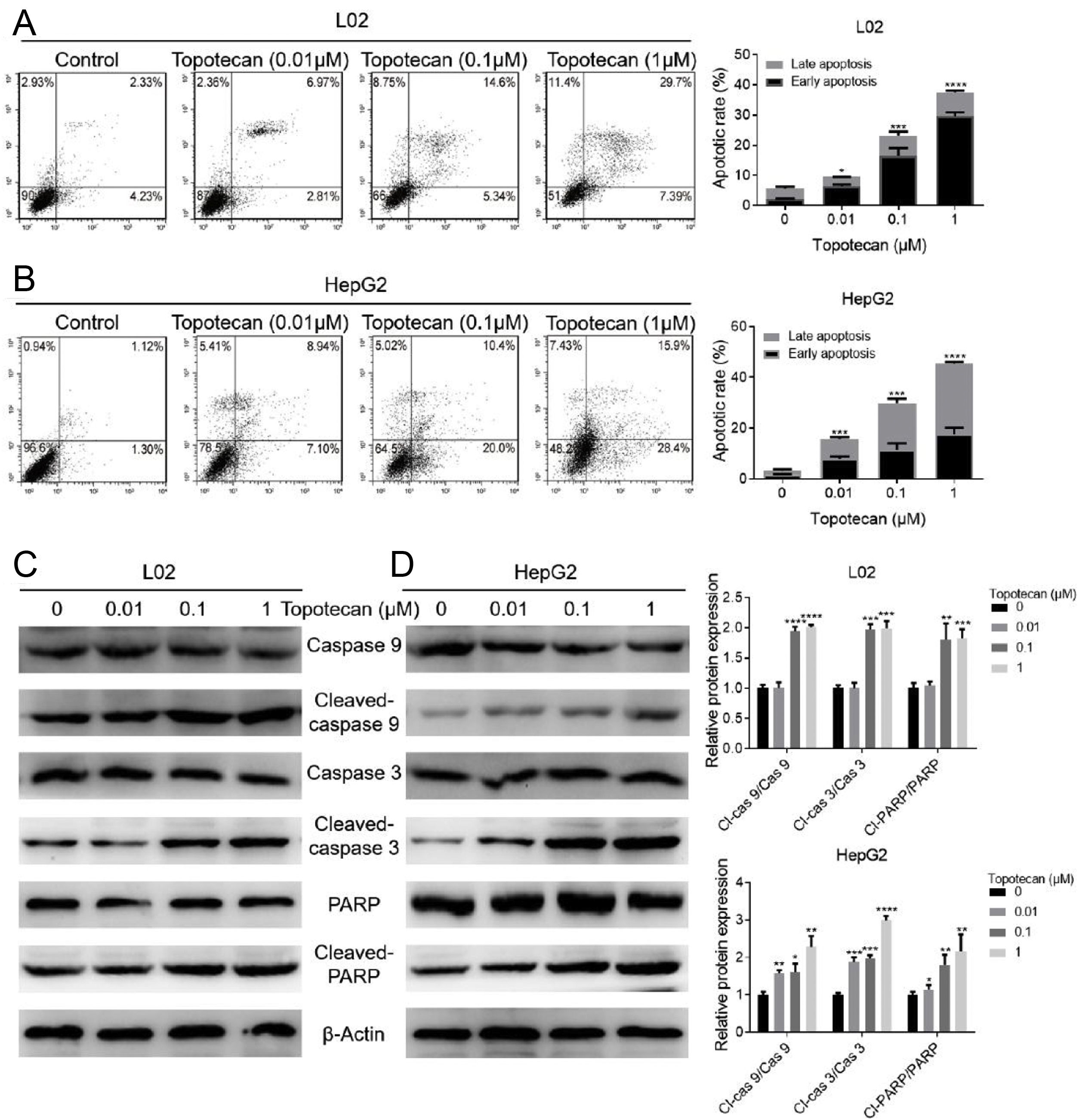
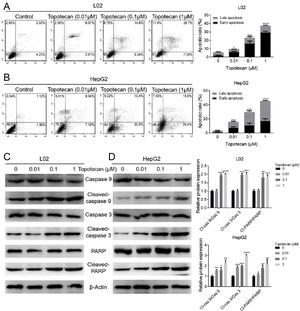
In order to investigate how topotecan inhibited cell growth of L02 and HepG2 cells, we performed flow cytometry to detect the influence of topotecan on apoptosis of L02 and HepG2 cells. As showed in Fig. 2A and B, topotecan induced apoptosis in both L02 and HepG2 cells, which indicated that topotecan could cause serious injury not only in hepatocellular carcinoma cells but also in normal hepatocytes. topotecan induced apoptosis was also verified at molecular level as the apoptotic markers Cleaved-caspase 3, Cleaved-caspase 9 and Cleaved-PARP were all up-regulated in L02 and HepG2 cells (Fig. 2C and D). Taken together, these results suggest that topotecan causes hepatocellular injury via inducing apoptosis.
Topotecan induced apoptosis in L02 and HepG2 cells. (A) and (B) L02 and HepG2 cells were treated with topotecan with different concentration for 24h. Apoptosis of cells was detected by flow cytometry. (C) and (D) L02 and HepG2 cells were treated with topotecan with different concentration for 24h. Apoptotic markers Cleaved-caspase 9, Cleaved-caspase 3 and Cleaved-PARP were detected by western blot. β-Actin was used as a loading control. Protein amount was quantified using Image J. Data represent the mean±SD of three independent experiments. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
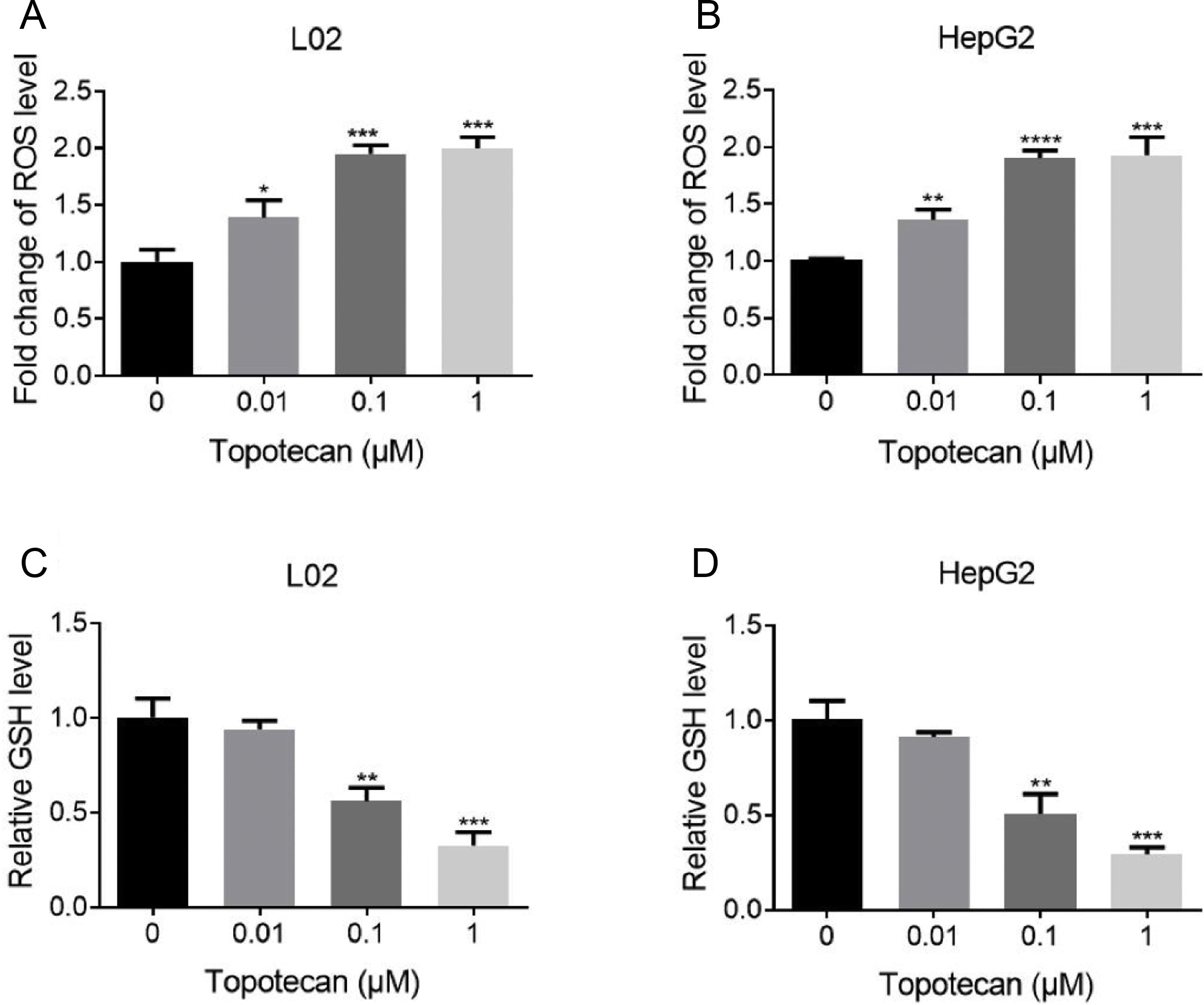
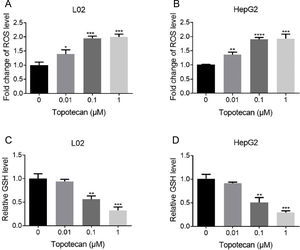
Next, we sought to study the mechanism of how topotecan induced apoptosis in L02 and HepG2 cells. It was reported that topotecan caused increased ROS level alone or combined with other medicines in various cell type.11,13 Thus, we detected ROS level in L02 and HepG2 cells upon topotecan treatment for 24h. As expected, topotecan caused increased ROS level in both L02 and HepG2 cells (Fig. 3A and B). In addition, the intracellular level of the most important antioxidant GSH was decreased in a dose-dependent manner (Fig. 3C and 3D). In summary, these results indicate that topotecan causes oxidative stress in L02 and HepG2 cells.
Topotecan increased ROS level and decreased GSH level in L02 and HepG2 cells. (A) and (B) L02 and HepG2 cells were treated with topotecan with different concentration for 24h. ROS level was detected using ROS detection kit. (C) and (D) L02 and HepG2 cells were treated with topotecan with different concentration for 24h. GSH level was detected using a GSH detection kit. Data represent the mean±SD of three independent experiments. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
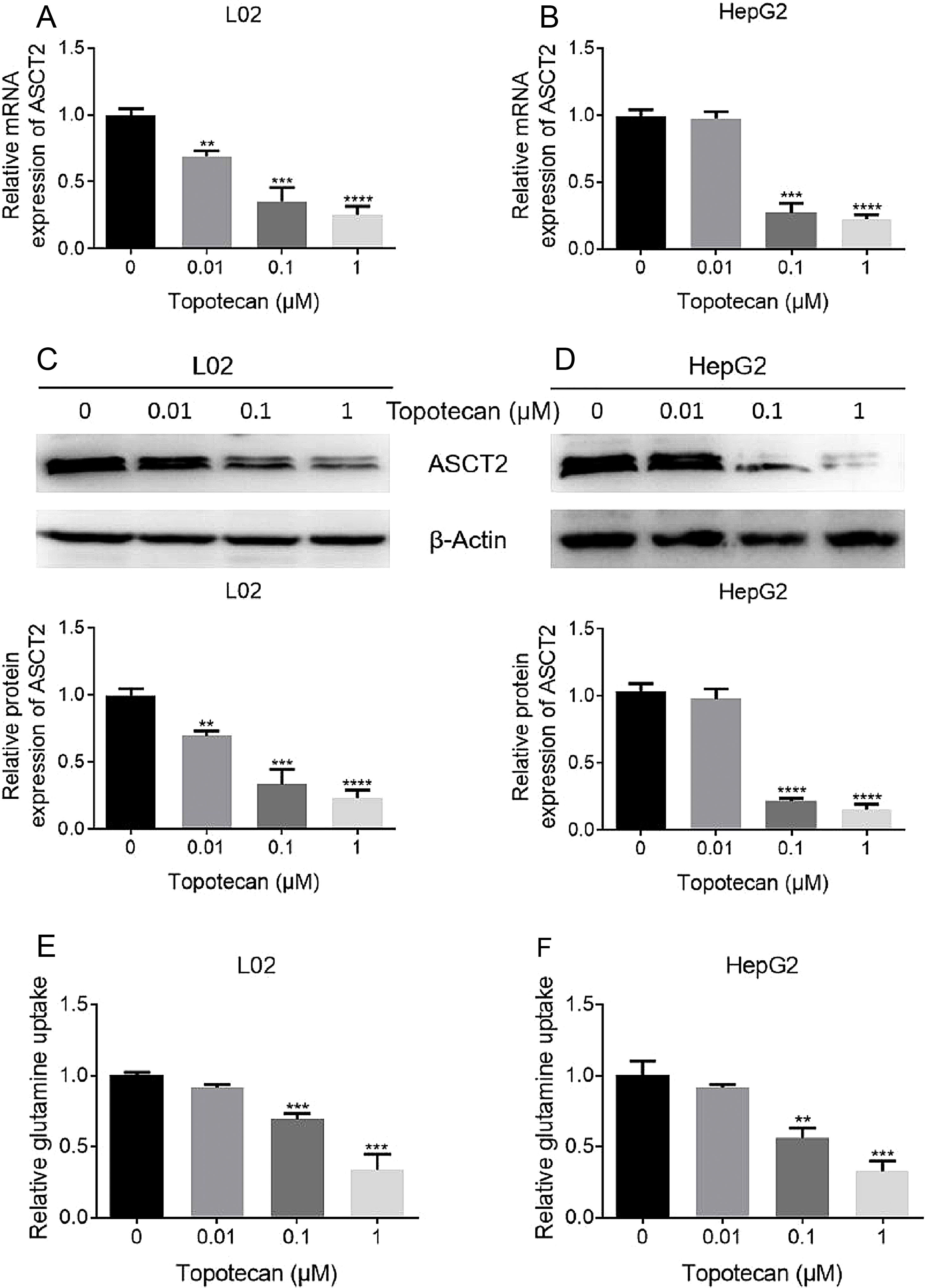
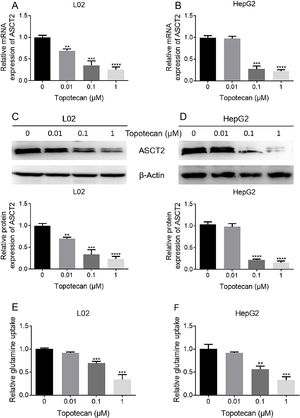
As glutamine is one of the three amino constituting GSH, dysfunction of glutamine uptake must cause decreased GSH production without doubt. So we checked whether topotecan caused a decrease of the expression of ASCT2, one of the most important glutamine transporters has been reported in hepatoma carcinoma cells which is also responsible for part of glutamine uptake in non-tumorigenic hepatocytes.14,15 As shown in Fig. 4A and B, topotecan inhibited ASCT2 expression at mRNA level in both L02 and HepG2 cells. The protein level of ASCT2 was also suppressed by topotecan in a dose-dependent manner (Fig. 4C and D). Besides, topotecan inhibited the uptake of glutamine as expected in both L02 and HepG2 cells. Taken together, these results suggest that topotecan inhibits ASCT2 expression in both L02 normal hepatocytes and HepG2 hepatocellular carcinoma cells.
Topotecan inhibited glutamine uptake via suppressing ASCT2 expression in L02 and HepG2 cells. L02 and HepG2 cells were treated with topotecan with different concentration for 24h. RNA and protein samples were harvested. mRNA expression of ASCT2 was detected by quantitative real-time PCR in L02 (A) and HepG2 (B) cells. Protein expression of ASCT2 was detected by western blot (C) and (D). (E) and (F) L02 and HepG2 cells were treated with topotecan with different concentration for 24h. Glutamine concentration in medium was detected using glutamine detection kit. Then glutamine uptake was calculated according to original concentration of glutamine in medium. β-Actin was used as a loading control. Protein amount was quantified using Image J. Data represent the mean±SD of three independent experiments. **P<0.01; ***P<0.001; ****P<0.0001.
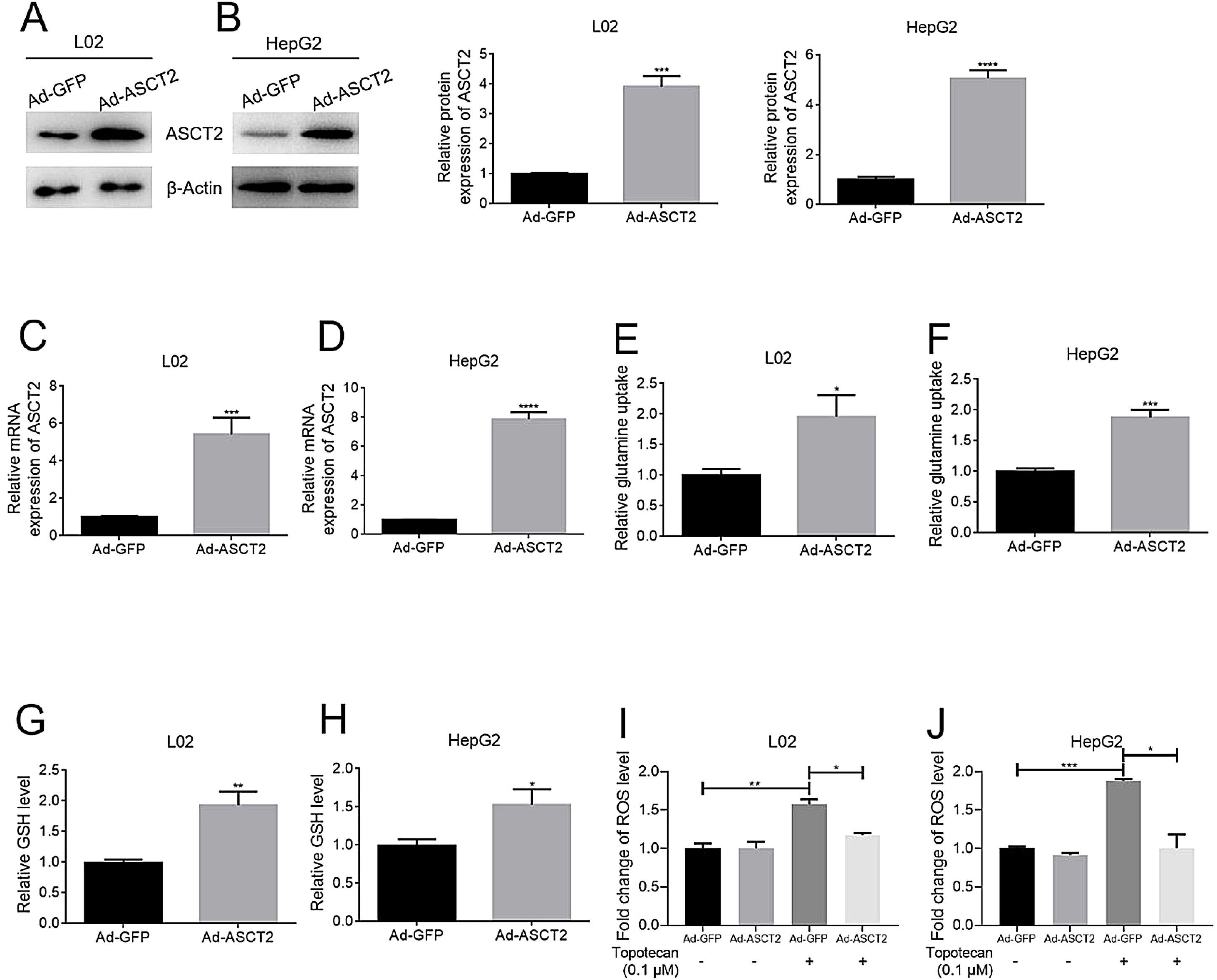
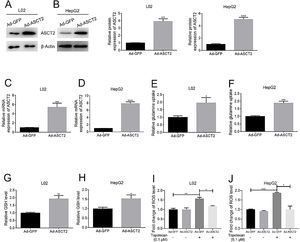
In order to study the role of ASCT2 played in topotecan-caused hepatocellular injury, we established ASCT2-overexpression cell model in both L02 and HepG2 cells using adenovirus vector. Both mRNA and protein level of ASCT2 were increased post adenovirus transfection (Fig. 5A–D). Overexpression of ASCT2 increased the uptake of glutamine in both L02 and HepG2 cells (Fig. 5E and F). The GSH level was also increased as expected in L02 and HepG2 cells. When treated with topotecan, ASCT2 overexpression inhibited ROS production in both L02 and HepG2 cells. Taken together, these results indicate that overexpression of ASCT2 increases GSH production and reduces topotecan-caused ROS production.
Overexpression of ASCT2 increased GSH production and reduced topotecan-induced ROS in L02 and HepG2 cells. L02 and HepG2 cells were treated with adenoviral expression vector for 48h, then changed fresh DMEM medium with 10% FBS for growth until 90% confluence. Protein and RNA samples were harvested for western blot and quantitative real-time PCR respectively. (A) and (B) ASCT2 expression post transfection was detected by western blot. (C) and (D) mRNA expression of ASCT2 post transfection was detected by quantitative real-time PCR. (E) and (F) Control cells and ASCT2 overexpression cells were seeded into 6-well plates until 60–70% confluence. Cells were supplemented with fresh complete DMEM medium and cultured for 24h. Glutamine in medium was detected using glutamine detection kit. Glutamine uptake was calculated according to original glutamine concentration in DMEM medium, then averaged to single cell. (G) and (H) Control cells and ASCT2 overexpression cells were seeded into 6-well plates until 80–90% confluence. Cells were lysed and GSH level was detected using GSH detection kit. (I) and (J) Control cells and ASCT2 overexpression cells were seeded into 6-well plates until 80–90% confluence. Cells were lysed and ROS level was detected using ROS detection kit. β-Actin was used as a loading control. Protein amount was quantified using Image J. Data represent the mean±SD of three independent experiments. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
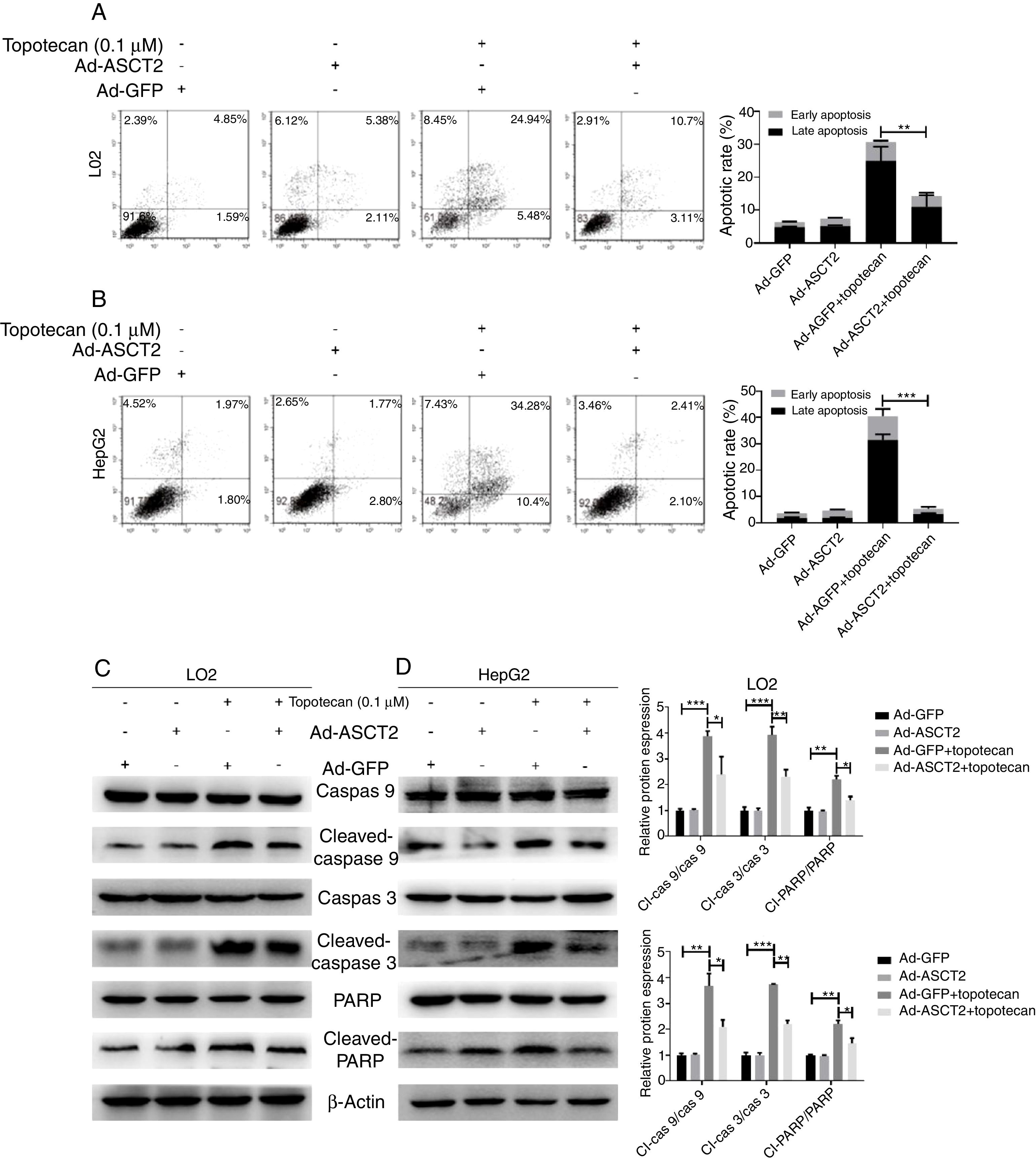
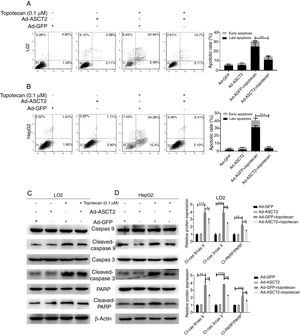
As ROS is an inducer of apoptosis, overexpression of ASCT2 inhibited ROS production upon topotecan treatment, we next explored whether overexpression of ASCT2 could protect against topotecan-caused apoptosis. As showed in Fig. 6A and B, overexpression attenuated topotecan-induced apoptosis of L02 and HepG2 cells. In control L02 cells, topotecan caused apoptosis rate up to around 30%, however, the apoptosis rate caused by topotecan was just around 15% (Fig. 6A). The protective function of ASCT2 overexpression was more obvious in HepG2 cells. Overexpression of ASCT2 almost absolutely protected against topotecan induced HepG2 apoptosis (Fig. 6B). At molecular level, topotecan-induced increase of Cleaved-caspase 3, Cleaved-caspase 9, Cleaved-PARP expression was reversed by ASCT2 overexpression (Fig. 6C and D). Taken together, these results demonstrate that overexpression of ASCT2 attenuates apoptosis caused by topotecan in both L02 and HepG2 cells.
Overexpression of ASCT2 reversed topotecan-induced apoptosis of L02 and HepG2 cells. (A) and (B) Control and ASCT2 overexpression cells were treated with or without topotecan (0.1μM) for 24h, cell apoptosis was detected by flow cytometry. (C) and (D) Control and ASCT2 overexpression cells were treated with or without topotecan (0.1μM) for 24h, cell samples were harvested for analysis of Cleaved-caspase 3, Cleaved-caspase 9 and Cleaved-PARP expression by western blot. β-Actin was used as a loading control. Protein amount was quantified using Image J. Data represent the mean±SD of three independent experiments. *P<0.05; **P<0.01; ***P<0.001.
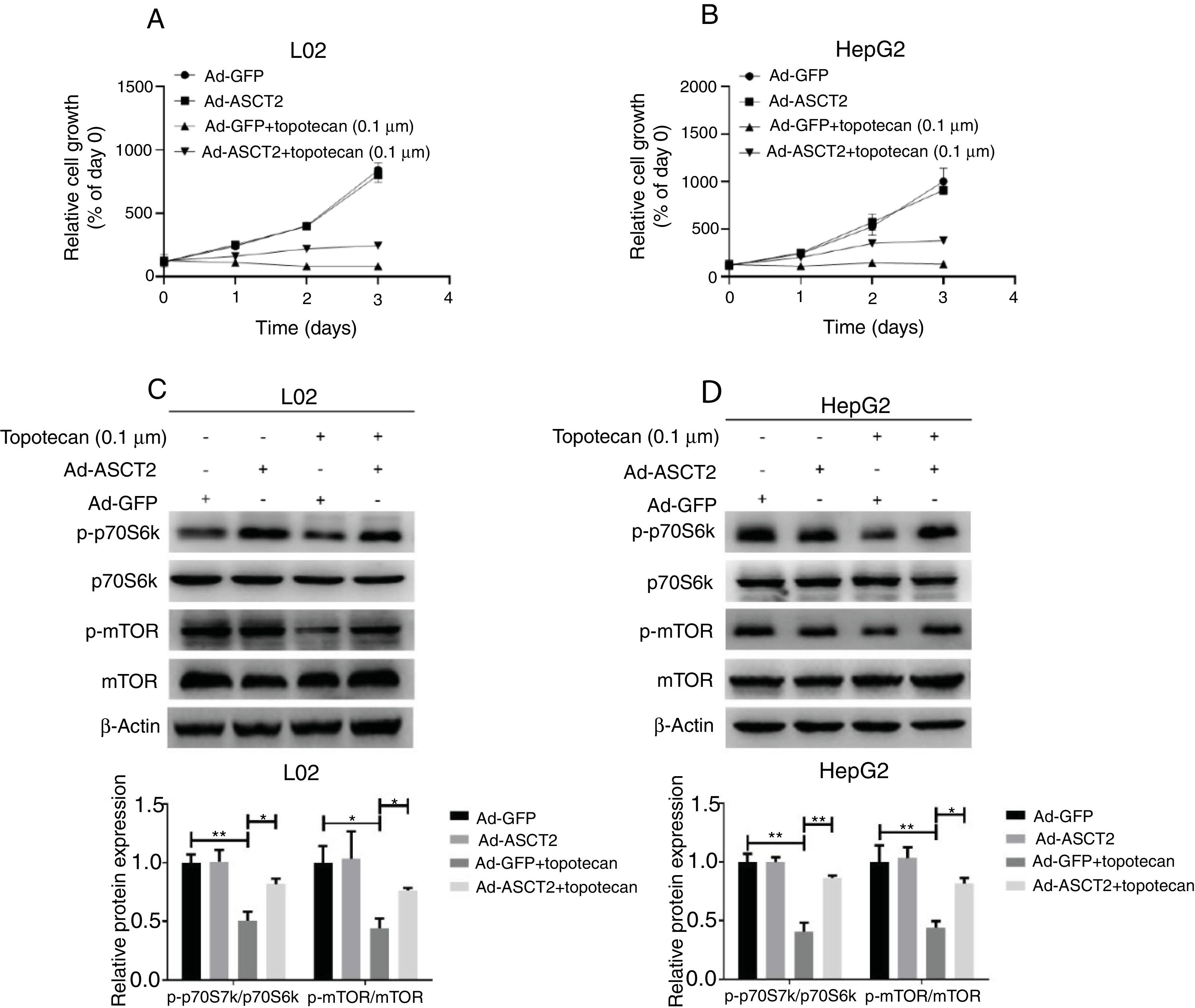
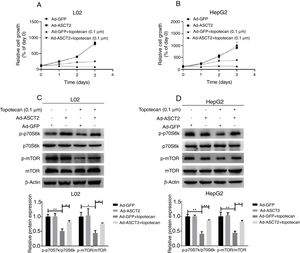
Next, whether overexpression of ASCT2 could reverse topotecan-caused growth inhibition was still unknown. Using a growth assay, we found that overexpression of ASCT2 sustained cell growth upon topotecan treatment. Although cells cannot grow rapidly upon topotecan treatment after ASCT2 overexpression, cells treated with topotecan still kept alive (Fig. 7A and B). Mechanistically, overexpression of ASCT2 sustained activated protein synthesis pathway as shown in Fig. 7C and D. Overexpression of ASCT2 kept phosphorylation of mTOR and p70S6k still at a high level. In summary, overexpression of ASCT2 attenuates the inhibitory effect of topotecan on the proliferation of L02 and HepG2 cells.
Overexpression of ASCT2 partly reversed topotecan-caused growth inhibition. (A) and (B) Control and ASCT2 overexpression cells were seeded into 12-well plates. After 24h, cells were treated with topotecan (0.1μM) for 72h. Cell number was counted every 24h post topotecan treatment. (C) and (D) Control and ASCT2 overexpression cells were treated with or without topotecan (0.1μM) for 24h, cell samples were harvested. The expression of p-p70S6k, p70S6k, p-mTOR and mTOR was detected by western blot. β-Actin was used as a loading control. Protein amount was quantified using Image J. Data represent the mean±SD of three independent experiments. *P<0.05; **P<0.01.
In our present study, we demonstrated that topotecan could cause hepatocellular injury via inducing cell death of hepatocytes and hepatocellular carcinoma cells mediated by ASCT2 down-regulation induced apoptosis.
There are two forms of apoptosis in cells, including death receptor pathway mediated by TNF-α and mitochondrial pathway. ROS is the most common apoptosis stimulating factor in cells. Most of absorbed oxygen is reduced back to water by electron from oxidation respiratory chain, but there is still a small number of oxygen undergo monovalent reduction by electron leaked out from oxidation respiratory chain to form the super oxygen anion. Then the super oxygen anion transforms to hydrogen peroxide (H2O2) by disproportionation, which is the major source of ROS.16 When treated with cytotoxic medicines, more ROS will be produced and induces cell apoptosis. GSH is the most important ROS scavenger inside living cells. Block of GSH production makes cells more sensitive to ROS-induced death. ASCT2 plays an important role in transporting glutamine no matter in normal cells or in cancer cells.10,17,18 Function damage of ASCT2 sensitizes cancer cell to ROS-induced apoptosis.19 Given the predominant role of hepatocytes in biotransformation and metabolism of xenobiotics, ROS production constitutes an important burden in liver physiology and pathophysiology and hence in the progression of liver diseases.20 Inhibition of ASCT2 will makes liver have no ability to face ROS damage. Topotecan-induced ASCT2 down-regulation puts liver cells in a more dangerous situation. Here, topotecan caused increased ROS level inside L02 and HepG2 cells, which leading to apoptosis. In order to elucidate the role of ASCT2 mediated glutamine uptake in topotecan-induced liver cells death, overexpression of ASCT2 was addressed. Overexpression of ASCT2 significantly reversed topotecan-induced liver cells death in both L02 and HepG2 cells. Given the oxidative stress topotecan may cause in liver, an ASCT2 agonist which could increase glutamine uptake will be useful for clinical patients. But up to now, there is no ASCT2 agonist has been developed. So, developing ASCT2 agonists is a meaningful thing for future study. Despite there is no ASCT2 agonist, topotecan combined antioxidants could also be effective for alleviating topotecan-induced liver injury. But this hypothesis still needs preclinical study and clinical study for verification. There are still some limitations in our study. The mechanism through which topotecan regulates ASCT2 expression in liver cells was not investigated in our study and this will be an interesting subject in our future study.
This study illustrates an unreported mechanism of topotecan-induced hepatocellular injury, which is that topotecan induces apoptosis via ASCT2 down-regulation mediated oxidative stress. These findings further our knowledge about the hepatotoxicity of topotecan and provide strategies for prevention or alleviation.
ConclusionTopotecan induces hepatocellular injury via ASCT2 mediated oxidative stress. This is the first time we demonstrate that topotecan can inhibit ASCT2 expression in hepatocytes and hepatocellular carcinoma cells.
Conflict of interestThe authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
This research project was financially supported by the National Natural Science Foundation of China (No. 31870080), Natural Science Research Subject from Education Department of Anhui Province (No. KJ2017A516) and Undergraduate innovation project in Anhui Province (Nos. S201910879201, S201910879216).