Different studies have described psychiatric comorbidities in inflammatory bowel disease (IBD) patients, but most of them focus mainly on depression and anxiety. Even though major mental disorders are considered one of the main factors that decrease quality of life (QoL), its role in IBD patients remains unclear. We sought to identify the prevalence of different mental disorders as well as its relationship with QoL.
Patients and methodsSubjects were recruited from the IBD Clinic. IBD Questionnaire 32 and structured clinical interview (SCID) for DMS-IV Text Revision were applied. Demographic and clinical data were collected via self-report questionnaires and medical records. The correlation between mental disorders and QoL (IBDQ-32 score) was evaluated using the Spearman correlation test.
ResultsIn all, 104 patients were recruited, 12 with Crohn's disease, and 92 with ulcerative colitis. The prevalence of any major mental disorder was 56.7%: anxiety (44.2%), mood (27.9%), substance use (12.2%), and other psychiatric diagnoses (17.3%), and 29.8% of the patients presented three or more comorbid diagnoses. Mental Disorder (p=0.005), mood disorder (p=0.004), anxiety disorder (p=0.009), were found to be significantly associated with lower QoL. Substance use disorder was associated with lower Digestive QoL (p=0.01). Major depressive disorder (p=0.004), social phobia (p=0.03), PTSD (p=0.02), and Generalized Anxiety Disorder (p<0.001), were found to be significantly associated with lower QoL.
ConclusionsIBD patients had important psychiatric comorbidity that significantly affects their QoL. These results warrant a systematic evaluation of psychiatric conditions in IBD patients.
La comorbilidad psiquiátrica ha sido descrita en Enfermedad Inflamatoria Intestinal (EII), pero la mayoria de los reportes sólo se enfocan en la depresión y ansiedad. Los trastornos mentales son considerados uno de los principales factores que disminuyen la Calidad de Vida (CV), pero el papel que tienen en EII es hasta el momento incierto. Identificamos la prevalencia de diferentes trastornos mentales y su relación con la CV.
Pacientes y métodosLos pacientes fueron reclutados de la clínica de EII. El cuestionario IBDQ-32 y la Entrevista Clínica Estructurada (SCID) para los trastornos mentales del DSM IV Texto Revisado fueron aplicados. Variables sociodemográficas y clínicas fueron obtenidas por cuestionarios autoaplicados y expedientes clínicos. Se correlacionó los trastornos mentales y la CV utilizando la prueba de Correlación de Spearman.
ResultadosSe incluyeron 104 pacientes, 12 con Enfermedad de Crohn y 92 con colitis ulcerativa. La prevalencia global de trastornos mentales fue 56.7%: ansiedad (44.2%), afecto (27.9%), uso de sustancias (12.2%) y otros trastornos mentales (17.3%). De ellos 29.8% presentaron 3 o más trastornos comórbidos. Se identificó a los trastornos mentales (p=0.005), trastornos afectivos (p=0.004), trastornos ansiosos (p=0.009), asociados significativamente con menor CV. Los trastornos por uso de sustancias estuvieron asociados a menor CV-digestiva (p=0.01). Depresión mayor (p=0.004), fobia social (p=0.03), PTSD (p=0.02), ansiedad generalizada (p<0.001), se asociaron a menor CV.
ConclusionesLos pacientes con EII tienen elevada comorbilidad psiquiátrica, la cual afecta su CV. Estos resultados justifican la evaluación sistemática de las condiciones psiquiátricas.
Inflammatory Bowel Disease (IBD) comprises two types of chronic intestinal disorders: Crohn's Disease (CD) and Ulcerative Colitis (UC).1 These conditions affect up to 0.3% of the population in the western part of the world and are characterized by chronic inflammation of the gastrointestinal tract with periods of relapse and remission of symptoms like diarrhea, abdominal pain, fever, and fatigue. The treatment for IBD involves medications like corticosteroids, biologics, and surgery.2,3 All of these symptoms and treatments can affect several aspects of patients’ lives and increases the risk of presenting a psychiatric condition.
Chronic medical conditions are associated with a high prevalence of anxiety, mood, and substance use disorder. The Manitoba IBD Cohort Study identified higher rates of anxiety disorders and major depression in IBD subjects than national samples from New Zealand and the United States.4 Another study by Bernstein et al.5 compared IBD patients with a general population and found a higher prevalence of different mental disorders like depression, anxiety, and bipolar disorders in IBD patients. Subjects with a psychiatric condition like the formerly mentioned report lower quality of life (QoL), which is defined by the World Health Organization (WHO) as “the individual's perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals”. It is a broad-ranging concept affected in a complex way by the person's physical health, psychological state, personal beliefs, social interactions, and the relationships of their environment.6 In an early systematic review and meta-analyses of QoL in IBD, Knowles and colleagues7 identified that QoL was significantly lower for those with IBD relative to healthy controls, in contrast, there were no evident differences in QoL between those with IBD and those with other medical chronic conditions. Some clinical characteristics had been associated with QoL like disease activity, length of the disease, stress, anxiety, and depressive symptoms.7–9 Regardless of the report of symptoms, mental disorders are frequently underdiagnosed in this population and their role in the QoL of IBD patients remains unclear. Since mental health conditions are considered one of the main causes of impairment worldwide, it is important to evaluate their impact on the QoL of IBD patients. Therefore, this study aimed to identify the prevalence of different mental disorders and their association with the QoL of subjects with IBD.
Patients and methodsParticipantsWe conducted a cross-sectional analytic study on 104 IBD patients who attended to IBD Clinic at the National Institute of Medical Science and Nutrition Salvador Zubiran, in Mexico City. Patients diagnosed as CD or UC based on clinical, radiographic, endoscopic, and histological criteria, aged≥18 were included.2
MeasuresClinical variables of the disease along with socio-demographic and personal history were registered. Afterward, a trained psychiatrist evaluated the patients who fulfill inclusion criteria using the Structured Clinical Interview (SCID-I) for DSM-IV. This instrument is a semi-structured diagnostic interview used to know the most common major psychiatric diagnoses based on DMS IV-TR classification.10 The SCID-I includes modules assessing mood disorders (e.g. major depressive disorder (MDD), dysthymia, bipolar disorder), psychotic disorders, anxiety disorders (e.g. social phobia, panic disorder, agoraphobia, simple phobia, post-traumatic stress disorder (PTSD), generalized anxiety disorder (GAD), substance use disorder (SUD) (alcohol use disorder (AUD), non-alcohol use disorder), obsessive-compulsive and related disorders (e.g. obsessive-compulsive disorder OCD), feeding and eating disorders (anorexia nervosa, bulimia nervosa and non-specified eating disorder EDNOS), somatic symptoms and related disorders – somatoform disorder – (e.g. somatization disorder, hypochondriasis, conversion disorder, pain disorder, and factitious disorder). To measure Quality of Life, we used the Inflammatory Bowel Disease Questionnaire 32 (IBDQ-32), which is a specific and reliable tool used to measure QoL in IBD patients.11 This instrument comprises 32 items grouped in 4 dimensions: digestive, systemic, social, and emotional. Every item has a response graded from 1 to 7; 1 indicates the “worst situation” and 7 indicates the “best situation”. The total sum is calculated by the sum of each score and it can vary in a range within 32–224; a higher score indicates a better quality of life. We used a Spanish version validated in Mexico.12
Ethical considerationsThis protocol was approved by The Committee of Ethics and The Committee of Investigation of the National Institute of Medical Science and Nutrition. Written consent was obtained from subjects who participated in the study and the research was conducted according to the Declaration of Helsinki standards.
AnalysisStatistical analysis was performed using SPSS version 24. Descriptive statistics were used to analyze demographic and clinical data. Parametric variables are expressed as mean and standard deviation (SD), non-parametric variables are expressed as median with interquartile range (IQR). Chi-square or Fisher's exact test was used to compare categorical variables. The continuous variables were compared with the Mann–Whitney U test. Univariate and multivariable logistic regression was used to determine if associations existed between patient characteristics and mental disorders. The correlation between mental disorders and QoL (IBDQ-32 score) was statically evaluated using the Spearman correlation test. All analyses were considered significant when p<0.05.
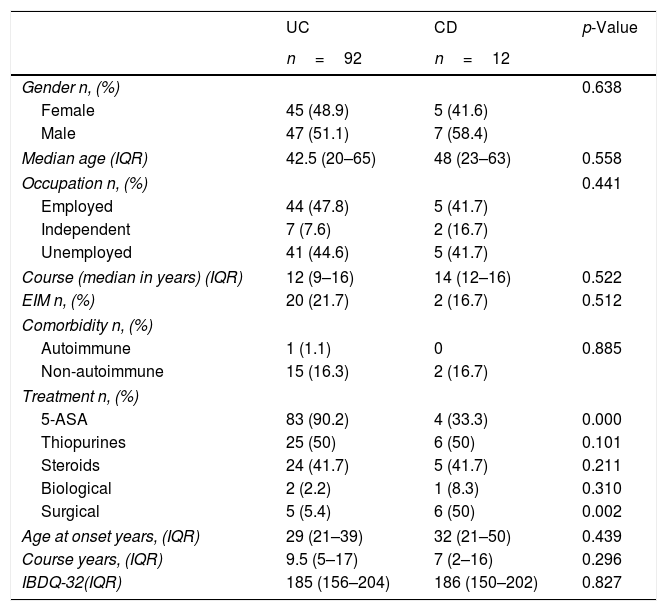
ResultsSociodemographic, clinical characteristics and QoLOur sample included 104 consecutive patients; no patients were excluded. Of them, 92 had UC (88.4%) and 12 (11.6%) CD. Among our population, 54 (51.92%) were males with a median age of 41.8 years (IQR 20–65). IBD onset had a median of 29 years (IQR 21–40) and 9 years was the median duration of disease (IQR 4–17). Median IBDQ-32 score was 185 (IQR 156–204), no differences were identified in the total IBDQ-32 by IBD type (p=0.827). Table 1 shows the demographics, clinical, and QoL characteristics and differences based on IBD type.
Comparison between sociodemographic, clinical variables and Quality of Life and IBD type.
| UC | CD | p-Value | |
|---|---|---|---|
| n=92 | n=12 | ||
| Gender n, (%) | 0.638 | ||
| Female | 45 (48.9) | 5 (41.6) | |
| Male | 47 (51.1) | 7 (58.4) | |
| Median age (IQR) | 42.5 (20–65) | 48 (23–63) | 0.558 |
| Occupation n, (%) | 0.441 | ||
| Employed | 44 (47.8) | 5 (41.7) | |
| Independent | 7 (7.6) | 2 (16.7) | |
| Unemployed | 41 (44.6) | 5 (41.7) | |
| Course (median in years) (IQR) | 12 (9–16) | 14 (12–16) | 0.522 |
| EIM n, (%) | 20 (21.7) | 2 (16.7) | 0.512 |
| Comorbidity n, (%) | |||
| Autoimmune | 1 (1.1) | 0 | 0.885 |
| Non-autoimmune | 15 (16.3) | 2 (16.7) | |
| Treatment n, (%) | |||
| 5-ASA | 83 (90.2) | 4 (33.3) | 0.000 |
| Thiopurines | 25 (50) | 6 (50) | 0.101 |
| Steroids | 24 (41.7) | 5 (41.7) | 0.211 |
| Biological | 2 (2.2) | 1 (8.3) | 0.310 |
| Surgical | 5 (5.4) | 6 (50) | 0.002 |
| Age at onset years, (IQR) | 29 (21–39) | 32 (21–50) | 0.439 |
| Course years, (IQR) | 9.5 (5–17) | 7 (2–16) | 0.296 |
| IBDQ-32(IQR) | 185 (156–204) | 186 (150–202) | 0.827 |
IBD, Inflammatory Bowel Disease; UC, Ulcerative Colitis; CD, Crohn's Disease; IQR, Interquartile Range; EIM, Extraintestinal manifestations; IQR, interquartile range; IBDQ-32, Inflammatory Bowel Disease Questionnaire.
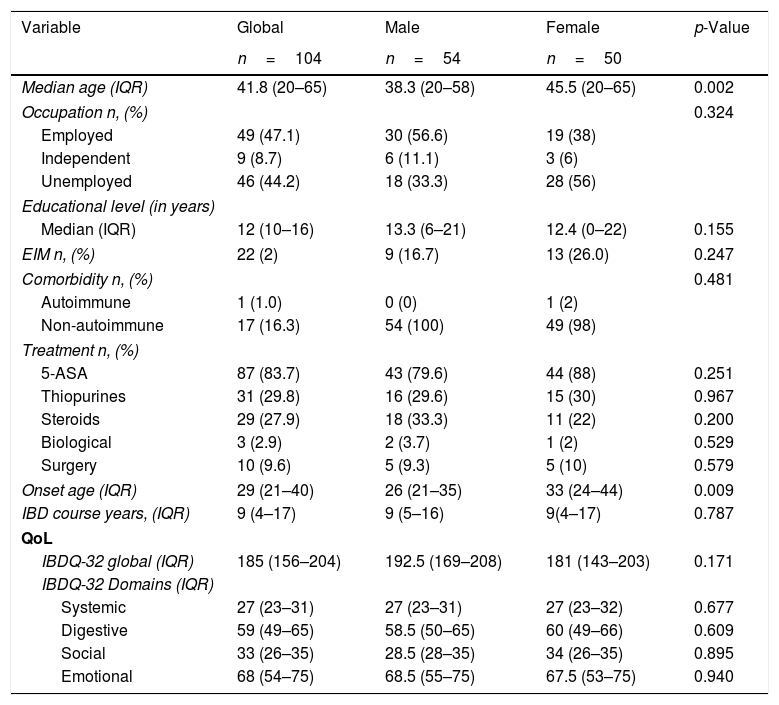
Male patients were younger than females at the time of study [38.3(IQR 20–58) vs. 45.5(IQR 20–65) years, p<0.002], and at diagnosis [26(IQR 21–35) vs. 33(IQR 24–44) years, p<0.009]. We found no differences in IBDQ-32 score (p<0.171); and their different domains (Systemic p=0.677; Digestive p=0.609; Social p=0.895 and Emotional p=0.940) based on gender. Clinical, sociodemographic, and QoL based on gender are shown in Table 2.
Clinical, sociodemographic and Quality of Life differences based on gender.
| Variable | Global | Male | Female | p-Value |
|---|---|---|---|---|
| n=104 | n=54 | n=50 | ||
| Median age (IQR) | 41.8 (20–65) | 38.3 (20–58) | 45.5 (20–65) | 0.002 |
| Occupation n, (%) | 0.324 | |||
| Employed | 49 (47.1) | 30 (56.6) | 19 (38) | |
| Independent | 9 (8.7) | 6 (11.1) | 3 (6) | |
| Unemployed | 46 (44.2) | 18 (33.3) | 28 (56) | |
| Educational level (in years) | ||||
| Median (IQR) | 12 (10–16) | 13.3 (6–21) | 12.4 (0–22) | 0.155 |
| EIM n, (%) | 22 (2) | 9 (16.7) | 13 (26.0) | 0.247 |
| Comorbidity n, (%) | 0.481 | |||
| Autoimmune | 1 (1.0) | 0 (0) | 1 (2) | |
| Non-autoimmune | 17 (16.3) | 54 (100) | 49 (98) | |
| Treatment n, (%) | ||||
| 5-ASA | 87 (83.7) | 43 (79.6) | 44 (88) | 0.251 |
| Thiopurines | 31 (29.8) | 16 (29.6) | 15 (30) | 0.967 |
| Steroids | 29 (27.9) | 18 (33.3) | 11 (22) | 0.200 |
| Biological | 3 (2.9) | 2 (3.7) | 1 (2) | 0.529 |
| Surgery | 10 (9.6) | 5 (9.3) | 5 (10) | 0.579 |
| Onset age (IQR) | 29 (21–40) | 26 (21–35) | 33 (24–44) | 0.009 |
| IBD course years, (IQR) | 9 (4–17) | 9 (5–16) | 9(4–17) | 0.787 |
| QoL | ||||
| IBDQ-32 global (IQR) | 185 (156–204) | 192.5 (169–208) | 181 (143–203) | 0.171 |
| IBDQ-32 Domains (IQR) | ||||
| Systemic | 27 (23–31) | 27 (23–31) | 27 (23–32) | 0.677 |
| Digestive | 59 (49–65) | 58.5 (50–65) | 60 (49–66) | 0.609 |
| Social | 33 (26–35) | 28.5 (28–35) | 34 (26–35) | 0.895 |
| Emotional | 68 (54–75) | 68.5 (55–75) | 67.5 (53–75) | 0.940 |
IBD, inflammatory bowel disease; QoL, Quality of Life; UC, ulcerative colitis; EC, Crohn's disease; EIM, extraintestinal manifestations; IQR, interquartile range; IBDQ32, inflammatory bowel disease questionnaire 32 items. p<0.05.
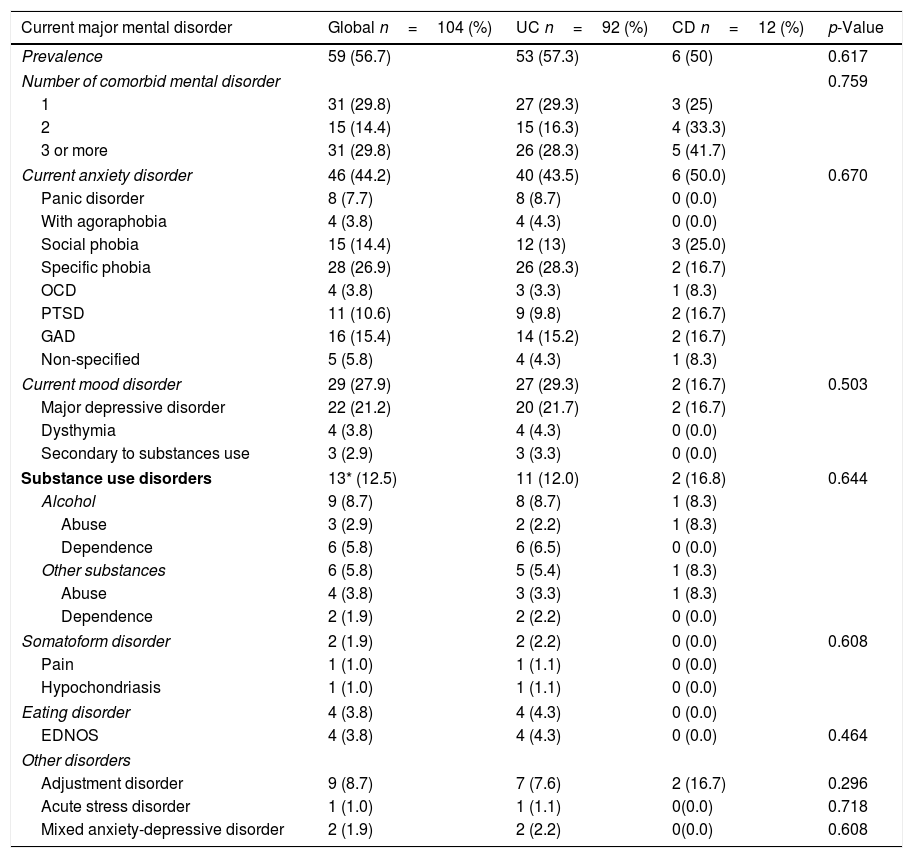
The overall prevalence of major mental disorders was n=56 (56%) with a higher trend toward UC n=53 (57.3%) compared to CD n=6 (50%); p<0.617. The most frequent mental disorder was anxiety disorders n=46 (44.2%); followed by mood disorders n=29 (27.9%) and SUD n=13 (12.5%). All the major mental disorders by category and type diagnosis identified are shown in Table 3.
Global prevalence of different mental disorders and differences among IBD disease type.
| Current major mental disorder | Global n=104 (%) | UC n=92 (%) | CD n=12 (%) | p-Value |
|---|---|---|---|---|
| Prevalence | 59 (56.7) | 53 (57.3) | 6 (50) | 0.617 |
| Number of comorbid mental disorder | 0.759 | |||
| 1 | 31 (29.8) | 27 (29.3) | 3 (25) | |
| 2 | 15 (14.4) | 15 (16.3) | 4 (33.3) | |
| 3 or more | 31 (29.8) | 26 (28.3) | 5 (41.7) | |
| Current anxiety disorder | 46 (44.2) | 40 (43.5) | 6 (50.0) | 0.670 |
| Panic disorder | 8 (7.7) | 8 (8.7) | 0 (0.0) | |
| With agoraphobia | 4 (3.8) | 4 (4.3) | 0 (0.0) | |
| Social phobia | 15 (14.4) | 12 (13) | 3 (25.0) | |
| Specific phobia | 28 (26.9) | 26 (28.3) | 2 (16.7) | |
| OCD | 4 (3.8) | 3 (3.3) | 1 (8.3) | |
| PTSD | 11 (10.6) | 9 (9.8) | 2 (16.7) | |
| GAD | 16 (15.4) | 14 (15.2) | 2 (16.7) | |
| Non-specified | 5 (5.8) | 4 (4.3) | 1 (8.3) | |
| Current mood disorder | 29 (27.9) | 27 (29.3) | 2 (16.7) | 0.503 |
| Major depressive disorder | 22 (21.2) | 20 (21.7) | 2 (16.7) | |
| Dysthymia | 4 (3.8) | 4 (4.3) | 0 (0.0) | |
| Secondary to substances use | 3 (2.9) | 3 (3.3) | 0 (0.0) | |
| Substance use disorders | 13* (12.5) | 11 (12.0) | 2 (16.8) | 0.644 |
| Alcohol | 9 (8.7) | 8 (8.7) | 1 (8.3) | |
| Abuse | 3 (2.9) | 2 (2.2) | 1 (8.3) | |
| Dependence | 6 (5.8) | 6 (6.5) | 0 (0.0) | |
| Other substances | 6 (5.8) | 5 (5.4) | 1 (8.3) | |
| Abuse | 4 (3.8) | 3 (3.3) | 1 (8.3) | |
| Dependence | 2 (1.9) | 2 (2.2) | 0 (0.0) | |
| Somatoform disorder | 2 (1.9) | 2 (2.2) | 0 (0.0) | 0.608 |
| Pain | 1 (1.0) | 1 (1.1) | 0 (0.0) | |
| Hypochondriasis | 1 (1.0) | 1 (1.1) | 0 (0.0) | |
| Eating disorder | 4 (3.8) | 4 (4.3) | 0 (0.0) | |
| EDNOS | 4 (3.8) | 4 (4.3) | 0 (0.0) | 0.464 |
| Other disorders | ||||
| Adjustment disorder | 9 (8.7) | 7 (7.6) | 2 (16.7) | 0.296 |
| Acute stress disorder | 1 (1.0) | 1 (1.1) | 0(0.0) | 0.718 |
| Mixed anxiety-depressive disorder | 2 (1.9) | 2 (2.2) | 0(0.0) | 0.608 |
UC, ulcerative colitis; CD, Crohn's disease; OCD, obsessive compulsive disorder; PTSD, post-traumatic stress disorder; GAD, generalized anxiety disorder; EDNOS, eating disorder not otherwise specified * 3 cases presented alcohol and benzodiazepines use.
The prevalence of major mental disorders was: for any major mental disorder (female 60% versus male 53.7%, p<0.519); for mood disorders (female 36% versus male 20.4%, p<0.077), for anxiety disorders (female 50% versus male 38.9%, p<0.257), for SUD (female 10% versus male 14.8%, p<0.460), for somatoform disorders (female 4.0% versus male 0%, p<0.140), and for eating disorders (female 4.0% versus male 3.7%, p<0.938); no statistical differences were identified.
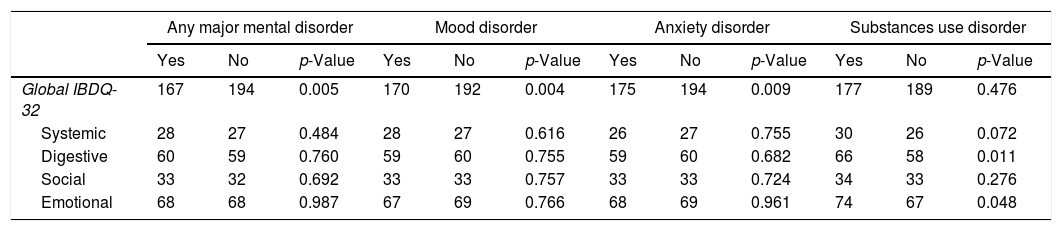
QoL and major mental disorder comorbiditySubjects with any major mental disorder, MDD, and anxiety disorders had a lower QoL than those without those (p<0.005, p<0.004, and p<0.009) respectively. The presence of any SUD was significantly associated with a lower IBDQ-32 for the bowel (p<0.011) and emotional dimensions (p<0.048). Differences in IBDQ-32 and its domains by the presence of any major mental disorder are shown in Table 4.
Differences between Quality of Life and presence of a major mental disorder.
| Any major mental disorder | Mood disorder | Anxiety disorder | Substances use disorder | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | p-Value | Yes | No | p-Value | Yes | No | p-Value | Yes | No | p-Value | |
| Global IBDQ-32 | 167 | 194 | 0.005 | 170 | 192 | 0.004 | 175 | 194 | 0.009 | 177 | 189 | 0.476 |
| Systemic | 28 | 27 | 0.484 | 28 | 27 | 0.616 | 26 | 27 | 0.755 | 30 | 26 | 0.072 |
| Digestive | 60 | 59 | 0.760 | 59 | 60 | 0.755 | 59 | 60 | 0.682 | 66 | 58 | 0.011 |
| Social | 33 | 32 | 0.692 | 33 | 33 | 0.757 | 33 | 33 | 0.724 | 34 | 33 | 0.276 |
| Emotional | 68 | 68 | 0.987 | 67 | 69 | 0.766 | 68 | 69 | 0.961 | 74 | 67 | 0.048 |
IBDQ-32, Inflammatory Bowel Disease Questionnaire.
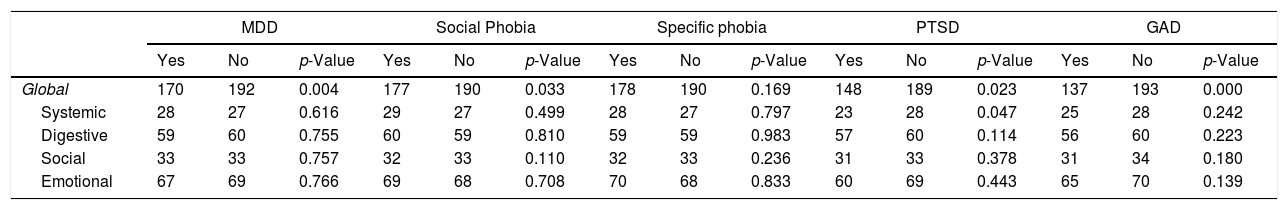
Participants who meet the criteria for MDD, social phobia, PSTD, and GAD had lower IBDQ-32: p=0.004, p=0.033, p=0.023, and p=0.000 respectively, when compared with participants without these mental disorders. Differences in IBDQ-32 domains by different mental disorders showed in Table 5.
Differences between Quality of Life and domains by the main psychiatric diagnosis.
| MDD | Social Phobia | Specific phobia | PTSD | GAD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | p-Value | Yes | No | p-Value | Yes | No | p-Value | Yes | No | p-Value | Yes | No | p-Value | |
| Global | 170 | 192 | 0.004 | 177 | 190 | 0.033 | 178 | 190 | 0.169 | 148 | 189 | 0.023 | 137 | 193 | 0.000 |
| Systemic | 28 | 27 | 0.616 | 29 | 27 | 0.499 | 28 | 27 | 0.797 | 23 | 28 | 0.047 | 25 | 28 | 0.242 |
| Digestive | 59 | 60 | 0.755 | 60 | 59 | 0.810 | 59 | 59 | 0.983 | 57 | 60 | 0.114 | 56 | 60 | 0.223 |
| Social | 33 | 33 | 0.757 | 32 | 33 | 0.110 | 32 | 33 | 0.236 | 31 | 33 | 0.378 | 31 | 34 | 0.180 |
| Emotional | 67 | 69 | 0.766 | 69 | 68 | 0.708 | 70 | 68 | 0.833 | 60 | 69 | 0.443 | 65 | 70 | 0.139 |
IBDQ-32, inflammatory bowel disease questionnaire; MDD, major depressive disorder; PTSD, post-traumatic stress disorder; GAD, generalized anxiety disorder.
The strongest predictors of GAD were the presence of MDD (OR 8.5; 95% CI: 2.6–27), followed by specific phobia (OR 6.0, 95% CI: 1.9–18.7) and social phobia (OR 5.2; 95% CI: 1.5–17.9). No other clinical or sociodemographic characteristics were associated with con GAD. No predictors for other mental disorders were identified.
Correlation between mental disorders and QoLDifferent factors were inversely correlated with IBDQ-32 global scores, such as GAD (p<0.05, Rho Spearman −.401), PTSD (p<0.05, Rho Spearman −.288) and social phobia (p<0.01, Rho Spearman −.210).
DiscussionOur study describes the prevalence of major mental disorders and their association with QoL in patients with IBD. Our overall major mental disorders prevalence is 56.7%; which is higher than the results of different population-based surveys, like the National Psychiatric Epidemiologic Survey in the USA (13.9%), Mexican Psychiatric Survey (5.8%, in the last 30 days) and the expected 17.6% in the general population worldwide.9,13,14 Different studies have tried to determine psychiatric comorbidities in IBD patients, but most of them, focus mainly on the identification of depression and anxiety symptoms/disorders, measured by self-administered instruments.4,9,8 To our knowledge, this is the first study that describes the prevalence of different major mental disorders and their comorbidity in IBD patients using a gold standard diagnostic interview.
In our study, 44.2% of the patients presented more than one current mental disorder (most common were anxiety and mood disorders). Meanwhile, previous clinical and epidemiological studies have shown that the prevalence of an anxiety disorder was 59% in patients with MDD.15
Anxiety disorders were the most prevalent diagnoses in our sample, which is in accordance with the previous population-based study that reported a higher rate of anxiety disorders among subjects with IBD compared with the general population.4,16 Our findings differ from previous reports that identified a prevalence 9.7% for specific phobia, 3.7% for GAD, 2.6% for social phobia, 4.0% for PTSD, 9.4% for OCD, 1.6% for panic attack, and 23% for agoraphobia.9,17
One of the most frequent conditions that we identified is GAD, which was associated with MDD, social phobia, and specific phobia. In contrast, a population-based study from Canada associated female sex, a history of sexual abuse, and the presence of moderate or severe pain with GAD.18 Even when the mechanisms of association between GAD and IBD are not clear, one of the explanations of this relationship involves a micronutrient deficiency and alterations in gut microbiota.19
On the other hand, MDD was the most prevalent (27.9%) mood disorder in our study with higher rates than those reported by Neuendorf and colleagues in their systematic review9 (15%), and the Manitoba Cohort Study4 (9.1%). Our MDD prevalence is higher than the worldwide general population prevalence of 5.9%,20 and The Mexican Psychiatric Survey of 9.1%.13 AUD (abuse and dependence) was identified in 8.7%, similar to the prevalence reported in the worldwide general population13 and IBD population. Substance use, excepting tobacco, has been slightly studied in IBD. A somatoform disorder was present in 1.9% of the evaluated patients. This prevalence is lower than 3.5% of the worldwide prevalence. To our knowledge, this is the first study that evaluated this condition in IBD patients. In our sample, we identified 4 UC cases (2 females and 2 males) who had an EDNOS. Worldwide, EDNOS has a prevalence of 2.4%, in all, a total of 219 cases of comorbid EDNOS and IBD have been described21 and this association is important since a delayed diagnosis leads to a worse prognosis.
The association between psychiatric conditions and IBD is likely due to several factors. Whether anxiety and/or depression disorder contribute to IBD onset or whether it occurs after diagnosis is still controversial.8 Stress has been shown to impact intestinal permeability and immune factors that are both important contributors to IBD.17 Still, future research is needed to explore the neurobiological pathways linking both conditions. Another possible connection between mental disorders and IBD are the psychiatric adverse effects which are common during systemic corticosteroid therapy, the most frequently reported are mood changes, cognition disturbances, sleep problems, and behavior problems as well as frank delirium or even psychosis. In our population, a causal relationship between the use of corticosteroids and the presence of affective symptoms could not be established.
QoL is an important indicator of patient outcome, and factors such as disease activity are relevant since it has been identified as one of the determining factors in the reduction of QoL. Chronicity, relapsing-remitting, and the unpredictable course of symptoms like fatigue, diarrhea, and pain can leave individuals worried about many aspects of life such as bowel control, social isolation, fear of developing cancer or needing surgery. These situations contribute to the presence of lower QoL as supported by the results of comparative studies of UC with other chronic illnesses. Patients with UC reported significantly more worrying over disease complications, depression, and embarrassment than patients with rheumatoid arthritis, asthma, and migraine headaches.17,22 Nonetheless, the comorbidity with anxiety, mood, and substance use can worsen the QoL2 in IBD patients.9,14 Fu et al.23 identified that anxiety and depression symptoms act as a mediator in the relationship between disease activity, social support, and QoL.
One of the main findings in our study was the identification of lower QoL in subjects with any mental disorder, MDD, and anxiety disorder. In the case of SUD, we found a negative impact in the QoL specifically for the digestive and emotional domains. Different explanations have been hypothesized for the relationship between alcohol consumption and the number and intensity of digestive symptoms. In UC, alcohol can alter the immune system with a subsequent increase in the risk for a relapse, while in CD the high sugar content of alcoholic beverages causes osmotic diarrhea.24
Another interesting finding was the identification of worst QoL in women, this could be associated with the different gender social role in this population and more stress in their lives.25 Other studies have reported that women have lower QoL than men during inactive disease, but was reduced to an equally low level during a flare.26
Considering the impact of IBD on the daily life of patients, health care professionals should be able to identify the presence of the different major mental disorders since its timely treatment could lead to an improvement in QoL. Guidelines on the management of CD have included recommendations for the evaluation and treatment of depression and anxiety.2,27 We believe an update of these guidelines can include UC and emit recommendations for the screening and management of SUD and eating disorders.
Our study has several strengths, such as the formal evaluation of different major mental disorders with a semi-structured interview and the use of a specific tool to evaluate QoL in IBD. Conversely, we acknowledge the lack of objective markers to identify disease activity and the use of a cross-sectional design as limitations. As a result, we were not able to correlate mental disorders, disease activity, and QoL.
In conclusion, IBD includes intestinal and extraintestinal symptoms, as well as high mental and QoL involvement. The prevalence was high for anxiety disorder, mood disorder, SUD, and other well-recognized disorders in IBD patients. The presence of any major mental disorder has a negative impact on QoL, while the male gender was a protective factor. Our findings suggest that clinicians may need to evaluate the global mental health of IBD patients, considering not only mood and anxiety disorders. Further studies are needed to determine a physiopathological link between mental disorder and IBD.
Authors’ contributionsGarcía-Alanis M and Yamamoto-Furusho J; designed the report, collected data and drafted the manuscript. García-Alanis M, Chiquete-Anaya E and Toapanta-Yanchapaxi L, interpreted and contributed to statistical analyses. Quiroz-Casian L, Castañeda-Gonzalez H, Arguelles-Castro P, Sarmiento-Aguilar A, and Bozada-Gutierrez K collected data, interpreted data, and review the final version. All the authors review and approved the final version of the article and due care was ensured for the integrity of the work.
Financial supportNone.
Conflict of interestNone of the authors have a conflict of interest related to the subject matter of the present work.
Arturo Reyes MD, Alejandra Monserrat Rodríguez-Ramírez MD and Geogina González MD for their support.