Recommendations are advice that is given and considered to be beneficial; however, they are still suggestions and are therefore open to different interpretations. In this sense, the final objective of the review has been to try to homogenize, with the evidence available, the approach to the diagnosis and medical/surgical treatment of one of the most complex manifestations of Crohn’s disease, such as simple and complex perianal fistulas.
Las recomendaciones son consejos dados por considerarse beneficiosos y no dejan de ser sugerencias, abiertas por tanto a diferentes interpretaciones. En ese sentido, el objetivo final de la revisión ha sido, con las evidencias disponibles, intentar homogeneizar al máximo la aproximación al diagnóstico y tratamiento medicoquirúrgico de una de las manifestaciones más complejas de la enfermedad de Crohn como son las fístulas perianales simples y complejas.
The concept of perianal disease (PAD) includes anorectal abnormalities (fissure, fistula or abscess, skin folds and perianal maceration) present in patients with Crohn's disease (CD) of any other location. The same denomination can be applied when the anorectal findings are compatible with said disease without there still being objective evidence of it. About 9% of patients diagnosed with CD start with anal or perianal pathology, and this can even precede the onset of intestinal symptoms by several years. Most patients with CD have some perianal abnormality that is usually asymptomatic in up to 70% of cases. The incidence of anal problems in CD varies greatly according to the published series (20–80%). This incidence is also variable depending on whether CD affects the small intestine (22–71%) or the colon (47–92%).1
The natural history of PAD is only partially known, and varies depending on the type of lesion and its severity. In an initial cohort study (1970–1993) that collected all patients with CD from Olmsted County (Minnesota), 20% developed perianal fistulas, with the cumulative risk at 10 and 20 years at 21 and 26%, respectively; 34% of the patients developed recurrent fistulas; 83% required surgical treatment (minor surgery in most cases), and up to 23% required a bowel resection.2 In this sense, in a review carried out in Spain that included 2391 patients with a diagnosis of CD and with a time of evolution of their disease of 12 years, 24% developed perianal fistulas (half of them complex) frequently associated with abscesses and anal canal stenosis.3
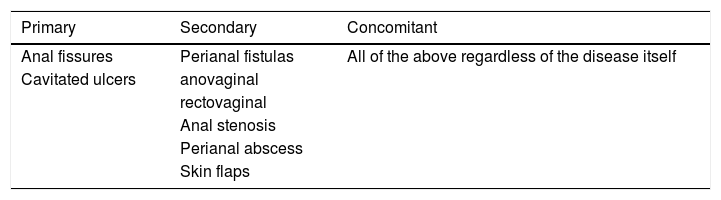
PAD includes three types of lesions: primary, secondary and concomitant or casual4: a) primary lesions, that derive from the primary pathological process found in the intestine and reflect the general activity of CD itself; b) secondary lesions, which are mechanical or infectious complications of primary lesions, rather than a direct manifestation of CD, and c) concomitant lesions, which refer to any of the previous lesions, but without a direct relationship with CD, being able to precede the onset of the disease regardless of this (Table 1).
Clinical manifestationsPAD manifests as anal surface erosions, skin folds, fissure, abscess, fistula or anal stenosis. In general, they are usually not very symptomatic or completely asymptomatic lesions, of atypical location, and their multiplicity is common. Diagnosis is easy if the patient is already diagnosed with CD. If it is an initial form, some features may indicate that it is PAD associated with CD, such as the multiplicity of lesions, the lateral location of the fissures, deep anal or perianal ulcers, anal stenosis, multiple fistula openings, and little or no symptoms despite the striking appearance of the lesions.4
The most frequent anal lesion is the skin folds, and the second, in order of frequency, is the fistulas that are usually chronic and painless, unless there is pressure due to an underlying anal abscess. In the latter case they are painful, they can present with fever or low-grade fever and the examination usually shows the external fistula opening with inflammatory signs and low pressure. There are often several external fistula openings not only in the perianal region but in more remote areas, such as the buttocks, thighs or genitals. In women, anovaginal or rectovaginal fistulas are also common, especially if there is rectal involvement.
DiagnosisAnal, perianal and perineal region examinationThe examination of the anoperineal region should follow a systematic process to obtain the maximum performance, with it being advisable to describe the situation and the number of external fistula openings, palpate possible indurated tracts between the external fistula opening and the anus, perform rectal touch, perform an anoscopy instilling physiological serum or 50% diluted hydrogen peroxide for each of the external fistula openings, appreciating the exit through the internal opening, performing rectoscopy to evaluate the presence of proctitis and trying to classify the fistulas as simple or complex.5
Examination/review under anaesthesia (EUA)Anorectal examination under anaesthesia by an expert colorectal surgeon is mandatory in the full evaluation of PAD in CD, and is the baseline test with which to contrast imaging techniques. During this review, the different types of fistula tract and the existence of associated cavities will be recognised and classified, and we can also drain abscesses, place/change setons, take biopsies and perform rectoscopy. EUA should be indicated preferentially before the onset of pain with suspected abscess, and its delay is not justified by not having any complementary imaging test.6 Likewise, when treatment with biologics is to be initiated, any perianal septic process must be ruled out by means of imaging tests and EUA indicated if there is any doubt for septic focus drainage or placement of setons. EUA has an accuracy greater than 90% to delimit the tracts and locate septic foci, superior to any other complementary examination.7 In cases where EUA cannot correctly identify fistulas or abscesses, mainly because there is induration, stenosis or scarring fibrosis, it may be necessary to repeat the examination or resort to imaging tests.
Imaging techniquesComputed axial tomography (CT) of the pelvis minorThe CT scan has a very limited role in the evaluation of PAD.8 Its resolution capacity is low, and it exposes patients to ionising radiation, avoided with magnetic resonance imaging (MRI) and endoanal ultrasound (EAUS). It is an outdated complementary examination to evaluate perianal CD.
MRI of the pelvis minorPelvic MRI is a non-invasive technique of high diagnostic precision, and is considered the reference imaging technique of PAD.9 It has proven very effective in detecting purulent collections in the pelvis, demonstrating hidden fistulas, evaluating the extent of the proximal disease or the level of the fistula, especially in patients with perianal sepsis and recurrent symptoms, and can differentiate between activity and fibrosis.10,11 Its diagnostic precision is around 80–100%.11 There are several classifications of perianal fistulas that are based on radiological indices measured by MRI.9 The most commonly used radiological index is the Van Assche index, which combines anatomical features of the fistula tracts and radiological findings related to fistula inflammation.11 MRI is the most accurate imaging technique to evaluate PAD, and should be the first technique to be performed for its characterisation.9
Endoanal ultrasoundEAUS is a useful alternative to MRI to diagnose perianal fistulas in the hands of an expert examiner, although its cost effectiveness may be limited due to its lower depth penetration.12 EAUS allows for a detailed visualisation of the anal sphincter complex, with an accuracy between 86 and 95% for the identification of the type of fistula tract and between 62 and 94% for the location of the internal fistula openings.13 However, it cannot properly identify supralevator abscesses or those located more than 4cm from the anal canal, since its focal length is limited. The cost effectiveness of this examination may also be limited by the presence of an anal stenosis. The possibility of performing EAUS with 3D image reconstruction, local hydrogen peroxide infusion and the use of colour Doppler imaging can improve its accuracy.14 The precision of transperineal ultrasound is similar to that of the EAUS and may offer advantages over MRI for diagnosing anovulvar fistulas.15 As mentioned, EUA, EAUS and MRI have similar precision in the study of anal fistulas. The combination of more than one of these techniques may be the most appropriate approach for patients with CD with complex fistula or abscess (being able to reach 100% accuracy).9 The use of one technique or another will depend on the availability and experience of the team treating the patient.
Pelvic MRI and EAUS are superior to physical examination to assess the response to PAD treatment (activity in the tract is ruled out even if the external opening is closed), although there are no studies comparing both techniques in this scenario.9 Pelvic MRI is often used to evaluate PAD's response to medical treatment, although the time at which this should be done, nor which radiological index is most optimal for analysis, is not well defined.9
EndoscopyThe assessment of rectal activity by endoscopy is essential for the subsequent management of PAD. Endoscopy can assess the severity and extent of luminal activity, especially rectal, in addition to detecting some complications such as cancer or stenosis. The presence of proctitis is a predictive factor of non-healing of fistulas and high rates of proctectomy.15
In clinical practice, the evaluation of perianal fistulas should combine physical examination of the anal and perianal region, performing an endoscopy to evaluate the rectum and pelvic MRI for anatomical description and inflammatory activity. EAUS is a useful alternative to MRI, with similar accuracy in the study of perianal fistulas.
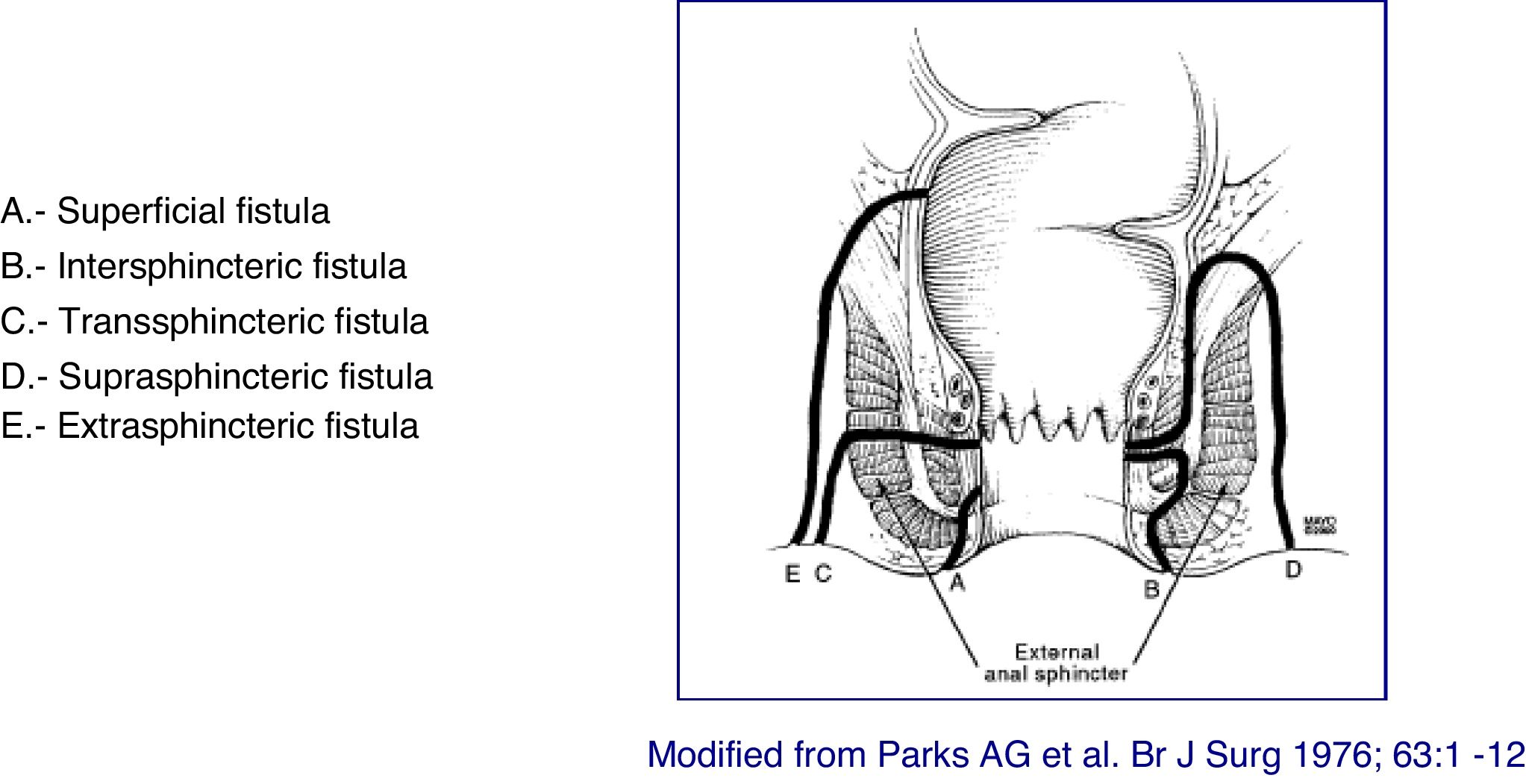
Classification of lesionsThe anatomical description of the fistulas should include the type of fistula, their anatomical relationship with the anal canal (internal and external sphincter), the external openings and their location, the identification of the internal openings and the presence of secondary tracts and abscesses. The tract of the fistula be described regarding its presence in the intersphincteric, transsphincteric, suprasphincteric, or extrasphincteric space, and it is important to indicate the presence of abscesses and their relationship with the sphincters. The description of the external and internal openings follows the "anal clock" system, in which, with the patient in lithotomy position, the anterior perineum is located at 12 o'clock and the posterior perineum at 6 o'clock. Likewise, the secondary tracts must be defined in relation to the plane of the elevating muscle (infra or supra-elevating) and by its horizontal extension (in horseshoe). Another important aspect during the description is to verify whether or not there is suppuration through the external openings, as well as the evaluation of the rectum. A correct classification of fistulas allows for a more appropriate treatment and better outcomes. Given the great heterogeneity of lesions in perianal CD, it is advisable to use some type of scale or classification that allows an objective establishment of the lesions presented by the patient, as well as their evolution and their response to treatment.
One of the first pathological classifications was proposed in 1978 by Hughes of the Cardiff group (Hughes-Cardiff classification). It evaluated the presence or not of ulceration, fistula or abscess and stenosis, together with the presence of bowel disease, associated anal pathology and lesion activity.16 This classification has not been validated, and, although it is one of the best known, its application in routine practice is difficult and of limited interest in terms of patient management.
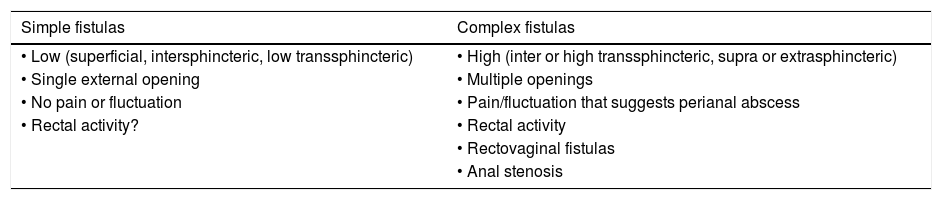
The classification based on the Perianal Disease Activity Index (PDAI) functionally assesses the effect of PAD on fundamental aspects of the patient's life17 (Table 2). The technical review of the American Gastroenterological Association (AGA) published in 2003 proposed a simpler classification identifying fistulas as "simple" or "complex"18 (Table 3). Although possibly none of the proposed classifications meets 100% of the requirements suitable for that purpose, it is advisable to use some in order to unify criteria in a working group and to be able to assess in an objective way the evolution of the lesions and their response to the different treatments.
Perianal disease activity index (PDAI).
| Discharge |
| 0. Absent |
| 1. Minimal mucous |
| 2. Moderate mucous or pus |
| 3. Significant |
| 4. Faecal incontinence |
| Pain/restriction on activities |
| 0. No restriction on activities |
| 1. Little discomfort, no restriction |
| 2. Moderate discomfort, some limitations |
| 3. Marked discomfort and restriction |
| 4. Severe pain and limitation |
| Degree of induration |
| 0. No induration |
| 1. Minimal induration |
| 2. Moderate induration |
| 3. Marked induration |
| 4. Fluctuation/abscess |
| Restriction on sexual activity |
| 0. No restriction on sexual activities |
| 1. Mild restriction |
| 2. Moderate limitation |
| 3. Marked limitation |
| 4. Total limitation |
| Type of perianal disease |
| 0. Absent/skin flaps |
| 1. Anal fissure or mucosal tear |
| 2. <3 perianal fistulas |
| 3. ≥3 perianal fistulas |
| 4. Anal sphincter ulceration |
Source: Irvine.17
American Gastroenterological Association (AGA) classification of perianal fistulas.
| Simple fistulas | Complex fistulas |
|---|---|
| • Low (superficial, intersphincteric, low transsphincteric) | • High (inter or high transsphincteric, supra or extrasphincteric) |
| • Single external opening | • Multiple openings |
| • No pain or fluctuation | • Pain/fluctuation that suggests perianal abscess |
| • Rectal activity? | • Rectal activity |
| • Rectovaginal fistulas | |
| • Anal stenosis |
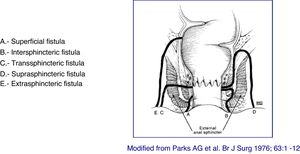
At present, the most anatomically accurate and clinically useful system is the Parks classification, which uses the internal and external anal sphincter as a reference and considers five types of fistulas: superficial, intersphincteric, transsphincteric, suprasphincteric and extrasphincteric19 (Fig. 1). However, this classification does not provide information on the complexity of the fistula (secondary tracts or presence of abscesses) or the presence of proctitis. Usually, colorectal surgeons use this anatomical classification. Another anatomical classification of fistulas is that of St. James’s University Hospital,20 which is based on the findings of the MRI with contrast; it is used by many radiologists, although it is not validated and its applicability in clinical practice is limited. There is no ideal classification, since anatomics lack the aspect of functionality, and vice versa. Due to its simplicity and ease of use, the Parks and AGA classifications are the most widely used.
Severity assessmentThe Crohn's disease clinical activity indices (CDAI, Harvey-Bradshaw, Van Hees) do not reflect the severity of perianal lesions, so specific indices are required, such as the PAD activity index, the PDAI (Table 2).17 This index has been validated in patients undergoing treatment with metronidazole and comprises five categories: two related to quality of life and three related to the severity of PAD, each of them being graduated on a five-point scale (0, asymptomatic; 4 severe symptoms). One of the main limitations of this index is that an adequate cut-off point has not been defined in order to establish the relevant clinical response, although it has been observed that using clinical evaluation (active suppuration of the fistula and/or signs of local inflammation) as a reference, a cut-off point of the PDAI>4 is associated with a clinical accuracy of 87%.
One alternative is the Present index, which assesses the number of openings suppurating both spontaneously and with digital pressure in the perianal region, considering an effective response to the reduction in the number of them (in 50% or more) in two or more consecutive visits (partial response) and the closure of fistulas such as the absence of spontaneous suppuration or after digital pressure (complete response).21 It is an index which is dependent on the examiner and, although it is accepted in the context of clinical trials, it is not validated. One of the main limitations of this index is that it can classify a fistula as closed but the tract persists with activity in more proximal sections. Therefore, to establish the complete closure of a fistula, an imaging technique is required, specifically pelvic MRI, and 12 months have been suggested arbitrarily as the evaluation time.9 The combination of an anatomical description of the fistulas with data reflecting inflammatory activity is defined in the Van Assche index, although its correlation with the PDAI is weak.11 A classic index, the Anal Disease Activity Index, developed by the Alexander-Williams group and which defines anal involvement by means of a pain analogue scale, has limited clinical applicability.22
Despite the limitations described with the different indices available, the PDAI is currently validated, and, although it should be used to assess severity, its use in clinical practice is limited. The alternative of using the Present index is an option, bearing in mind the aforementioned limitations. For this reason, the most appropriate approach to assess severity should not be limited to the use of clinical indices but must be completed with an imaging technique.
Specific evaluation of fistulasFistulas can originate from a penetrating fissure or an infected anal gland, and there are often several external fistula openings not only in the perianal region but in more remote areas, such as the buttocks, thighs or genitals. They represent the second manifestation of PAD in order of frequency after skin folds. These fistulas are chronic and painless, unless there is pressure pus, and most of them are usually low and simple (especially in the ileal and ileocolic location), although there is a relatively high proportion of complex fistulas (more common in CD of the colon).23 In the latter cases, a study of the degree of complexity is required by imaging techniques, mainly by MRI or EAUS.9,18
Normally, complex fistulas, suppurative or not, have more than one fistula opening more than 3cm from the anus, with more than one tract, and are relatively well tolerated. Whenever possible, especially if it is a complex fistula, a loose seton will be placed between the external and internal opening in order to ensure adequate drainage, decrease the probability of abscess and the appearance of new fistula tracts. If the rectum is affected, the fistulas are more likely to be more aggressive and a septic state that requires radical actions, such as derivative ileostomy or proctectomy, originates.24 In clinical practice, the AGA classification in simple and complex fistulas is used, although the Parks classification is also used to describe the relationship of sphincters with the fistula tract.12,24
Medical treatmentSalicylatesOral salicylates do not seem to have any effect on PAD, although some studies suggest that topical treatments, in specific cases, can improve it, probably by decreasing rectal inflammation.12,25 Thus, they are not suitable for the direct treatment of PAD, nor as an exclusive treatment for it, but they could be useful when topically administered in certain patients with proctitis.
CorticosteroidsCorticosteroids have been associated with a worsening of PAD because they make it difficult to close the fistulas, increase the flow rate through them, favour the appearance of abscesses and, therefore, increase the need for perianal surgery.12,26 They can be administered for luminal activity, but taking into account that, predictably, they will not improve PAD and that they can even worsen it, it is advised to prescribe them for the minimum essential time. There are only clinical observations, case series, expert opinions and mentions in reviews.
AntibioticsAntibiotics, especially ciprofloxacin and metronidazole, are used as the first-line treatment of PAD, in conjunction (and as an initial bridge) with surgery and the use of thiopurines and anti-tumour necrosis factor (anti-TNF).6 Fistulas are fundamentally colonised by gram-positive bacteria and there is no change in bacterial flora after antibiotic treatment, which suggests that this may be effective by other mechanisms or, perhaps, that in the PAD of inflammatory bowel disease they act as bacteriostatic and non bactericidal.27
Several observational studies, with small sample sizes (5–26 patients), and two meta-analyses show the usefulness of metronidazole and ciprofloxacin, alone or in combination, in the treatment of PAD, even with complete closure of fistulas at 6–8 weeks after starting treatment, although the disease relapses when reducing or discontinuing the drug.6,12,28–31 The initial response to antimicrobials is good, but the side effects (digestive intolerance with high doses of metronidazole or neuropathy that may appear with low but maintained doses) and the emergence of resistance, as well as the recurrence of the disease after cessation or decrease of the dose, may limit its chronic use.27
Its role has been established as a bridge until other medical treatments, such as thiopurines, or as an enhancer of anti-tumor necrosis factor alpha (anti-TNF〈), are effective. An open-label and prospective study conducted at 20 weeks in 52 patients showed that the combination of antibiotics and azathioprine is more effective than antibiotics exclusively (48% vs. 15%, p=0.03), suggesting its usefulness as a bridge until the thiopurine exerts its effect.32 In another study, also of small sample size (24 patients with Crohn's with PAD), a greater decrease in PDAI was observed in the group treated with ciprofloxacin and infliximab (IFX) than in that of IFX alone, but without obtaining significant differences when analysing the overall response.33 A multicentre, double-blind, placebo-controlled study, which included 76 patients with CD and PAD, demonstrated the superiority of the combined treatment of adalimumab (ADA) with ciprofloxacin over ADA alone in the treatment of perianal fistulas. In week 12, clinical response was observed in 71% of those treated with ADA and ciprofloxacin and in 47% of those who received ADA and placebo (p=0.047). Remission rates were also higher in the group taking antibiotics (65% vs 33% ADA alone, p=0.009).34
The available evidence suggests that antibiotics, if possible, will always be prescribed for diagnosis of PAD or a new outbreak, as a bridge to immunomodulatory medical or biological treatment and surgical drainage, if appropriate. Alone as an adjunctive treatment in fistulas.
ThiopurinesThe thiopurines, azathioprine and its mercaptopurine metabolite, seem to improve long-term PAD symptoms and reduce the number of perianal interventions without associating complete fistula closure.35 In the 1980s, several studies were published showing a partial or complete closure of perianal (31–71%) or rectovaginal (25–50%) fistulas at three months of treatment with mercaptopurine, at doses of 1.5mg/kg.36–39 In 1995, a meta-analysis on thiopurines in CD was published and, as a secondary finding, a significant, partial or complete improvement of the fistulas was observed. A total of 54% of those who had received thiopurines showed improvement, compared to 21% of those in the placebo group (odds ratio [OR]: 4.44; 95% CI: 1.5–13.2).40 Two subsequent meta-analyses on thiopurines in the treatment of CD, in which the efficacy of thiopurines in PAD is secondarily evaluated, show significant differences in favour.41,42 Therefore, randomised clinical trials that have as a primary objective to determine if there is real improvement in PAD with thiopurines are needed. Currently, the ECCO guidelines and the Toronto consensus8,23 recommend their use as a first-line treatment of PAD, together with antibiotics, in complex perianal fistulas and in simple fistulas with associated proctitis, although the evidence is contradictory and the degree of recommendation is low.
Cyclosporine and tacrolimusA 2005 meta-analysis by Cochrane, which included four controlled trials, determined that cyclosporine is not effective in active CD, and its use in PAD is not justified.43
In 2011, a systematic review on the effect of tacrolimus in inflammatory bowel disease was published.44 It also analysed studies related to PAD, notably the one by Sandborn et al.,45 as it is a controlled clinical trial (although of small sample size), the one by González Lama et al.,46 for suggesting that prolonged treatment achieves a greater number of fistula closures, and the one by Lowry et al.47), which, although retrospective, takes into account the concomitant use of mercaptopurine, with good outcomes. The conclusions of the review are that, when administered long-term (six months), orally, improvement and remission of perianal fistulas of 43–57% and 10–29%, respectively, are obtained. All the studies mention the side effects (tremor, paraesthesia, nephrotoxicity, etc.), which generally yield by reducing the dose of tacrolimus or by withdrawing the drug. Topical treatment with tacrolimus can be effective, as an adjuvant, in the anus of an individual with Crohn's disease, with ulcers or fissures, but not in fistulas. Therefore, tacrolimus, orally (0.1–0.3mg/kg/day), could be an option to consider in cases of fistulas refractory to biological treatment.
Anti-TNFThe anti-TNF drugs revolutionised the treatment of PAD in 1999. A systematic review and meta-analysis has highlighted that anti-TNFs are effective in the treatment of fistulas.27,48 IFX and ADA have proven useful in inducing and maintaining PAD remission48; data on certolizumab are scarce and inconclusive.48
In 1999, Present et al.49 published the first randomised controlled clinical trial with 94 patients, with at least one active fistula, treated with IFX at doses of 5 or 10mg/kg, compared to a placebo-treated control group. Of those treated with IFX, 62% showed improvement in PAD and 56% achieved closure of the fistulas in a median time of two weeks from the start of the response. Later, the ACCENT II study confirmed the closure of the fistulas with the induction of IFX (5mg/kg, at 0, 2 and 6 weeks; initial response of 69%) and showed that IFX (5mg/kg every 8 weeks) was superior to placebo in the prevention of recurrence at 12 months with complete fistula closure of 36 and 19% (p=0.009) and response of 46 and 23% (p=0.01) of those treated with IFX and placebo, respectively. However, almost half of the patients treated with IFX experienced a recurrence during the first year.50,51 IFX has also been shown to reduce the number of hospitalisations and surgery.52 All these results have been supported by studies based on clinical practice.53,54
ADA has demonstrated its effectiveness in PAD as a secondary objective of two randomised clinical trials.6 In 2007, the results of the CHARM study were published, in which 30% of the 113 patients with PAD had closure of fistulas after 26 weeks of treatment, versus 13% of placebo, maintaining this effect for the 56 weeks of the study.55 The CHOICE study obtained similar results, with a fistula closure associated with an improvement in the quality of life in 39% of patients.56 However, in the CLASSIC-1 study57 and in the GAIN study,58 no significant differences were found between ADA and placebo. An open-label Spanish study observed that 41% of the 22 patients with complex fistulas showed improvement with induction of ADA (160–80mg) and 23% had remission.59 In another Spanish, prospective study, which included 16 patients, the evolution of patients with PAD who started treatment with ADA after previous failure to IFX was analysed, observing a significant reduction in fistula drainage and PDAI at 48 weeks.60 A retrospective and multicentre Spanish study analysed the effectiveness of ADA in the treatment of perianal fistulas (83% complex) in 46 patients naïve to anti-TNF treatment. The response to treatment was 72% (54% remission, 18% partial response) and 49% (41% remission, 8% response) at 6 and 12 months, respectively. In patients with complex fistulas, the response was 66% at 6 months and 39% at 12 months.61 Given that most of the results are positive, the guidelines consider it a good option in the treatment of PAD.6,23
The efficacy of certolizumab in the closure of fistulas has been analysed in the PRECiSE 1 and 2 studies, without observing significant differences with respect to placebo in the induction of remission.62,63 In the analysis of a subgroup of 58 patients, of PRECiSE 2 (55 of them with PAD), responders to the induction with certolizumab, randomised to placebo vs anti-TNF, at week 26, fistula closure was observed in 36% of those who received certolizumab versus 17% of those who received placebo (p=0.038), but this difference disappeared in the follow-up.64 A recent systematic review confirms these data.48 In view of the available evidence, the recommendation for the use of certolizumab in the treatment of PAD is debatable.
There are limited and controversial data on the efficacy of the combined use of immunosuppressants and anti-TNF for the treatment of perianal fistulas. In the ACCENT II study, the response at one year is similar among patients with combined treatment and those receiving IFX monotherapy.51 However, other studies have observed a positive association between the combined treatment and the closure of the fistulas, being of special interest in patients with associated proctitis and naïve to immunomodulatory treatment.65 Therefore, although the data are limited and of poor quality, based on the available evidence on the greater efficacy of the combined therapy in luminar CD66 and that higher levels of anti-TNF (impact of concomitant immunosuppressive therapy on pharmacokinetics) have been associated with a higher probability of fistula closure, in this clinical scenario the use of combined therapy at the start of anti-TNF treatment is suggested.23
VedolizumabVedolizumab is an anti-integrin 〈4®7 humanised monoclonal antibody of intravenous administration, indicated in ulcerative colitis and moderate-severe CD.67,68 There are currently no results from any study that has attempted to directly assess the efficacy of vedolizumab in PAD. A total of 12% of patients in the GEMINI-2 pivotal study had PAD at the start of the study. In a post-hoc evaluation, it was observed that, at week 52, those who had received the biologic every eight weeks (n=17) had fistula closure in 41.2% of cases, compared to 22.7% (n=22) of those who received it every four weeks and 11% of the placebo group (n=18).69
The results of the US VICTORY Consortium indicate that PAD is one of the factors that are associated with less response with vedolizumab.70 Furthermore, the post-hoc analysis of the 35 patients (20.2%) with PAD from the OBSERV-IBD study showed that 42.9% at 14 weeks and one third at 52 weeks were in remission of the PAD after being treated with vedolizumab.71 A multicentre, observational study by the Groupe d'Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif [Therapeutic Study Group of Inflammatory Conditions of the Gastrointestinal Tract, GETAID] that analyses the efficacy of vedolizumab as a rescue treatment in 102 patients with complex perianal fistulas in activity observes clinical remission in 23/102 (22.5%) and withdrawal of setons in 9/61 (15 %) patients who had them at the beginning of treatment.72 A clinical trial is underway to evaluate the efficacy of vedolizumab in PAD (NCT02630966).
Therefore, there are not enough studies to advise vedolizumab as a first-line treatment in complex PAD, although the results in clinical practice suggest that it may be useful as a rescue treatment in certain cases.
UstekinumabUstekinumab is a monoclonal antibody against the p40 subunit of interleukins 12 and 23, indicated in the treatment of active, moderate to severe CD, in adult patients who have had an inadequate response, have a loss of response or are intolerant to conventional treatment or to TNF〈 antagonists, or present medical contraindications to these treatments.
There is no prospective study that specifically evaluates the efficacy of ustekinumab in PAD. Indirect information has been obtained from the UNITI-1 and UNITI-2 clinical trials in which 10.8–15.5% of patients had active fistulas at the start of the study. A total of 24.7% of patients with PAD experienced complete fistula closure at week 8, compared to 14.1% of the placebo group (p=0.073).73 In the CERTIFI study, response was observed at week 22 in 9/19 (47%) patients with fistulas of the ustekinumab group versus 6/20 (30%) of patients treated with placebo.74 Likewise, in the IM-UNITI study, there was a better response in the group treated with ustekinumab at week 44 (12/15 patients [80%]) versus placebo (5/11 [45.5%]), but without significant differences (p=0.64).75
Of the clinical practice studies, a multicentre and retrospective Spanish study with subcutaneous ustekinumab in different regimens and that includes 116 patients with CD that shows that, of the 18 patients with active PAD at the beginning of the biological treatment, 11 (61%) improve, stands out.76 The multicentre, observational BioLAP study of the GETAID confirms the efficacy of ustekinumab, as a rescue treatment, in inducing clinical remission in 56/148 (38%) patients with complex perianal fistulas at six months of treatment, facilitating the withdrawal of setons in 37% of patients who had them at the beginning of the treatment.77
Prospective controlled studies are needed to analyse the efficacy of ustekinumab in PAD to be able to conclude whether or not it is useful in the first-line treatment. On the other hand, it is necessary to analyse whether the currently accepted regimen for CD (an intravenous infusion the dose of which is based on weight and, subsequently, 90mg/sc every 8–12 weeks) obtains better outcomes in PAD than the previous regimens, which varied depending on the practice of each hospital and used as a reference the doses used in dermatology and rheumatology.
Therefore, there are not enough studies to recommend ustekinumab as a first-line treatment in PAD. Clinical practice studies suggest its use as a rescue option in complex fistulas with failure of anti-TNF treatment.
Other medical treatmentsThere are some publications of cases or case series that show improvement of PAD with thalidomide or methotrexate, among others, but there is not enough evidence to recommend them in clinical practice.18,68
Surgical treatmentIt must always be kept in mind that surgical treatment of fistulas should be associated with the medical treatment of PAD and luminal disease. Both treatments will vary depending on the complexity of the fistulas, the evolutionary course and the activity of the affected intestinal section.
In relation to the different surgical techniques applicable to perianal CD, there is hardly any scientific evidence of which is the best method. Most publications do not collect large series, they are not prospective and the ultimate goal can vary widely from one author to another.78,79 A fistula of cryptoglandular origin has a different treatment from the fistula related to CD. In the first, the objective is to heal the fistula while preserving continence, while in the second, it is necessary to add, ahead of these objectives, the improvement of quality of life and avoiding proctectomy, as well as ileostomy. Before initiating a local or specific surgical treatment of the fistulas, the extent and activity of the luminal disease must be evaluated, especially if there is rectal involvement. Similarly, before performing any surgical technique on the fistula it is an essential requirement to drain the septic foci and place setons, except in cases where the fistula is not productive. If there is proctitis, it is recommended to be as conservative as possible locally, since very torpid evolution of wounds that can take months to close, or that even end in proctectomy, have been described.80
Other factors that influence the definitive surgical management are based on the severity of the symptoms, the complexity of the fistula and the state of continence. It is for these reasons that we would only operate on those fistulas that determine or alter the patient's quality of life, preserving continence, and always after having controlled local active sepsis. Any fistula that is well tolerated may not require any surgical intervention, while those that are symptomatic, but without associated complications, are better treated medically.80 Normally, the greater the complexity of the fistula, or the more interventions that have been performed, the worse the outcomes of the intervention tend to be.
A rule to keep in mind is that the rectum must always be evaluated before anal fistula surgery, since the existence of proctitis should limit aggressive surgery; loose setons are a good resource in those cases and medical treatment is the first line option.
Simple fistulasThey do not usually have associated proctitis and fistulotomy is the recommended technique, with more than 80% of outcomes satisfactory.80 However, if it is a woman and the fistula is anteriorly located, the risk of incontinence is very high and its performance is not recommended.12 The recurrence figures are highly variable, although in most series they are less than 20%, and the problems of incontinence after this surgery are usually mild, ranging from 0 to 50%.12,23,68
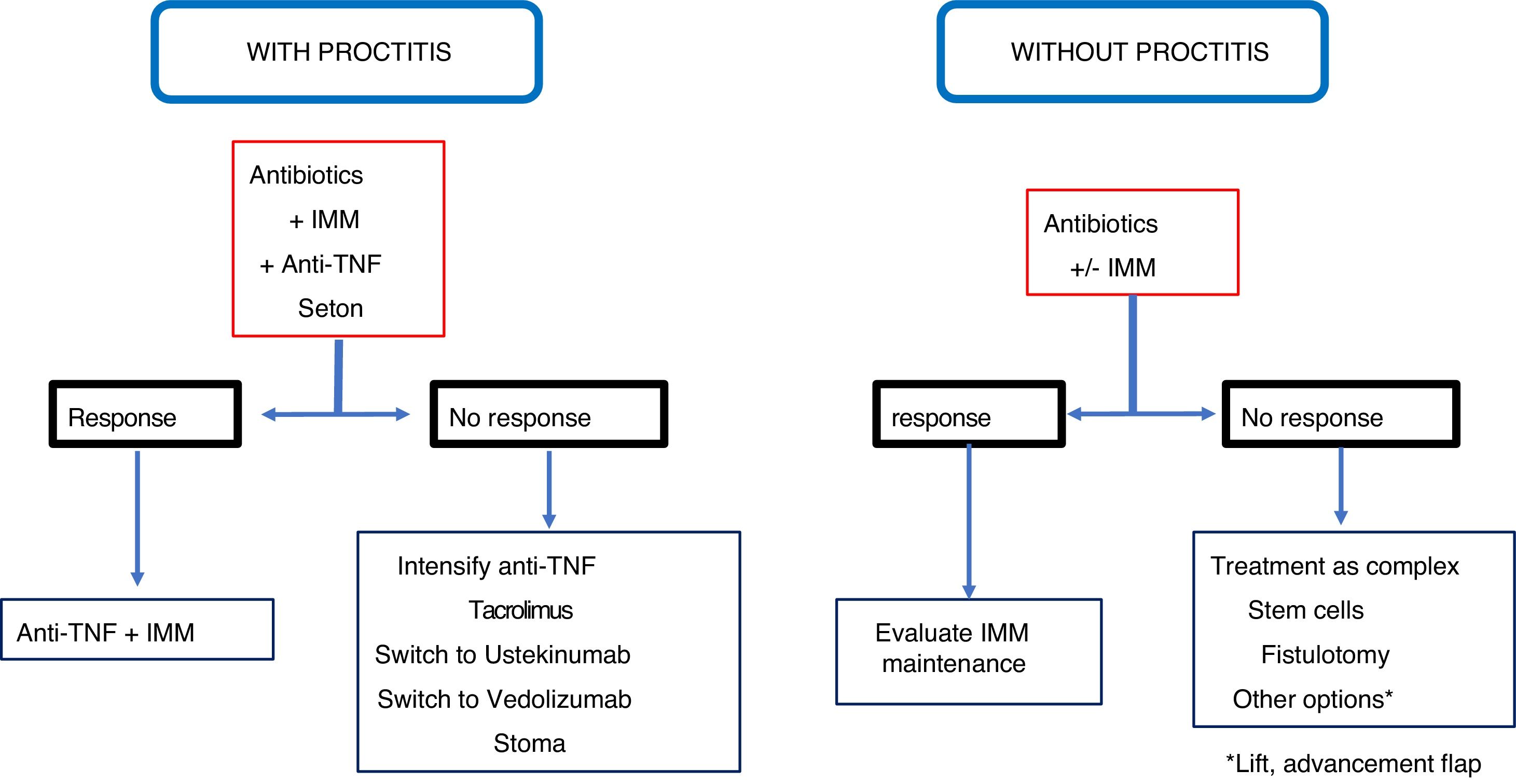
Other techniques that can be used in simple fistulas, very infrequently, are the placement of loose setons, the rectal advancement flap or the ligation of intersphincteric fistula tract (LIFT), which are reserved for when there is a risk of incontinence or very limited proctitis.12,23,81 None of these three techniques injures the sphincter mechanism. In a systematic review, Soltani and Kaiser82 observe that the advancement flap in Crohn's disease fistulas has a healing rate in 64% of cases and is associated with 9.4% of incontinence, although the quality of the articles analysed is not sufficient to achieve a high level of evidence. Regarding the LIFT technique, there is not enough experience in CD to be able to come to a decision on the outcomes.81 Therefore, the most advisable surgical technique for a simple fistula is fistulotomy if there is no proctitis, and a loose seton if the latter is present.
Complex fistulasIn these cases, fistulotomy and sharp setons are contraindicated due to the high risk of incontinence. If there is no associated proctitis or anal or anorectal stenosis, the mucosal advancement flap may be a good option, although the success figures range between 25 and 75%, and incontinence occurs in 10% of cases.82,83 This surgical technique is the most widely evaluated for the treatment of fistulas of PAD.
Recently, a new procedure or technique of video-assisted anal fistula treatment (VAAFT) has been used, which does not damage the sphincter mechanism and can be used alone or in combination with the flap. Although experience is limited and the outcomes seem to be good, with an 81% closing of fistulas84, its future is yet to be determined.
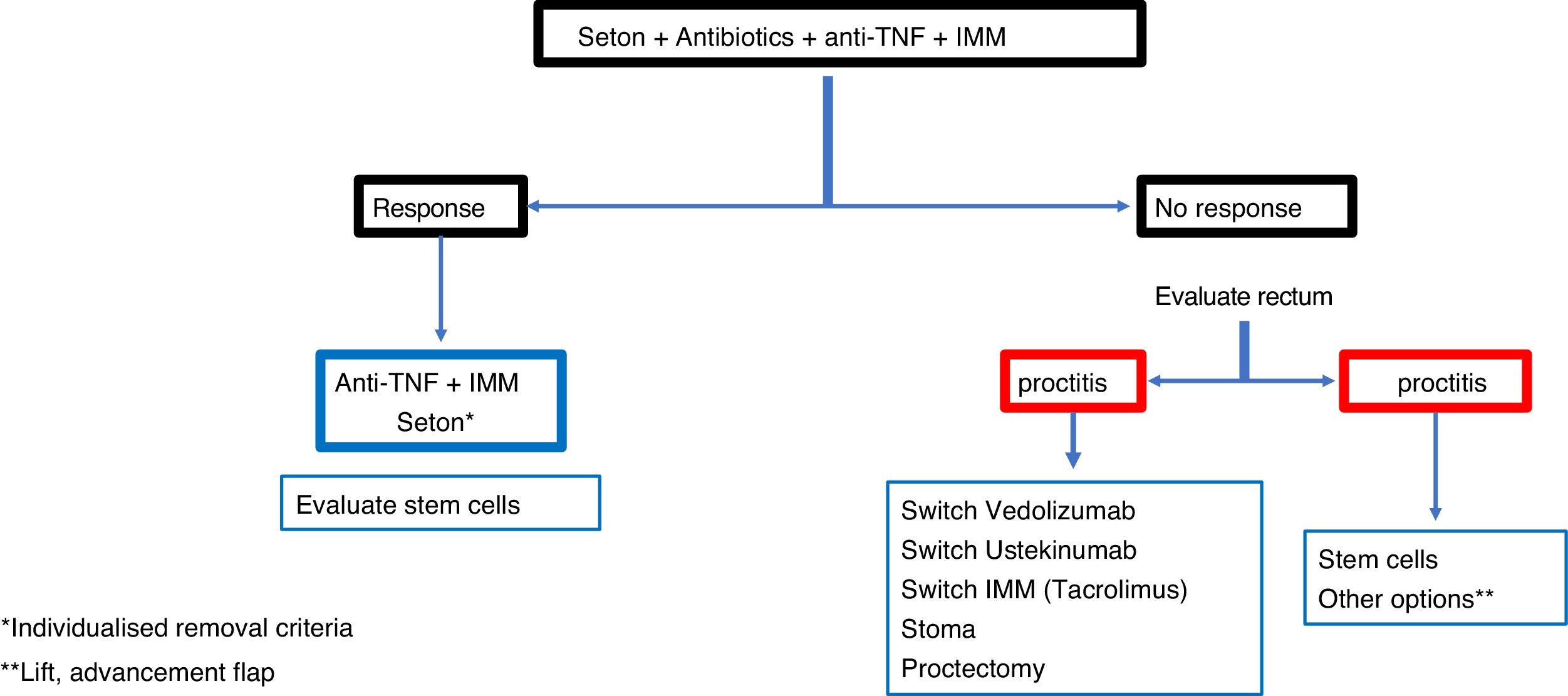
In a complex fistula, even without proctitis, most authors prefer loosely knotted setons to act as a drain along with drug treatment.80,85 However, it is common for these fistulas to have rectal involvement, in which case the treatment of choice is the seton drain. In these patients, fistulotomy or rectal advancement flap should never be performed, because the figures for incontinence (40%), unhealed wounds (60%) and proctectomies (60%) are very high.82
In cases of severe perianal sepsis with complex fistulas, seton drains are placed to avoid large perineal wounds, prevent the spread of abscesses or fistulas and reduce pain.86 In addition, the sphincter function is thereby preserved and anal continence is maintained. The different series show improvement in 90% of patients avoiding or delaying proctectomy or faecal diversion.85,86 A good option to consider is the combination of biological therapies, such as anti-TNF, together with the application of loose setons, since the outcomes of healing or closing of the fistulas is much better if both procedures are combined.12,78,79
There is controversy about how long the seton should be kept in place, because once removed there is a high rate of recurrence.80,87 Some think it should be maintained indefinitely, although others think it should be used as the first step for a definitive subsequent surgery. From a practical point of view, the "entrapment" of the seton suggests the need for removal.
In a complex fistula, if there is no associated proctitis, the advancement flap is the most appropriate procedure, while, if there is proctitis, the placement of a loose seton is recommended.
Anovaginal or rectovaginal fistulasThey occur in 9% of patients. The majority are anovaginal and do not require treatment if they are very low or cause few symptoms. The rectovaginal fistulas are associated with deep ulcerations or proctitis and their appearance is an unfavourable prognostic factor. If there is rectal involvement, it is best to place a seton or consider a derivative stoma that may eventually end up not closing or practising a proctectomy.88 If the rectum is healthy or minimally affected, there is good sphincter function and there is no active PAD, it can be repaired by endorectal, vaginal or cutaneous flap, obtaining healing figures of between 30 and 70%.89 However, experience is limited and evidence virtually absent. In the case of relapsed fistulas, a muscle interposition (gracilis, bulbocavernosus or sphincteroplasty) may be considered.90 Therefore, in a rectovaginal fistula, as in other types of fistulas, there are no absolute rules or a suitable standardised technique. Proctitis, fistula height and symptomatology will influence the decision, with it being possible to use a mucosal advancement flap in the absence of proctitis up to the seton or bypass with stoma in the presence of rectal activity.
The indication of a derivative stoma in anal fistulas is reserved for aggressive cases which are difficult to manage with conservative methods. It should be considered in the event of severe perianal sepsis not medically controllable or with drainage of the septic foci, in cases of recurrent deep ulcerations and in complex or rectovaginal fistulas that seriously alter the quality of life, when there is no response to standard medical-surgical treatment.88
Other surgical proceduresA large number of them have been described, but none have enough evidence to make strong recommendations. Literature and experience are limited and more studies are needed to confirm their true usefulness.23,68,81 Among others, tissue adhesives or fibrin sealants, the placement of a plug made from porcine collagen and the injection of fragmented autologous fat around the fistula tract have been used, resulting in highly variable outcomes.
In the case of fibrin sealants, it is where there is the most experience, and the figures for definitive healing range between 17 and 57% of cases; with the collagen plug the figures vary between 25 and 80%, and with the autologous fat the results seem encouraging, with 80% good outcomes, but experience is minimal.91,92 Although there is hardly any evidence for these procedures, given their safety and since they do not disturb the sphincter mechanism, they can be used in some specific cases. Anecdotally, ablation of the fistula tract with laser has been used in Crohn's disease patients, obtaining healing rates close to 70% after a first intervention and greater than 90% if the patients in whom the fistula recurred were operated on again.93 However, these patients were associated with a mucosal advancement flap, so it is not clear whether the closure was favoured by the laser or by the flap itself.
Local anti-TNFThere are seven case series published, with low sample sizes (from 9 to 33 patients), in which the results are communicated with the local injection of INX (between 15 and 25mg every four weeks)94–97 or ADA (20 or 40mg every two weeks)98–104 and the closure of fistulas is referred to in 31–75% of cases, with the advantage that injections can be repeated. Further studies are necessary to recommend its use in the routine treatment of PAD.
Stem cellsThe administration of autologous or heterologous mesenchymal stem cells (MSC) in the perianal fistulas of CD is a therapy that has been in development since the early 2000s. MSCs possess immunomodulatory capacity, suppressing the activation and proliferation of T cells, dendritic cell differentiation and natural Killer cells proliferation.81 These properties could be very relevant in fistulising CD, because in the pathogenesis of fistulas there is activation of dendritic cells and lymphocytes, as well as proliferation of natural killer cells.101 In the early 2000s, the first studies with stem cells obtained from bone marrow and adipose tissue in the perianal fistulas of CD were published, obtaining very promising results.102–104
The first randomised and placebo-controlled clinical trial was published in 2016.105 It compared the efficacy of a single injection of 120 million allogeneic MSCs derived from adipose tissue, versus placebo, in 107 and 105 patients, respectively. The treatment was a success in 51.5% of patients treated with MSCs, compared to 35.6% in the placebo group. The benefit of treatment was maintained at week 52 in 56.3% of patients in the MSC group, compared to 38.6% of patients in the placebo group.106 Darvadstrocel (Cx601) is a cell therapy that is presented as a suspension of adult human expanded allogeneic stem cells of mesenchymal origin extracted from adipose tissue that obtained marketing authorisation in March 2018.
The use of cell therapies in perianal fistulas of CD is emerging as an attractive and very promising treatment. In principle, its indication for the treatment of complex perianal fistulas in adult patients with inactive or mild luminal CD (special reference to proctitis) is approved when the fistulas have presented an inadequate response to a conventional treatment (seton, antibiotic, azathioprine) or first-line biologic (anti-TNF).
Hyperbaric oxygen therapyIt has been used in the treatment of CD, assuming that there is an alteration in tissue oxygenation that facilitates the proliferation of anaerobes. The elevation of the partial O2 pressure during a period of time of 90min in hyperbaric oxygen chamber (2.5 absolute atmospheres) has demonstrated its efficacy in inducing remission in 10 patients with perianal fistulas (50% complete remission; 20% partial remission; 20–40 sessions).107 Although hyperbaric O2 may be useful as a last option in patients with chronic perianal CD refractory to other treatments or as a complement to surgery, controlled studies should be performed before recommending this treatment option in the management of PAD.108
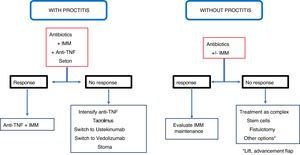
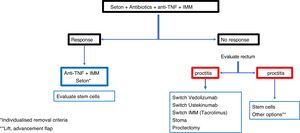
ConclusionRecommendations are pieces of advice given as they are considered beneficial. However, they are still suggestions, and are therefore open to different interpretations. In that sense, the final objective of the review has been, with the available evidence, to try to homogenise as much as possible the approach to the diagnosis and medical-surgical treatment of one of the most complex manifestations of Crohn's disease, i.e. perianal fistulas. Figs. 2 and 3 summarise the approach to the management of simple and complex fistulas based on the conclusions derived from this review.
Conflicts of interestJavier Gisbert has given scientific advice, support for research and/or training activities for MSD, AbbVie, Hospira, Pfizer, Kern Pharma, Biogen, Takeda, Janssen, Roche, Sandoz, Celgene, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, Vifor Pharma, Almirall, Nycomed, AstraZeneca, Casen Recordati, Mayoly and Allergan.
Joaquín Hinojosa has given scientific advice, support for research and/or training activities for MSD, AbbVie, Ferring, Faes Farma, Shire Pharmaceuticals, Chiesi, Otsuka Pharmaceutical, Pfizer-Hospira, Kern Pharma, UCB Pharma, Vifor Pharma, Sandoz, Biogen, Janssen, Takeda, Celgene and Dr. Falk Pharma.
Marta Maia Boscá declares that she has participated in educational activities, scientific meetings and committees financed by MSD, Ferring, AbbVie, Janssen and Takeda.
Nuria Maroto declares that she has participated in educational activities, scientific meetings and committees financed by MSD, Ferring, AbbVie, Janssen and Takeda.
Pilar Nos has given scientific advice, support for research and/or training activities for MSD, AbbVie, Ferring, Faes Farma, Otsuka Pharmaceutical, Pfizer, Janssen, Takeda and Dr. Falk Pharma.
Beltrán Belén has given scientific advice, support for research and/or training activities for AbbVie, Otsuka, Pfizer,
Takeda, and MSD.
Miguel Mínguez has given scientific advice, support for research and/or training activities for Takeda, AbbVie and MSD.
The other authors have no conflicts of interest to declare.
Please cite this article as: Boscá MM, Alós R, Maroto N, Gisbert JP, Beltrán B, Chaparro M, et al. Recomendaciones del Grupo Español de Trabajo de Enfermedad de Crohn y Colitis Ulcerosa (GETECCU) para el tratamiento de las fístulas perianales de la enfermedad de Crohn. Gastroenterol Hepatol. 2020;43:155–168.