Despite high prevalence of iron deficiency anemia (IDA) in patients with acute or chronic gastrointestinal bleeding (GIB), IDA and iron deficiency (ID) are frequently untreated. Reasons may be misconceptions about the impact and diagnosis of IDA and the efficacy of new treatments. Addressing these misconceptions, this article summarizes current evidence for better understanding and management of GIB-associated IDA. Despite only few controlled studies evaluated the efficacy of iron treatment in patients with GIB, there is consistent evidence suggesting that: (a) IDA should be diligently investigated, (b) effective treatment of ID/IDA improves outcomes such as health-related quality of life and can avoid severe cardiovascular consequences, and (c) intravenous iron should be considered as well-tolerated treatment in this setting. Overall, the misconceptions and practices outlined in this article should be replaced with strategies that are more in line with current guidelines and best practice in GIB and other underlying conditions of ID/IDA.
A pesar de la alta prevalencia de anemia por déficit de hierro (ADH) en pacientes con hemorragia digestiva (HD) aguda o crónica, la ADH y el déficit de hierro (DH) son frecuentemente infratratados. Diversos conceptos erróneos sobre el impacto, el diagnóstico y la eficacia de los nuevos tratamientos de la ADH probablemente lo justifican. Para abordar estos errores conceptuales, este artículo resume la evidencia actual para una mejor comprensión y manejo de la ADH. A pesar de que existen pocos estudios controlados que hayan evaluado la eficacia del tratamiento con hierro en pacientes con HD, hay evidencia que sugiere que: (a) la ADH debe ser investigada diligentemente; (b) el tratamiento eficaz del DH/ADH mejora la calidad de vida relacionada con la salud y puede evitar relevantes complicaciones cardiovasculares, y (c) el hierro intravenoso debe ser considerado como un tratamiento bien tolerado en este contexto. En general, los conceptos erróneos y las prácticas inadecuadas descritas en este artículo deben ser reemplazados por estrategias que estén más en línea con las directrices actuales y buenas prácticas clínicas en HD y otras condiciones causantes del DH/ADH.
Gastrointestinal bleeding (GIB) is a common complication of many gastrointestinal conditions.1–7 One of the most comprehensive U.S. data for peptic ulcer disease and GIB, collected from 2001 to 2009, indicated approximately 66 cases per 100,000 population for upper GIB and 223/100,000 for any GIB,8 with substantial increases to hospitalization costs and length of stay.9 While hospitalizations due to upper GIB (particularly due to peptic ulcer bleeding and perforation) decreased over the recent years, varices and lower GIB (particularly colonic diverticular and angiodysplasia bleeding) remained common or even increasing conditions.10,11 There have been few advances in GIB management, yet costs are likely to have increased.
GIB is the most frequent reason for iron deficiency (ID) and iron deficiency anemia (IDA) in adult men and postmenopausal women.12,13 In turn, IDA is a common cause of referral to gastroenterologists and internists,12 can lead to hospitalization, contribute to fatigue, affect health-related quality of life (QoL),14–16 and can be associated with increased mortality in certain populations.17–19 The role of IDA as a common co-morbidity across a wide range of gastrointestinal and liver disorders has been reviewed recently.20 Accordingly, early detection and cost-effective management of anemia and ID would be in the interest of both patients and health care systems.
While the occurrence of IDA in inflammatory bowel disease (IBD) is well reported,21–23 prevalence data on IDA associated with GIB are scarce. Part of the challenge in recording and characterizing GIB-associated IDA is the heterogeneity of underlying gastrointestinal conditions.5,12,14 IDA is typically attributed to occult bleeding from either the upper or lower GI tract.7 Furthermore, age and age-related malnutrition or medications, such as acetylsalicylic acid (ASA) or nonsteroidal anti-inflammatory drugs (NSAIDs), and antithrombotics/anticoagulants, can promote or aggravate GIB and consequently IDA.24 Other gastrointestinal-related causes of IDA include extensive bowel resection, associated dietary restrictions, malabsorption, and Helicobacter pylori infection.25–29 Finally, IDA can be a post-hemorrhage consequence of an insufficient management of major overt GIB event.
Due to the paucity of data and guidelines covering GIB across different underlying gastrointestinal conditions, several open questions and misconceptions on the diagnosis and management of ID and IDA in patients with GIB arose over time (Table 1). This review aims to summarize currently available and burgeoning evidence for better understanding of GIB-associated IDA and improvement of patient outcomes, in order to address this modifiable but often unconsidered risk. The summaries and conclusions are a result of expert discussions based on published literature and expert experience. They reflect the authors’ collective, independent opinion with unanimous agreement on how to address these open questions and misconceptions.
Common misconceptions and clarification about iron deficiency and iron deficiency anemia in patients with gastrointestinal bleeding.
| 1. Prevalence and impact of anemia |
| 1a. Anemia is generally mild in patients with GIB |
| → Anemia is frequently moderate or severe in patients with GIB |
| 1b. The impact of IDA on the quality of life of GIB patients is very limited |
| → The impact of IDA on the quality of life of GIB patients is important |
| 2. Diagnosis |
| 2a. Doctors are sensitive to detect and treat anemia during hospitalization and discharge of patients with acute and overt GIB |
| → Doctors are usually not sensitive to detect and treat anemia during hospitalization of patients with GIB |
| 2b. Ferritin is accurate enough for the diagnosis and monitoring of ID in GIB |
| → Ferritin alone is not accurate enough for the diagnosis of ID in GIB and for the monitoring after i.v. iron administration |
| 2c. Anemia but not ID is the only relevant laboratory finding in GIB |
| → Iron deficiency with or without anemia is a relevant laboratory finding in GIB |
| 3. Treatment goals and efficacy |
| 3a. All patients with GIB and IDA are quite similar and, therefore, one treatment approach for IDA fits all |
| → Patients with GIB and IDA are heterogeneous and require an individualized treatment approach for IDA |
| 3b. When treating IDA with iron supplementation in patients undergoing a GIB event, the aim is just to increase but not necessarily normalize Hb values |
| → When treating IDA with iron supplementation in GIB, the aim is to achieve normal Hb values |
| 3c. Intravenous iron administration should be reserved for GIB patients with very severe anemia (e.g. Hb <8g/dL) |
| → Intravenous iron administration should not be reserved only for GIB patients with very severe anemia |
| 3d. When treating IDA with i.v. iron administration, a dose of 500 or up to 1000mg is enough |
| → Total doses of i.v. iron administration should be individualized but frequently are higher than 1000mg; most available clinical data are based on single doses of up to 1000mg iron |
| 3e. The correction of IDA after i.v. iron administration is practically immediate |
| → The correction of IDA after i.v. iron administration is rapid but takes some time (several weeks) |
| 3f. The efficacy of i.v. iron for the treatment of IDA in GIB, in contrast with non-GIB patients, has not been demonstrated |
| → The effectiveness of i.v. iron for the treatment of IDA in patients with GIB is demonstrated |
| 3g. Intravenous iron treatment is unsafe |
| → Intravenous iron treatment is safe |
| 3h. Red blood cell transfusion is indicated in hemodynamically stable patients when Hb drops below 8–9 g/dL |
| → Blood transfusion is not indicated in hemodynamically stable patients when Hb is≥7–8g/dL |
| 3i. After initiation of blood transfusion, no further i.v. iron treatment is needed |
| → Even after blood transfusion, i.v. iron treatment is needed in most cases |
| 3j. When oral iron is administered, higher doses than usual should be prescribed in patients with GIB |
| → When oral iron is administered, higher doses than usual should not be prescribed in patients with GIB |
| 3k. The response of IDA to oral iron supplementation is almost invariably excellent in GIB |
| → The response of IDA to the oral iron supplementation is variable and depends on factors such as age, gender, concomitant medications and comorbidity |
| 3l. Treatment with i.v. iron has no role in the patients with GIB seen in the emergency room for self-limiting bleeding who are discharged early, without being admitted to hospital |
| → Treatment with i.v. iron may be crucial in patients with IDA seen in the emergency room for self-limiting bleeding who are discharged early, without even being admitted to hospital |
| 4. Other |
| 4a. The role of the gastroenterologist ends when the GIB event is controlled (stopping GIB is the priority, anemia is not relevant) |
| → The role of the gastroenterologist is not only to control the GIB event but also to treat IDA and ID |
| 4b. Intravenous iron formulations are expensive and not cost-effective |
| → Intravenous iron can be cost-effective |
GIB, gastrointestinal bleeding; Hb, hemoglobin; i.v., intravenous; ID, iron deficiency; IDA, iron deficiency anemia.
While anemia in adults is defined by hemoglobin (Hb) <13g/dL in men or <12g/dL in women (moderate anemia Hb <10g/dL, severe anemia Hb <8.0g/dL), Hb levels <12g/dL in men and <10g/dL in women should be investigated more urgently, since lower Hb levels suggest more serious disease.12 The World Health Organization's most recent Global Burden of Disease study showed a 33% global anemia prevalence in 2010 with 15% of episodes being classified as moderately to severely anemic.30
Data specific for patients with either acute overt or chronic (non-oncological) GIB and IDA are scarce. Among patients who had been admitted for non-variceal acute upper GIB (AUGIB) to a Danish university hospital, 50% had a Hb <10g/dL at admission and 80% were still anemic at discharge (median Hb 10.6g/dL).1 A recent multicenter study in Spain (REGIS),31 which investigated characteristics of 379 patients hospitalized for gastrointestinal diseases, showed a 60% anemia prevalence at admission with half of those patients being severely anemic (Hb <10g/dL). Patients with occult GIB (chronic bleeding of unknown origin) had a low mean Hb of 8.0g/dL. Despite the absence of similar studies in other countries, it is very likely that these results can be extrapolated to other geographic areas.
Authors’ conclusion: Anemia is frequently moderate or severe in patients with GIB.
Misconception 1b: The impact of iron deficiency anemia on health-related quality of life of patients with gastrointestinal bleeding is very limitedThe impact of IDA on QoL is often underestimated or ignored. Although patients tend to adapt to low Hb levels,32,33 this is rather an adaptation to a lower QoL.
QoL studies in patients with specific GIB-lesions and IDA or related symptoms are scarce and small.34,35 A sub-study of a trial on iron treatment in patients with acute GIB showed that patients who were non-anemic after three months treatment had a higher overall health-related QoL mean index than anemic patients.34 Although this difference was not statistically significant, it is noteworthy that the QoL mean index remained stable in non-anemic patients but decreased substantially in anemic patients over the following three months, resulting in significantly different proportions of patients who achieved normal age- and gender-matched health-related QoL. In patients with IBD or other conditions associated with IDA, QoL studies suggest that IDA has a substantial impact on QoL.32,33,36–39 Studies in iron-treated patients with IBD showed significant improvements of QoL compared to baseline.38,40 One study even correlated single-unit increases in Hb levels and improvements in quality of life scores independent of changes in disease activity. Considering that IDA can affect several body systems,33 one can expect a similar impact of IDA on QoL in patients with GIB. Notably, even before the decline of Hb to anemic levels, ID can already affect QoL as well as cognitive and behavioral functioning.41–43
Authors’ conclusion: The impact of IDA on the quality of life of GIB patients is important.
DiagnosisMisconception 2a: Doctors are sensitive to detect and treat anemia during hospitalization and discharge of patients with acute and overt gastrointestinal bleedingEvidence suggests just the opposite: among patients with GIB in the REGIS study, the prevalence of severe anemia did not change significantly from admission to discharge.31 Anemia was frequently ignored or left untreated in patients hospitalized for gastrointestinal diseases with 58% still anemic (17% severely anemic) at discharge. Similarly, another study in patients hospitalized with non-variceal AUGIB showed that >80% were anemic at the time of discharge.1 Moreover, most patients with non-variceal AUGIB develop IDA within 30 days after the episode,44 suggesting suboptimal treatment of IDA for a considerable proportion of hospital admissions.
These findings in GIB are in line with a recent study on the management of IBD-associated anemia and ID in 9 European countries.45 This study showed that the diagnostic tests and criteria used to assess or confirm ID/IDA varied considerably, implying guidelines were not adhered. Furthermore, high frequencies of severe anemia (15%) and absolute ID (76%) suggest insufficient monitoring and iron repletion in routine disease management. In contrast to the available published recommendation preferring intravenous (i.v.) over oral iron treatment,21 only 28% received i.v. iron.
Authors’ conclusion: Doctors are usually not sensitive to detect and treat anemia during hospitalization of patients with GIB.
Misconception 2b: Ferritin is accurate enough for the diagnosis and monitoring of iron deficiency in gastrointestinal bleedingIn patients with AUGIB, not only baseline levels of Hb and ferritin but also levels of Hb and transferrin saturation (TSAT) five days after GIB were predictive for the development of IDA 30 days after the GIB episode (patients did not receive i.v. iron but transfusions when being hemodynamically instable).44 Also, patients with acute GIB may have an unknown or untreated chronic inflammation or comorbidities that can result in falsely normal or increased ferritin levels because ferritin is an acute phase reactant.46 Accordingly, diagnostic criteria for ID should be adapted to the potential or actually confirmed presence of inflammation (e.g. via elevated levels of C-reactive protein, endoscopic findings, diarrhea). TSAT and red blood cell indices such as the hemoglobin content of reticulocytes or the percentage of hypochromic cells are less prone to such influences. Accordingly, half of 22 professional-association guidelines on the diagnosis and treatment of ID in different indications propose TSAT as an alternative or complementary diagnostic test besides serum ferritin.47 The British Society of Gastroenterology guideline for the management of IDA in digestive diseases proposes ID as serum ferritin below 15–50μg/L, depending on inflammatory status.12 In IBD-associated anemia, consensus recommendations suggest that the lower limits of serum ferritin and TSAT in patients with evidence of inflammation should be 100μg/L and 16–20%, respectively.21,48,49 Without evidence of inflammation or liver damage, a serum ferritin <30μg/L is indicative of ID.50 In addition to Hb, iron status and markers of inflammation or liver damage (C-reactive protein, alanine aminotransferase), a full evaluation should also include vitamin B12 and folic acid levels as recommended by the European guidelines for patients with IBD.48,49
Overall, iron status should be monitored to minimize the risk of newly developed or recurrent ID and IDA in the context of the patient's condition and management of GIB. For monitoring, the British Society of Gastroenterology proposes that Hb levels and red blood cell indices are assessed at 3-monthly intervals for one year, then after a further year, and immediately in case symptoms of ID/IDA reoccur.12
In the primary care setting, where nutritional deficiencies, cancer/chemotherapy, or chronic renal failure can be easily ruled out, ferritin values, combined with standard laboratory tests and a complete blood count, can be considered accurate enough to diagnose ID or IDA.51
Authors’ conclusion: Assessment of ferritin levels as sole iron status parameter is not accurate enough for the diagnosis of ID in GIB and for the monitoring of iron status before or after i.v. iron administration.
Misconception 2c: Anemia but not iron deficiency is the only relevant laboratory finding in gastrointestinal bleedingNotably, any chronic illness can disrupt iron homeostasis and ultimately reduce the availability of iron for effective erythropoiesis and hemoglobin production,50 and untreated ID will likely progress to IDA. Therefore, regular assessment of iron status (ferritin and TSAT) is supported, with adequate workup and consideration to the clinical consequences of disturbances in iron status.12 The relevance of ID beyond just being a laboratory measure is indicated by studies in different populations, showing that ID can affect QoL and cognitive functioning even before the onset of anemia. Furthermore, iron repletion improved functional as well as QoL outcomes in ID patients with and without anemia.41–43,52,53 A recently presented study among patients with IBD in a Spanish outpatient clinic showed that 37% were iron deficient but non-anemic, and these patients had significantly lower QoL (CCVEII-9) and fatigue (FACIT-F) scores than patients with normal iron and Hb status.54
Authors’ conclusion: Iron deficiency with or without anemia is a relevant laboratory finding in GIB.
Treatment goals and efficacyMisconception 3a: All patients with gastrointestinal bleeding and iron deficiency anemia are quite similar and, therefore, one treatment approach for iron deficiency anemia fits allGuidelines for the treatment of AUGIB hardly address IDA, and in case they do, red blood cell (RBC) transfusion is the only suggested treatment for patients with Hb levels below 7–8g/dL.55 However, it is essential to consider also other patient characteristics that can differently influence the etiology of GIB and IDA, e.g. advanced age, comorbidities (especially heart failure and chronic obstructive pulmonary disease) or medications such as anticoagulants (given to approximately 30% of patients with treated IDA),56,57 ASA, NSAIDs and proton pump inhibitors (PPIs).58,59 Although anticoagulants are transiently suspended during active bleeding, they are reintroduced shortly after a bleeding period, particularly in patients with heart failure, atrial fibrillation or cardiac valve diseases, increasing the risk of recurrent bleeding and IDA. Furthermore, conditions associated with acute or chronic inflammation and co-medication with PPIs that increase the gastric pH can affect the efficacy of oral iron.50,60 Intravenous iron therapy is less dependent on such patient characteristics and has a substantial different gastrointestinal side effect profiles than oral iron,21 however, approved dose limits differ between different i.v. iron preparations according to their stability.61 With respect to oral iron, one should consider the stool coloring effect that may either frighten patients suspecting to have a new bleeding event (even if they have been informed about that effect by their physician) or conversely, miss this typical sign of bleeding since they judge it as side effect of the treatment.
Authors’ conclusion: Patients with GIB and IDA are heterogeneous and require an individualized treatment approach for IDA.
Misconception 3b: When treating iron deficiency anemia with iron supplementation in patients undergoing a gastrointestinal bleeding event, the aim is just to increase but not necessarily normalize hemoglobin valuesThe overall aims of treating IDA are to normalize Hb levels, and to replenish iron stores.12 A study investigating patients hospitalized for new or worsening heart failure showed a sharp reduction in mortality among patients with Hb levels that are closer to normal (13g/dL) at hospital admission.62 In two separate studies following-up patients with chronic heart failure (CHF), multivariate analysis showed that anemia (Hb <12g/dL) is an independent risk factor for mortality and heart failure-related readmission, with rates of approx. 30% in anemic and 15% in non-anemic patients. The benefit of even incremental increases of Hb levels has been shown in different patient populations where Hb increase and not necessarily Hb normalization correlated with improvements of QoL.38,63 Among patients with acute GIB who had received a three-month anemia treatment, approximately 60% of patients with resolved anemia but only about 30% of patients with unresolved anemia had normal QoL scores three months’ post-treatment.34
After hospital discharge, follow-up of IDA patients should address the goal to raise and maintain normal Hb levels (>13g/dL in men, >12g/dL in women).12,21,49 Otherwise, anemia may recur relatively fast, as observed in patients with Crohn's disease and ulcerative colitis.64
Authors’ conclusion: When treating IDA with iron supplementation in GIB, the primary aim is to achieve normal Hb values, and the additional aim should be to normalize iron status (as reflected by ferritin or TSAT levels).
Misconception 3c: Intravenous iron administration should be reserved for gastrointestinal bleeding patients with very severe anemia (e.g. hemoglobin <8g/dL)In the past, i.v. iron was reserved for very severe anemia because of putative side effects of early developed, high molecular weight iron dextrans that were associated with risks of allergic reactions.65 Even nowadays, anemia seems to be left ignored or treated only in severe cases.31 Because newer i.v. iron preparations are generally well tolerated when used according to their label,12,66 patients with chronic GIB who are intolerant or not responding to oral iron should receive i.v. iron for the correction of IDA and repletion of body iron stores.12 As a simple heuristic, i.v. iron might be indicated in all cases when Hb levels are <10g/dL and in cases when Hb levels are >10g/dL together with relevant cardiac or respiratory comorbidities. Observational studies in oncology patients have shown that i.v. iron treatment can consistently improve Hb levels across different baseline Hb categories (Hb <10g/dL, 10–11g/dL).67,68
In clinical practice, GIB is often associated with chronic kidney disease (CKD)69–71 a disease area with a long history and vast experience in the effective and safe use of i.v. iron for the treatment of ID and IDA (with i.v. iron given alone or in combination with an erythropoiesis-stimulating agent [ESA]).72 Because iron is an essential element required for a wide range of cellular and physiological functions,73 rapid resolution of ID with appropriate iron treatment should be considered even in the absence of anemia.21 In patients with chronic heart failure, treatment of ID with i.v. iron improved functional outcomes and QoL in both patients with and without anemia.52,53
Authors’ conclusion: Intravenous iron administration should not be reserved only for GIB patients with very severe anemia.
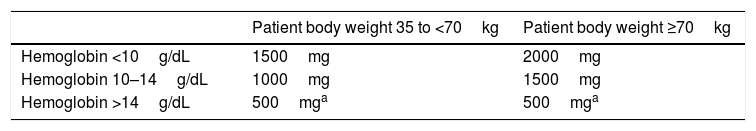
Misconception 3d: When treating iron deficiency anemia with i.v. iron administration, a dose of 500 or up to 1000mg is enoughIn fact, in most studies on anemia treatment with new i.v. iron preparations, iron was administered in individual doses of 500–1000mg.40,74,75 However, in patients with acute or chronic bleeding, iron deficits can easily exceed 1000mg as calculated by the Ganzoni formula,76 which rather underestimates the iron needs.40,74,75 A novel and simpler dosing guide for total dose estimates based on Hb and body weight (Table 2) given in single doses of 500–1000mg iron has been tested in patients with IBD40 and adopted in the labels of i.v. iron preparations.77 Option for high iron doses for rapid and efficient iron replenishment are ferric carboxymaltose and, although data are scarce, low-molecular-weight iron dextran. Several clinical trial and observational data that evaluated efficacy and safety of high-dose iron administration used these compounds.40,78,79
Authors’ conclusion: Total doses of i.v. iron administration should be individualized but frequently are higher than 1000mg; most available clinical data are based on single doses of up to 1000mg iron.
Misconception 3e: The correction of iron deficiency anemia after i.v. iron administration is practically immediateAlthough the elevation of Hb levels after i.v. iron treatment occurs generally faster than with oral iron,12,80 the utilization of administered iron and the process of erythropoiesis need some time. Therefore, Hb levels do not increase or normalize immediately after treatment as it would be the case after RBC transfusion. The administered iron carbohydrate complexes are taken up and degraded by the cells of the reticuloendothelial system, followed by utilization of iron for repletion of iron stores or erythropoiesis in the bone marrow.81 Analytical control (e.g. assessment of Hb and iron status parameters) should be performed at least 10 days after administering i.v. iron (in most cases after hospital discharge).
Up to 2 months after i.v. iron administration, elevated ferritin values may be observed as a normal transitory response without indicating iron overload.82,83 Additional iron parameters that should be checked to evaluate the erythropoietic response to i.v. iron are TSAT, hemoglobin content of reticulocytes (should increase quickly after iron treatment) or the percentage of hypochromic cells (slow decrease after iron treatment).14,46
Authors’ conclusion: The correction of IDA after i.v. iron administration is rapid but takes some time (several weeks).
Misconception 3f: The efficacy of i.v. iron for the treatment of iron deficiency anemia in gastrointestinal bleeding, in contrast with non-gastrointestinal bleeding patients, has not been demonstratedPublished clinical evidence on the use of i.v. and other iron treatment in patients with either acute or chronic GIB and IDA is scarce compared with that in IBD. However, data from a published trial in patients with non-variceal AUGIB79 and recent congress presentations56,57,84–86 suggest its clinical benefit. The published trial was designed as a double-blind, 3-arm study (1×1000mg i.v. ferric carboxymaltose; 100mg BID oral ferrous sulfate for 12 weeks; placebo).79 From as early as week 4 onwards, median Hb levels were significantly higher among iron- vs placebo-treated patients. Unfortunately, most patients in this study were not iron deficient at baseline, and no significant differences in Hb outcome between the two iron groups have been detected. However, from week 1 onwards, ferritin levels in the i.v. iron group were higher than those in the oral iron and the placebo group. Furthermore, ferritin levels of oral iron-treated patients decreased by 50% from baseline to week 4. This is an important aspect, because a study in patients with IBD revealed that patients with low ferritin levels (<100μg/L) at the end of treatment experienced earlier recurrence and reinitiation of iron therapy.64 Anticipated results from an ongoing trial (ClinicalTrials.gov NCT01690585) investigating i.v. ferric carboxymaltose in patients with GIB will add evidence to this area.
Recently presented data suggest effectiveness of ferric carboxymaltose in improving Hb levels in patients after acute GIB,85,86 angiodysplasia57 and outpatients with GIB due to different conditions referred from a gastroenterology department for anemia treatment.84 In patients after acute GIB, a randomized comparison vs oral iron showed faster Hb response and normalization of TSAT by Day 7 post treatment with ferric carboxymaltose,85 and in another study significant Hb increase in patients >75 years of age, Charlson index >3 or hospital admission Hb <10g/dL was reported.86
Clearly, the lack of published evidence affects clinical practice. A retrospective study in Denmark1 showed that only a minority of patients (16%) hospitalized for non-variceal AUGIB received a recommendation to begin oral iron supplementation. Intravenous iron was not even considered and more than 80% of admitted patients were anemic at the time of discharge.
The lack of guidance on the management of GIB-related IDA is illustrated by the fact that among 29 guidelines from different professional associations, only three refer to gastrointestinal diseases; two of them referring to IBD-associated IDA21,49 and only one to digestive diseases in general.12 In lieu of clinical data and guidelines in patients with GIB, there is published evidence of effective blood/iron management and i.v. iron treatment in patients bleeding from another source (e.g. colon cancer87,88 or surgery89). Such results can be reasonably extrapolated to anemic patients with GIB.
Authors’ conclusion: The effectiveness of i.v. iron for the treatment of IDA in patients with GIB is demonstrated.
Misconception 3g: Intravenous iron treatment is unsafeThe persistent misconception about safety of i.v. iron is mainly based on historical reports of anaphylactic reactions and high serious adverse event rates associated with high-molecular weight iron dextrans65 (no longer marketed in many regions), and a superparamagnetic iron oxide preparation90 (marketing authorization withdrawn in Europe at the manufacturer's request).91 With other preparations, the incidence of serious adverse events is very rare and hypersensitivity usually self-limited.61,92–94 Furthermore, the European Medicines Agency reviewed the benefit-risk balance of i.v. iron-containing medicinal products, concluding that the benefits of i.v. iron continue to outweigh the risks in the treatment of ID when the oral route is insufficient or poorly tolerated.66
Authors’ conclusion: Intravenous iron treatment is safe.
Misconception 3h: Red blood cell transfusion is indicated in hemodynamically stable patients when hemoglobin levels drop below 8–9g/dLIn general, it is not recommended to simply transfuse to a threshold Hb level; clinicians need to consider age, comorbidities, cause and severity of bleeding, etc. in any case. If RBC transfusion is deemed necessary, a restrictive policy should be considered, unless ischemic heart disease is a comorbidity.95,96 Results of the TRIGGER trial95 suggest that the Hb threshold for transfusion can be safely lowered (Hb <8.0g/dL) without adversely affecting clinical outcomes in patients with acute upper GIB. In another study, restricted transfusions (Hb <7.0g/dL) in patients with acute upper GIB were associated with lower mortality.97
Although, a restrictive transfusion policy means that many patients will remain anemic for some time, it does not mean ignoring IDA and doing nothing. In contrast, it provides time to manage IDA with available transfusion alternatives, mainly i.v. iron. A retrospective study of patients with moderate (Hb <10g/dL) to severe (Hb <8.0g/dL) IDA who required chronic transfusion support (>2 RBC transfusions within one year) in five Italian transfusion centers showed the effectiveness of i.v. iron treatment in the reduction of RBC requirements.98 Treatment with ferric carboxymaltose was associated with a significant reduction of the median annual number of transfused RBC units from 6 (interquartile range 4–15) to 0 (0–5) and median Hb levels nevertheless increased significantly from 8.7g/dL (8.1–9.2g/dL) to 11.0g/dL (9.9–11.7g/dL).
A restrictive transfusion policy is in line with the guideline for the management of IDA in digestive diseases, recommending to reserve blood transfusions for patients who have symptomatic IDA despite treatment with iron or for patients who are at risk of cardiovascular instability due to the degree of their anemia.12 Such a policy is also backed by the ‘Choosing Wisely’ initiative of the American Board of Internal Medicine (ABIM) and the American Association of Blood Banks, asking to limit the administration of RBC units to the absolutely necessary minimum and a restrictive Hb threshold of 7.0–8.0g/dL.99 Furthermore, RBC transfusions should not be used as treatment for iron deficiency in patients without hemodynamic instability.
Authors’ conclusion: Blood transfusion should be restricted to hemodynamically instable patients and the absolutely necessary minimum number of RBC units. If transfusion is deemed necessary in hemodynamically stable patients who do not present with cardiovascular risk factors, a restrictive Hb threshold of 7.0–8.0g/dL should be considered.
Misconception 3i: After initiation of blood transfusion, no further i.v. iron treatment is neededIn clinical practice and guidelines, RBC transfusions are often mentioned as treatment without considering or addressing needs for further iron repletion or maintenance treatment.100 However, RBC transfusions raise Hb levels only transiently without correcting the underlying pathology and iron depletion at the same time, making the recurrence of IDA more likely.12,64 Accordingly, iron status parameters and whole blood counts should be monitored as part of transfusion follow-up.48 Notably, a single RBC unit (300mL) contains approximately 150mg iron, whereas modern i.v. iron preparations can be given at doses up to 1000mg iron per administration. With i.v. iron, the production of appropriate red blood cells (erythropoiesis) and the repletion of iron stores is facilitated.81
It is prudent to keep in mind that transfusions are a transitory measure and not intended to restore normal Hb values. Only the minimum number of RBC units that are needed to reach a safe Hb level and hemodynamic stabilization of the patient should be transfused. Restoration of normal Hb levels (and iron stores) can be achieved with following i.v. iron treatment as recommended12,89 and recently presented even for patients with acute GIB.86
Authors’ conclusion: Even after blood transfusion, i.v. iron treatment is needed in most cases.
Misconception 3j: When oral iron is administered, higher doses than usual should be prescribed in patients with gastrointestinal bleedingThere is little evidence to support high doses of oral iron in patients with GIB, as iron absorption may be generally impaired. Controlled efficacy studies of oral iron-based IDA treatment in adult, elderly, pediatric and pregnant populations support low-dose oral iron supplementation either in tablet or liquid form.101–104 In the absence of inflammation or chronic disease, the upper limit of daily iron absorption is approximately 10mg iron/day (under inflammatory conditions even less).102,105 Thus, a dose of 100mg elemental iron daily is sufficient and higher doses will only increase the risk of adverse gastrointestinal effects (e.g. nausea, vomiting, constipation and diarrhea).
In general, the response to oral iron (rate with normalized Hb levels) strongly depends on factors such as comorbidities, age and gender that often influence levels of inflammatory cytokines and thereby affect intestinal iron absorption and utilization.50,60
Authors’ conclusion: When oral iron is administered, higher doses than usual should not be prescribed in patients with GIB.
Misconception 3k: The response of iron deficiency anemia to oral iron supplementation is almost invariably excellent in gastrointestinal bleedingApart from gastrointestinal side effects and the risk of masking a new bleeding period due to stool coloring, main limitations of oral iron supplementation are slow response and poor tolerance/compliance (see Section 3j).102,105 In non-variceal AUGIB, a single 1000mg i.v. iron dose achieved equivalent Hb outcomes over three months as the twice daily oral intake of 100mg of iron (approx. 36g total iron dose).79 A recently presented randomized, controlled study in patients with IDA after acute GIB showed significantly better Hb normalization, Hb response (≥2g/dL) and TSAT increase in ferric carboxymaltose-compared to oral iron-treated patients.85 In chronic GIB, absorption of oral iron may be severely compromised and persistent blood loss will exceed the capacity of intestinal absorption of iron.106
Furthermore, patient adherence to long-term oral treatments is a general issue107,108 and particularly with oral iron due to its known gastrointestinal side effects such as constipation, nausea and diarrhea.109 In patients after acute GIB, abdominal pain and constipation were reported by 6.7% and 21% of oral iron-treated patients whereas no side effects were reported for the i.v. iron group. In line, treatment adherence was lower with oral vs i.v. iron (87% vs 100%).85
In daily life, patient adherence may be even lower than in the controlled environment of clinical trials and further limit the effectiveness of oral iron treatment. While oral iron (fumarate) is associated with increased clinical disease activity in IBD patients,110 studies on the risk of rebleeding in patients with GIB taking oral iron are lacking. Notably, even if Hb levels improve with oral iron, repletion of iron stores (ferritin levels) will hardly be achieved within a reasonable time period due to the poor iron absorption and generally large iron deficits, thus, increasing the risk of IDA recurrence.64,89 Considering that many GIB patients may have chronic comorbidities with an underlying inflammatory status, oral iron is not recommended.48 Despite these valid precautions, there may be cases where oral iron treatment may be sufficient if well tolerated.
Authors’ conclusion: The response of IDA to the oral iron supplementation is variable and depends on factors such as age, gender, concomitant medications and comorbidity.
Misconception 3l: Treatment with i.v. iron has no role in patients with gastrointestinal bleeding seen in the emergency room for self-limiting bleeding who are discharged early, without being admitted to hospitalDiagnosis and early intervention in the emergency room may be crucial in patients with sub-acute but self-limiting GIB and IDA, even though iron status analysis may not be available on a 24-h basis in all emergency departments. The need for blood conservation, particularly in patients not requiring acute transfusion, and proper resource and cost management in the emergency room suggest a role for i.v. iron in the management of acute as well as chronic GIB.55,111–113 While many emergency cases of self-limiting bleeding stop spontaneously, at least patient's iron status and risk of developing ID or IDA should be followed up by referring to a primary care physician after discharge. In a Spanish Day Hospital, 34.7% of patients treated with i.v. iron treatment after GIB where referred from an emergency department.56
In a recently presented fast-track anemia management program within an emergency department that emphasized early intervention with i.v. iron (No.=202 patients with moderate-to-severe anemia [Hb <11.0g/dL]), 44% of the referred cases related to chronic GIB.112 The use of i.v. ferric carboxymaltose was associated with a 3.9g/dL mean increase in Hb after 4 weeks and an 84% response rate. In addition, a low transfusion rate (17%) was achieved, demonstrating that i.v. iron use in emergency departments is possible, beneficial and safe.112 Although further data and longer follow-up are needed, clearly i.v. iron does have a role for patients with sub-acute, self-limiting GIB and IDA, with close follow-up needed to prevent relapse or transfusion.
Moreover, patients in the emergency room may have ID independent of whether they have any GIB because anemia is highly prevalent even in developed countries.114,115 This fosters the need for follow-up if not an early intervention.
Authors’ conclusion: Treatment with i.v. iron may be crucial in patients with IDA seen in the emergency room for self-limiting bleeding who are discharged early, without even being admitted to hospital.
OtherMisconception 4a: The role of the gastroenterologist ends when the gastrointestinal bleeding event is controlled (stopping gastrointestinal bleeding is the priority, anemia is not relevant)In parallel with the immediate needs of managing and treating the source of GIB, specific treatment goals and proper surveillance of patients are needed to correct IDA or ID in a timely manner. Considering that QoL declines very fast in anemic patients with GIB while it remains stable in non-anemic patients,34 the gastroenterologist should consider anemia as a serious comorbid condition and treat it with the same rigor as the bleeding. Control or resolution of GIB and anemia do not exclude each other. While treatment of the underlying disease is intended to contain or prevent further loss of blood, parallel iron replacement treatment is intended to resolve anemia and replenish iron stores.12 If patients are not followed-up at the gastroenterology department, referring physicians should be instructed to continue anemia and iron status monitoring as indicated by the relevant guidelines12 and initiate treatment as needed.
Authors’ conclusion: The role of the gastroenterologist is not only to control the GIB event but also to treat IDA and ID.
Misconception 4b: Intravenous iron formulations are expensive and not cost-effectiveWhile oral iron may appear inexpensive and convenient, it is associated with a slow Hb response and gastrointestinal adverse events that can increase indirect costs. A cost-minimization analysis of treating IDA in patients with colon cancer before surgery showed lower costs with a high-dose i.v. iron (ferric carboxymaltose) compared with oral iron (1827 vs 2101€/patient), mainly based on higher efficacy (Hb increase in a short period of time) that is reflected in shorter hospital stays and less transfusion needs.116 Conversely, higher costs were observed with an i.v. iron that requires more frequent administrations (iron sucrose; 2312€/patient); maybe because the dose of iron sucrose was calculated using the Ganzoni formula that seems to underestimate iron needs.
Another cost-effectiveness analysis in a fast-track anemia clinic for the management of moderate-to-severe IDA comparing an i.v. iron-based fast-track protocol with the previous transfusion-based standard care showed cost savings of 78€/patient with ferric carboxymaltose vs RBC transfusions (594 vs 672€/patient).113
Authors’ conclusion: Intravenous iron can be cost-effective.
ConclusionsGastroenterologists have a unique position in the follow-up and care of referred patients with GIB, particularly with the objective of identifying and improving the underlying cause(s). Current practice is not standardized and is suboptimal. First, more research and greater awareness of the challenges of ID and IDA in the GIB setting are needed, aiming to influence the clinical outcomes and QoL of these patients and minimize the need for blood transfusions. Second, all clinicians involved in patient care should engage in addressing misconceptions to achieve a positive impact on QoL and minimize the risk of persistent blood loss, inadequate iron status at hospital discharge and early anemia recurrence. Commitments from the health care network to better coordination with evidence-based guidelines that benefit patients with ID/IDA would be ideal.
While there are few controlled studies evaluating the efficacy of IDA treatment in GIB, consistent data exist showing that patients with GIB can benefit from diligent investigation and treatment of IDA, and allowing for some fundamental statements (Table 1) in response to common misconceptions. Oral iron is an acceptable ‘first-line’ option primarily for clinically stable patients with only mild anemia and without risk of rebleeding, gastrointestinal complications or sequelae, chronic inflammation, malabsorption or tolerability issues. When using i.v. iron, a faster improvement in Hb and iron status can be expected. While most patients with acute GIB experience significant blood loss prior to endoscopic interventions, transfusions should always be restricted to rescue treatments and combined with i.v. iron supplementation to replete iron stores. The Hb threshold for transfusion should be kept low if there are no cardiovascular comorbidities present.
In light of the available treatment options, the ‘status quo’ of ignoring ID and IDA in the GIB does not seem appropriate. The treatment goal should not only be to stop bleeding but to completely correct ID/IDA in a deliberate, efficient and fast manner and minimize the risk of anemia recurrence.
AuthorshipÁngel Lanas and Fermín Mearin contributed equally to this work.
Conflict of interestAll authors participated in the advisory board of Vifor Pharma España. Fermín Mearin has been part of the advisory board of Vifor Pharma España. Antonio Hervás has served as consultant of Vifor Pharma España. Miguel Montoro served as advisor for MSD and Almirall. Javier P. Gisbert has served as a speaker, a consultant and advisory board member for or has received research funding from MSD, Abbvie, Hospira, Kern Pharma, Takeda, Janssen, Pfizer, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, Vifor Pharma, Almirall, Nycomed, AstraZeneca, Casen Recordati, Allergan.
Angel Lanas, Luis Bujanda, Pilar Canelles, José Cotter, Carlos Martín de Argila declared no conflicts of interest.
The panel discussion meeting and medical writing support by Walter Fürst, SFL Regulatory Affairs & Scientific Communication, were funded by Vifor Pharma España. The manuscript was reviewed and commented by Mercedes Cucala, Medical Department, Vifor Pharma España.
Please cite this article as: Mearin F, Lanas Á, Bujanda L, Canelles P, Cotter J, Hervás A, et al. Preguntas y errores en el diagnóstico y manejo de la anemia en pacientes con hemorragia digestiva. Gastroenterol Hepatol. 2018;41:63–76.