To define clinical screening criteria for spondyloarthritis (SpA) in patients with inflammatory bowel disease (IBD) and vice versa, which can be used as a reference for referring them to the rheumatology or gastroenterology service.
MethodSystematic literature review and a two-round Delphi method. The scientific committee and the expert panel were comprised of 2 rheumatologists and 2 gastroenterologists, and 7 rheumatologists and 7 gastroenterologists, respectively. The scientific committee defined the initial version of the criteria, taking into account sensitivity, specificity, standardisation and ease of application. Afterwards, members of the expert panel assessed each item in a two-round Delphi survey. Items that met agreement in the first or second round were included in the final version of the criteria.
ResultsPositive screening for SpA if at least one of the following is present: onset of chronic low back pain before 45 years of age; inflammatory low back pain or alternating buttock pain; HLA-B27 positivity; sacroiliitis on imaging; arthritis; heel enthesitis; dactylitis. Positive screening for IBD in the presence of one of the major criteria or at least two minor criteria. Major: rectal bleeding; chronic diarrhoea with organic characteristics; perianal disease. Minor: chronic abdominal pain; iron deficiency anaemia or iron deficiency; extraintestinal manifestations; fever or low grade fever, of unknown origin and duration >1week; unexplained weight loss; family history of IBD.
ConclusionScreening criteria for IBD in patients with SpA, and vice versa, have been developed. These criteria will be useful for early detection of both diseases.
Definir criterios clínicos de cribado de espondiloartritis (SpA) en pacientes con enfermedad inflamatoria intestinal (EII) y vice versa, que sirvan de referencia en la derivación entre Reumatología y Aparato Digestivo.
Material y métodosRevisión sistemática de la literatura y Delphi a dos rondas. Formaron parte del comité científico 2 reumatólogos y 2 digestólogos; del panel de expertos, 7 reumatólogos y 7 digestólogos. El comité científico definió los componentes potenciales de los criterios, teniendo en cuenta aspectos de sensibilidad, especificidad, facilidad de uso y estandarización. A continuación, se realizó el Delphi. Aquellos ítems para los que hubo acuerdo en primera o segunda ronda formaron parte de la versión final de los criterios.
ResultadosCribado positivo de SpA si se cumple al menos uno de los siguientes: dolor lumbar crónico con inicio antes de los 45 años; dolor lumbar inflamatorio o dolor alternante en nalgas; HLA-B27 positivo; sacroilitis en pruebas de imagen; artritis; entesitis del talón; dactilitis. Cribado positivo de EII si uno de los criterios mayores o al menos dos de los menores. Mayores: rectorragia; diarrea crónica de características orgánicas; enfermedad perianal. Menores: dolor abdominal crónico; anemia ferropénica o ferropenia; manifestaciones extraintestinales; fiebre o febrícula, sin focalidad aparente y de más de una semana de duración; pérdida de peso no explicable; antecedentes familiares de EII.
ConclusionesSe han definido criterios de cribado de EII en pacientes con SpA y viceversa. Estos han de ser de utilidad en la detección precoz de dichas patologías.
Musculoskeletal symptoms are the extraintestinal manifestations most frequently associated with inflammatory bowel disease (IBD). Patients with IBD frequently develop spondyloarthritis (SpA). According to a meta-analysis published in 2016, the prevalence of peripheral arthritis is around 13%, sacroiliitis around 10% and ankylosing spondylitis 3%.1
In a cohort of 269 patients with IBD evaluated for joint pain, 50.5% were diagnosed with SpA; an average diagnostic delay of 5.2 years was observed.2
In another study of 122 patients with IBD, the prevalence of SpA was 28.7%, of whom 45.7% were not previously diagnosed despite a history of inflammatory lower back pain and/or peripheral arthritis.3
By comparison, according to the data of a meta-analysis published in 2015, the prevalence of IBD in ankylosing spondylitis is 6.8%.4 The studies also revealed a link between psoriatic arthritis and the onset of IBD, although to a lesser degree.5–7
And although not as long as with SpA, there is also a diagnostic delay in the case of IBD. In a series of 1591 patients with IBD, in 25% of cases this delay exceeded two years for Crohn's disease (CD) and one year for ulcerative colitis (UC).8 In another series of 1196 patients, the delay times were 18 months for CD and three months for UC.9
For both SpA and IBD it is very important to avoid diagnostic delay because it is associated with a worse clinical course and poorer response to treatment.10–13
Currently, there are no tools aimed at providing an early diagnosis for SpA in patients with IBD, and vice versa, that are adapted to the Spanish healthcare system. The objective of this article is to define clinical screening criteria for SpA in patients with IBD and vice versa, which serve as a reference in patient referral between the Rheumatology and Digestive System departments for the early detection of these diseases.
Material and methodsGeneral designSystematic review of the literature and consensus using the two-round Delphi method.
Selection of scientific committee and panel membersTo make this selection, the experience (both clinical and investigational) of the candidates with regard to the project subject matter and their research CVs (publications from the last five years and participation in research projects) were evaluated. In the case of the scientific committee members, their personal qualities and attitude towards working in a group were also evaluated. For the selection of panellists, the geographic representation of the Spanish territory and the type of hospital (level 1 or basic hospitals, level 2 or reference hospitals, and level 3 or high-tech hospitals) were also evaluated.
The scientific committee was made up of two rheumatologists and two gastroenterologists. The panel of experts included seven rheumatologists and seven gastroenterologists.
Literature reviewTwo systematic reviews of the literature were conducted, one of which focused on the screening tools or the suspected IBD gastroenterologist referral criteria, and the other focusing on screening tools or suspected SpA (not including psoriatic arthritis) rheumatologist referral criteria. Both were limited to a population under the age of 18. The searches (Appendix B) were performed using Pubmed, Embase and Cochrane Library, and include articles in Spanish and English published until January 2016.
Definition of the screening criteriaUsing the results obtained in the systematic review stage and considering their experience, the members of the scientific committee defined the potential components of the screening criteria. In this respect, the sensitivity, specificity, ease of use in normal clinical practice and standardisation (ensuring that variability in their application is as low as possible) criteria were considered.
The two-round Delphi method was then initiated. The selected panellists evaluated the proposed criteria electronically. The panellists were provided with systematic review reports from the literature in advance. The scores for each criterion were calculated according to the following scale: 1=absolute disagreement; 2=moderate disagreement; 3=neither agree nor disagree; 4=moderate agreement, and 5=absolute agreement.
For each of these criteria, the panellists could make comments about changes that may be considered necessary in the wording or reasons explaining their evaluation if deemed necessary, or provide additional evidence (not collected in the systematic review reports) that supported their scoring. The panellists could also propose additional criteria.
After analysing the results of this first Delphi round, the scientific committee evaluated the criteria for which there was no consensus, as well as the suggestions for additional criteria. A second document was then prepared outlining the criteria for which there had been no consensus in the first round, together with the changes made by the scientific committee according to the comments and suggested criteria. The first round scores and comments from the panellists for the non-consensual criteria were also included, as well as the scientific committee's response to these comments.
In the second round, this document was sent to the panellists, who once again evaluated the criteria for which no consensus had been reached, aware of the result of the first round so that they could compare their initial score with that of the other panellists and the considerations of the scientific committee. The criteria were evaluated according to the same scale used in the first round.
The anonymity of the participants was maintained throughout the entire Delphi process.
Analysis of the Delphi resultsThe level of consensus for each item was defined as the percentage of panellists who gave a score ≥4, and the level of disagreement as the percentage of scores ≤2.
The following thresholds were used to define consensus:
- –
Consensus: score ≥4 from at least 75% of the panellists.
- –
Disagreement: score ≤2 from at least 75% of the panellists.
Those items for which there was consensus in the first or second Delphi round formed part of the final screening criteria.
ResultsThe initial screening criteria defined by the scientific committee are outlined in Figs. 1 and 2.
Results of the first Delphi roundThe evaluations of 13 panellists were collected. Tables 1 and 2 show the level of consensus reached for each item proposed by the scientific committee.
SpA screening criteria for patients with IBD. Level of consensus.
| Criterion | Level of consensus |
|---|---|
| Chronic lower back pain starting before the age of 45 | 92% (12 of the 13 panellists) |
| Inflammatory lower back pain | 92% (12 of the 13 panellists) |
| HLA-B27 positive | 92% (12 of the 13 panellists) |
| Sacroiliitis in imaging tests | 92% (12 of the 13 panellists) |
| Arthritis | 100% |
| Achilles enthesitis | 100% |
| Dactylitis | 100% |
IBD: inflammatory bowel disease; SpA: spondyloarthritis.
IBD screening criteria for patients with SpA. Level of consensus.
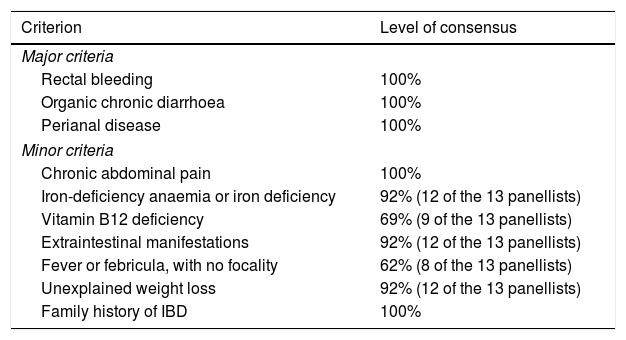
| Criterion | Level of consensus |
|---|---|
| Major criteria | |
| Rectal bleeding | 100% |
| Organic chronic diarrhoea | 100% |
| Perianal disease | 100% |
| Minor criteria | |
| Chronic abdominal pain | 100% |
| Iron-deficiency anaemia or iron deficiency | 92% (12 of the 13 panellists) |
| Vitamin B12 deficiency | 69% (9 of the 13 panellists) |
| Extraintestinal manifestations | 92% (12 of the 13 panellists) |
| Fever or febricula, with no focality | 62% (8 of the 13 panellists) |
| Unexplained weight loss | 92% (12 of the 13 panellists) |
| Family history of IBD | 100% |
IBD: inflammatory bowel disease; SpA: spondyloarthritis.
For the IBD screening, four of the panellists suggested that calprotectin be included, one panellist considered it necessary to include the age at which the symptoms started, and another suggested that the presence of mucus in faeces be included as a minor criterion.
Regarding the SpA screening, the following suggestions for inclusion in the criteria were made (each of these suggested by one panellist): axial morning stiffness lasting more than one hour; morning stiffness lasting more than 30min in the case of axial or peripheral articular symptoms; presence of previous or current uveitis; articular pain lasting at least three months and starting before the age of 45; and alternating buttock pain.
After evaluating the criteria for which no consensus was reached (level of consensus below 75%) and the comments of the panellists, the scientific committee decided:
- –
Not to make any changes to the criterion concerning vitamin B12 deficiency.
- –
To amend the criterion on fever or febricula, with no apparent focality, to the following: fever or febricula, with no apparent focality, lasting more than one week.
- –
For the rectal bleeding criterion, although the level of consensus was 100%, the following change was made based on the panellists’ comments: rectal bleeding, unless haemorrhoidal signs are evident and there are haemorrhoids on physical examination.
Regarding possible additional criteria, the scientific committee evaluated the suggestions received in the first round and it was decided that alternating buttock pain be included, combining it with the inflammatory lower back pain criterion so that the final wording of the criterion was as follows: inflammatory lower back pain (according to the Assessment of SpondyloArthritis international Society [ASAS] definition) or alternating buttock pain.
The scientific committee decided not to include faecal calprotectin in the IBD screening for patients with SpA because they believe it is a tool that is still not accessible to a large proportion of Rheumatology departments. Furthermore, they believe that including this item could run the risk of over-indication in the request for this test. As a result, it was decided that the request for calprotectin should currently be limited to the gastroenterology setting. The panellists were informed of this decision at the beginning of the second Delphi round. One of the panellists commented, “the fact that faecal calprotectin is not available at this time does not mean that it won’t be widely available in the next few months or years. It is such a simple tool with so much negative predictive value that it is now suggested to be used as screening for organic gastrointestinal disorders in patients with chronic diarrhoea, and it has been recommended that it become available in the Primary Care setting”.
Results of the second Delphi roundIn this Delphi round, the panellists once again evaluated the two non-consensual criteria from the first round, taking into account the change made by the scientific committee to the fever or febricula criterion. For this reason, the scores and comments of the other panellists were provided, as well as the scientific committee's response to these comments. They also evaluated whether to include alternating buttock pain in the inflammatory lower back pain criterion.
A total of 13 panellists took part. Table 3 shows the level of consensus for each of the criteria.
Level of consensus with the criteria. Second Delphi round.
| Criterion | Level of consensus |
|---|---|
| Vitamin B12 deficiency | 77% (10 of the 13 panellists) |
| Fever or febricula, with no apparent focality, lasting more than one week | 100% |
| Inflammatory lower back pain (according to the ASAS definition) or alternating buttock pain | 100% |
For vitamin B12 deficiency, one of the panellists, who scored this criterion with a two (moderate disagreement), supplied the systematic review by Battat et al. to justify his/her evaluation and to provide more information.14 This systematic review includes 42 studies that analyse serum cobalamin levels and the absorption test. According to the results of these studies, neither CD nor UC would predispose to a vitamin B12 deficiency. Considering this reference and the low specificity of this marker, the scientific committee believed it unnecessary to include the B12 deficiency in the IBD screening criteria for patients with SpA. The panellists were notified of this decision and none opposed it.
Final version of the screening criteriaThe screening criteria defined according to the process described above are outlined in Figs. 3 and 4.
DiscussionIn studies conducted in various countries (we have not been able to find published data relating to Spain), it was observed that a significant percentage of patients with IBD and musculoskeletal manifestations who were referred to the Gastroenterology department were not evaluated by the Rheumatology department.3,15 Furthermore, in accordance with the data from the Swiss IBD cohort, the presence of extraintestinal manifestations (including peripheral arthritis and ankylosing spondylitis) could be associated with a diagnostic delay in CD.8
This article proposes some IBD screening criteria for patients with SpA and SpA screening criteria for patients with IBD, with the aim of facilitating early diagnosis. These criteria are based on the systematic review of the literature and the experiences of the scientific committee members and the panel of experts. For their definition, we sought an ease of use in consultation and that variability among physicians in their application be as low as possible.
We were only able to find one study on tools for the early detection of SpA in patients with IBD.16 That study describes the development and analysis of the reliability of a questionnaire aimed at detecting cases of axial SpA. As in this article, the majority of the information collected relates to characteristics typical of axial inflammatory pain and the existence of peripheral arthritis, enthesitis or dactylitis. The differences include a longer self-administered questionnaire, since it contains some aspects not included in the screening criteria, specifically, family history of ankylosing spondylitis, morning back stiffness, response of pain to anti-inflammatory drugs and personal history of uveitis or psoriasis.
Regarding these differences, in patients from the ESPeranza cohort (multicentre state-run programme for the early diagnosis and treatment of SpA, in which patients with suspected SpA were referred from Primary Care to 25 SpA units over a three-year period), among the various characteristics of SpA, family history and response to nonsteroidal anti-inflammatory drugs (NSAIDs) had the lowest diagnostic value in patients with axial SpA during the initial stages (symptoms lasting 3–24 months).17 Moreover, uveitis was not included in the screening criteria because it is an extraintestinal manifestation of IBD itself. The starting point for the definition of these criteria was the ASAS recommendations for the referral of patients with suspected axial SpA.18
In the systematic literature review, we did not find any clinical tools for the early detection of IBD in patients with SpA. In the Netherlands, the Dudley Inflammatory Bowel Symptom Questionnaire (DISQ; a self-administered questionnaire developed and validated to evaluate intestinal symptoms in IBD) was validated in order to evaluate the presence and severity of intestinal symptoms in patients with axial SpA.19 Significant differences were found in the average score between healthy controls and patients with SpA, and between those with SpA and those with CD. Furthermore, the DISQ score was correlated with SpA activity. 31% of the patients with SpA obtained a score ≥11, which would reflect intestinal symptoms that are sufficiently severe to affect quality of life, and approximately 7.8% presented with symptoms compatible with active IBD (DISQ score ≥19). This questionnaire is not adapted to the Spanish population and we have not been able to find validation studies in other populations.
Calprotectin is a protein produced by monocytes, macrophages and neutrophils, which is released by these cells at the site of inflammation. Despite the contrasted usefulness of faecal calprotectin in the detection of IBD in patients with gastrointestinal symptoms,20 its validity as an IBD screening method in patients with SpA is not currently well defined. Its values are positively correlated with the SpA activity parameters and are higher in patients with no gastrointestinal symptoms; this elevation could be a risk marker for developing IBD.21,22 Taking into account the risk of over-indication and the lack of access for a large proportion of the Rheumatology departments, it was decided that the request for this test should currently be limited to the gastroenterology setting. Depending on its availability, practicability and suitability for use in Rheumatology consultations, its inclusion may be evaluated in the future.
Regarding the strengths of the study, it should be noted that the level of consensus was very high (over 90%) for all the criteria included. Some authors have suggested a threshold of 80% in order to validate the content when there are fewer than ten experts taking part in the consensus process.23,24
The Spanish Society of Rheumatology (Sociedad Española de Reumatología, SER) has sponsored this project, in collaboration with the Spanish Association of Gastroenterology (Asociación Española de Gastroenterología, AEG) and the Spanish Working Group on Crohn's Disease and Ulcerative Colitis (Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa, GETECCU). Gastroenterologists and rheumatologists who work in various autonomous communities took part, ensuring a sufficiently wide geographic representation for the scientific committee and panel of experts.
One limitation that must be mentioned is that there were no level-1 hospital specialists on the scientific committee or the panel. However, since these are criteria based fundamentally on clinical practice, their applicability does not depend on the technical complexity of the hospital.
New studies are required to complete the validation of the criteria defined (a study of their proper application by the specialists involved, as well as their sensitivity and specificity).
In conclusion, IBD screening criteria have been identified for patients with SpA and vice versa. These have to be of use in the early detection of these disorders in order to ensure that the patients can benefit from a comprehensive treatment of the disease in its initial stages. Their fundamentally clinical nature will enable their use by specialists at the different hospital levels.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments on humans or animals have been conducted in this research.
Confidentiality of dataThe authors declare that no patient data are contained in this article.
Right to privacy and informed consentThe authors declare that no patient data are contained in this article.
FundingThis project was funded by MSD, which did not take part in the design, collection and analysis of data, or the drafting of this article.
AuthorshipJ. Sanz Sanz and X. Juanola Roura are the main authors.
Conflicts of interestJSS declares that he has no conflict of interest with regard to the article. XJR received funding from MSD for a teaching collaboration and attendance at a conference. MM has taught three courses funded by MSD. FG received funding from MSD to participate in conferences and for research. DSM declares that he has no conflicts of interest with regard to the article. None of the members of the PIIASER Project Working Group have received payment for participating in the study.
Félix Manuel Francisco Hernández and María Rosa González Crespo for their work in the systematic literature review. Carlos Sánchez Piedra for his involvement in the drafting of the final study report. María Auxiliadora Martín Martínez for her methodological support. Mercedes Guerra Rodríguez for conducting the bibliographic searches in the systematic literature review.
Scientific committee: Jesús Sanz Sanz, Xavier Juanola Roura, Miguel Montoro, Fernando Gomollón.
Panel of experts: Carlos Montilla Morales (Hospital Clínico Universitario de Salamanca).
Raquel Almodóvar González (Hospital Universitario Fundación Alcorcón).
Juan Cañete Crespillo (Hospital Clínic de Barcelona).
Rubén Queiro Silva (Hospital Universitario Central de Asturias).
María del Carmen Castro Villegas (Hospital Universitario Reina Sofía).
Enrique Ornilla Laraudogoitia (Clínica Universidad de Navarra).
Miguel Mínguez (Hospital Clínico Universitario de Valencia).
Montserrat Aceituno (Hospital Universitario Mútua de Terrasa).
Marta Carrillo (Hospital Universitario de Canarias).
Yolanda Arguedas (Hospital General San Jorge de Huesca).
Manuel Barreiro (Complejo Hospitalario Universitario de Santiago).
Belén Beltrán (Hospital Universitario y Politécnico La Fe de Valencia).
Ignacio Marín (Hospital General Universitario Gregorio Marañón de Madrid).
The members of the PIIASER Project Working Group are listed in Appendix A.
Please cite this article as: Sanz Sanz J, Juanola Roura X, Seoane-Mato D, Montoro M, Gomollón F, Grupo de Trabajo del proyecto PIIASER. Criterios de cribado de enfermedad inflamatoria intestinal y espondiloartritis para derivación de pacientes entre Reumatología y Gastroenterología. Gastroenterol Hepatol. 2018;41:54–62.
This article is published simultaneously in Reumatología Clínica: 10.1016/j.reuma.2017.07.001, with the consent of the authors and editors.