INTRODUCTION
Gilbert's syndrome is a frequent hereditary chronic and benign disorder characterized by unconjugated hyperbilirubinemia in the absence of structural liver disease or overt hemolysis1. Diagnosis is made by exclusion, and no diagnostic test has been adopted as a universally accepted reference. However, the need for a follow-up confirmation of the absence of disease explains the use of a variety of tests that support the initial diagnosis; among them, there are tests based on the bilirubin response to fasting, nicotinic acid, phenobarbital or rifampin. The last one is the most frequently performed in our setting. Pérez et al2 reported that nicotinic acid and rifampin tests are comparable in the diagnosis of Gilbert's syndrome; it is never-theless important to point out that no consensus exists about the test procedure and the interpretation of results.
Rifampin test normally takes 4 h and is considered as positive if total bilirubin concentration rises above 32.5 µmol/l (1.9 mg/dl). However, many patients start from even higher basal levels, due to a marked inter- and intra-individual biological variation. Moreover, available data show that the major relative increase in total bilirubin concentration occurs in the first 2 h, and is less intense afterwards (4 to 6 h)3. Thus, magnitudes other than the absolute increase in total bilirubin concentration are needed for the rifampin test to be useful in Gilbert's syndrome. Therefore, we studied the progression of total bilirubin at 2 and 4 h after rifampin administration, in a sufficiently large series of patients with a clinical diagnosis of Gilbert's syndrome, with the aim to estimate whether the shortened, 2-h version of the test shows an equally useful pattern.
PATIENTS AND METHODS
Patients
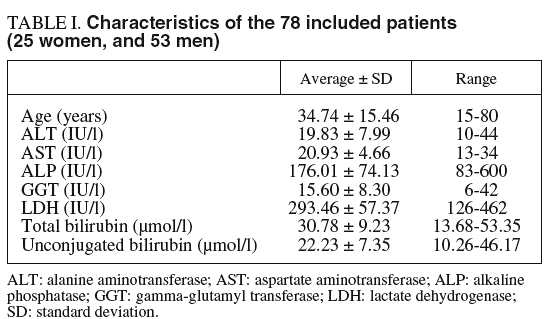
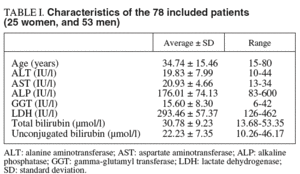
On a prospective basis, we studied 89 patients admitted to the specialist's office in the digestive disease service of a general hospital with clinical diagnosis of Gilbert's syndrome. Criteria for inclusion were: total bilirubin concentration greater than 22.2 µmol/l (1.3 mg/dl), with a conjugated bilirubin concentration normal or slightly elevated, at least twice in the last 6 months; absence of analytical data compatible with hepatic or hemolytic disease (aminotransferases, gamma-glutamyl transferase, alkaline phosphatase, lactate dehydrogenase, haptoglobin, blood cell count, reticulocytes, prothrombin time); no excessive alcohol consumption (less than 20 g/day) or hepatotoxic drug intake; and normal liver ultrasonography.
Pregnancy, lactation or allergy to rifampin were established as criteria for exclusion; 11 patients were excluded a posteriori because in vitro hemolysis rendered some of the collected samples useless, since in vi-
tro hemolysis exerts a strong negative analytical interference on the procedure used for total bilirubin measurement. Characteristics of the subject group are shown in table I.
Methods
Procedure for rifampin test
After a 12-h fasting, a basal blood sample was collected, and immediately 900 mg rifampin were administered per os. New blood collections were made at 2 and 4 h after rifampin intake. A catheter was inserted to avoid repeated punctures. Patients were not allowed to eat or drink during the test.
Analytical procedures
Total and conjugated bilirubin were measured in all samples. In addition, haptoglobin was measured in basal and 4-h samples in order to investigate a possible hemolytic effect of rifampin during the test. Measurements were carried out in an Advia®-1650 analyzer (Bayer Diagnostics, Tarrytown, New York, USA), according to the procedures, reagents and calibrators provided by the manufacturer. Methods for bilirubin (total and conjugated) were those described by Jendrassik and Grof4; as for haptoglobin, the method was immunoturbidimetric5.
Samples were analyzed within 2 h from their collection, included in the laboratory routine and not as a separate series; thus, manipulation, recording, centrifugation and analytical processing were the same as any other patient sample. Variations due to analytical bias or random error were within the acceptable criteria according to external quality assurance programs.
Mathematical and statistical methods
Concentration of unconjugated bilirubin was calculated as the difference between the measured concentrations for total and conjugated bilirubin. In order to standardize results, and to avoid the effects of intra- and inter-individual biological variation, response to rifampin was expressed as relative increases in total bilirubin concentration at 2 and 4 h with respect to basal values. Relationship between relative increases at 2 and 4 h was analyzed by Spearman's correlation.
RESULTS
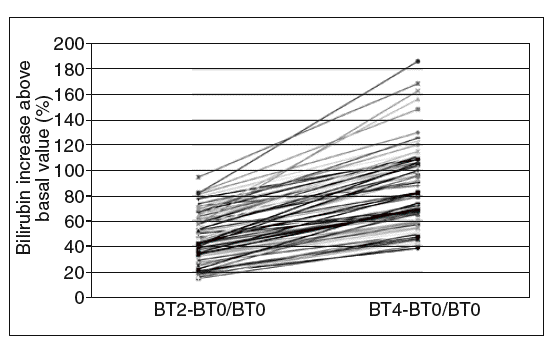
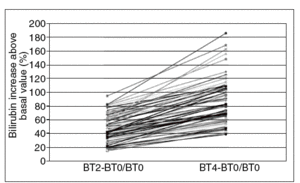
After administration of 900 mg rifampin, concentrations of total and unconjugated bilirubin (expressed as mean and 95% confidence interval [95% CI]) were, respectively, 43.8 (95% CI, 22.4-61.7) and 29.4 (95% CI, 14.5-51.0) µmol/l at 2 h and 55.1 (95% CI, 32.8-80.4) and 37.8 (95% CI, 18.8-64.8) µmol/l at 4 h. Thirty-one subjects had a basal concentration of total bilirubin above 32,5 µmol/l (95% CI, 1.9 mg/dl). On calculating relative increases (related to the basal value) 2 and 4 h after drug intake, it can be observed, as shown in figure 1, that all patients presented at least an increase of 15% at 2 h and 38% at 4 h. A positive correlation was found between the relative increases at 2 and 4 h (Spearman's rho = 0.902; p < 0.000).
Fig. 1. Relative increase in total bilirubin at 2 and 4 h after rifampin administration (900 mg). BT0: basal total bilirubin; BT2: total bilirubin after 2 h; BT4: total bilirubin after 4 h..
DISCUSSION
Gilbert's syndrome is probably inherited as an autosomal dominant trait and affects 5-7% of total population, more often in males6. Diagnosis of Gilbert's syndrome is based on exclusion rather than on a panel of tests7. Many different tests exist that can support the diagnosis, like those based on fasting, phenobarbital, nicotinic acid or rifampin. These 2 latter are comparable2. The last one has been carried out using varied doses and collection timing. Murthy et al3 gave 900 mg rifampin fasting, with blood drawing at 2, 4 and 6 h, and considering a total bilirubin concentration greater than 32.5 µmol/l (1.9 mg/dl) to discern between subjects without and with Gilbert's syndrome. In contrast, Erdil et al8 administered 600 mg rifampin and made bilirubin measurement 4 h later, assuming as a positive response a 50% increase in unconju-gated bilirubin above its basal value. Noguerado et al9 utilized a 300 mg dose and collected samples 3 h postrifampin, but they set no threshold value and concluded that rifampin is useless in diagnosing Gilbert's syndrome as compared to fasting test. Velilla et al10 drew blood at a basal point and 1, 2, 3 and 4 h after a 900 mg rifampin intake and did not establish a cut-off point between patients and controls, although in a later communication they stated that a decision level had been set at 29.1 µmol/l (1.7 mg/dl)7. Pérez et al2 used 900 mg rifampin and made basal and hour-by-hour collections until 4 h choosing no threshold value. Atmetlla et al11 administered 900 mg rifampin and selected neither cut-off limit.
In our study, all patients with Gilbert's syndrome showed a relative increase in total bilirubin concentration of at least 0.15 times their basal values at 2 h, and 0.38 at 4 h. As for haptoglobin levels, these were measured in 72 patients and the mean basal value was 0.98 g/l (95% CI, 0.10-2.83) and 4 h after rifampin administration 0,97 g/l (95% CI, 0.07-2.83) /L showing no difference, unlike the results obtained by Murthy et al3.
In conclusion, according to our results: a) all patients with Gilbert's syndrome showed a relative increase in total bilirubin concentration of at least 0.15 times their basal values, 2 h after rifampin administration; b) relative increases at 2 and 4 h are correlated and thus can give similar information, and c) there were no change in haptoglobin concentration between samples collected at basal state and 4 h samples after rifampin intake. Hence, we suggest that the relative increase of total bilirubin at 2 h can be useful in the diagnosis of Gilbert's syndrome.