Despite pharmacological advances, strictures in Crohn's Disease (CD) continues to be an important problem that leads in a high percentage of patients to undergo endoscopic and/or surgical treatments. There are currently no clinical scores or diagnostic tools that allow predicting which patients will develop this complication, and when a stricture is diagnosed, it is usually already well established and clinically relevant. The current role of pharmacological treatment is limited to treat inflammation and once there is significant fibrosis, the only therapeutic options are endoscopic and/or surgical. To establish a correct therapeutic algorithm and based on the current scientific evidence available, the Spanish Group Working on Crohn’s Disease and Ulcerative Colitis (GETECCU) has decided to conduct this position statement on the treatment of strictures in CD. This document embraces the three mentioned therapeutic approaches, medical, endoscopic and surgical. Recommendations and therapeutic algorithms are established to help us to choose the most appropriate option based on the characteristics of the stricture and the patient.
A pesar de los avances farmacológicos, la estenosis en la Enfermedad de Crohn (EC) sigue siendo un problema importante que obliga en un alto porcentaje de pacientes a realizar tratamientos endoscópicos y/o quirúrgicos. No existen en la actualidad índices clínicos o herramientas diagnósticas que nos permitan predecir qué pacientes desarrollarán esta complicación, y actualmente cuando una estenosis se diagnostica, ésta suele estar ya bien establecida y ser clínicamente relevante. El papel actual del tratamiento farmacológico se limita a tratar la inflamación y una vez existe una fibrosis importante las únicas opciones terapéuticas son las endoscópicas y/o quirúrgicas. Para poder establecer un correcto algoritmo terapéutico y en base a la evidencia científica disponible actual, el grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU) ha decidido realizar este documento de posicionamiento sobre el tratamiento de la estenosis en la EC. Este documento abarca los tres abordajes terapéuticos mencionados, médico, endoscópico y quirúrgico. Se establecen recomendaciones y algoritmos terapéuticos que nos permitan ayudar a elegir la opción más adecuada en función de las características de la estenosis y del paciente.
Strictures are among the most common complications in patients with Crohn’s disease (CD) and normally require a combined approach of medical, surgical and/or endoscopic treatment.1–4 They occur as a result of chronic transmural inflammation with subsequent tissue remodelling that presents with hypertrophy of mesenchymal cells featuring hyperplasia and fibrosis. They are most commonly located in the terminal ileum and in the ileocolic or rectal anastomosis. They are considered clinically significant when persistent narrowing of the bowel lumen occurs with pre-stricture dilation but in particular in the presence of symptoms of obstruction.
Strictures are seen in a third of patients 10 years after the disease is diagnosed. Among those who require ileal resection, more than 50% will require further surgery after 15 years. More than 40% of patients who undergo surgery will present recurrence of symptoms of obstruction after four years, which may lead to a need for further bowel resection and, with this, a long-term possibility of suffering from short bowel syndrome. Strictures are more common in CD than in ulcerative colitis and in disease limited to the small bowel than in the colon exclusively (64% versus 5%, respectively).1–3
The different treatment options should be considered based on the degree of inflammation and fibrosis. Although strictures in CD are neither purely inflammatory nor fibrotic, when there is a significant inflammatory component, medical treatment is the first-line treatment option. By contrast, surgical resection should be reserved solely for irreversible fibrotic strictures. Endoscopic treatment is a good alternative to surgery in this type of fibrotic stricture, as it shows a similar efficacy rate but is associated with fewer complications.5
To establish a proper treatment algorithm, based on the current available scientific evidence, the GETECCU has decided to prepare this position statement on the treatment of strictures in CD. This document covers the three treatment approaches mentioned: medical, endoscopic and surgical.
Medical treatment of strictures in Crohn’s diseaseTreatment of strictures in CD was classically based on steroids, bowel rest and, in the event of non-response, bowel resection. Advances in medical treatment of CD in the past 20 years have been aimed at decreasing inflammation. In any case, despite advances in new medical treatments, the only treatment options in predominantly fibrotic strictures are still surgery or endoscopic treatments.6,7 Strictures have been considered an inevitable result of long-term inflammation in patients who do not respond to anti-inflammatory treatments. Fibrosis associated with inflammatory bowel disease (IBD) has been thought to be an irreversible condition that often causes bowel obstructions. This paradigm will likely shift in the coming years with the advent of new anti-inflammatory treatments capable of modifying the natural course of the disease, as well as the development of antifibrotic therapies.
What should be taken into account before proposing any treatment in strictures in CD?It is important to conduct a full assessment of the patient's disease and, of course, strictures. To this end, it is essential to have complete laboratory testing results with inflammatory parameters (C-reactive protein [CRP], erythrocyte sedimentation rate [ESR], fibrinogen and faecal calprotectin) and a recent imaging study (preferably, magnetic resonance [MRI] enterography; alternatively, computed tomography [CT] enterography) enabling assessment of disease extent and providing information on stricture length and type.
To assess the patient's symptoms of obstruction, a scale already used and validated in some studies and offering a more objective picture of said symptoms can be used.8,9 It is essential to maintain proper patient nutrition and even to weigh the need for enteral nutrition. Despite the lack of scientific evidence in this regard, it seems reasonable to recommend a no-fibre diet or a low-fibre diet (with insoluble fibre only) with plenty of fluid intake.
Is medical treatment a treatment option that should be proposed in strictures in a patient with CD?An important aspect of stricture evaluation is assessment of the degree of inflammation in the area of the stricture using imaging tests. The signs of inflammation on CT or MRI are the comb sign (congestion of rectal vessels), bowel wall thickening and contrast hyperenhancement.10 If concomitant inflammation is confirmed, an anti-inflammatory treatment should be attempted initially, as it could decrease wall oedema, resulting in a reduction in wall thickness, and thus relieve symptoms of obstruction.11,12 Treatment with corticosteroids as induction therapy, followed by immunosuppressants or biologics if corticosteroid dependence occurs, would be the first step to be taken at all centres that treat patients with IBD.
Tumour necrosis factor (TNF) inhibitors such as infliximab and adalimumab have proven effective in inducing and maintaining remission in CD. Their usefulness and use in the management of stricturing lesions are debated. Some studies have found that TNF inhibitors could reduce rates of stricture development if treatment is started early; on the other hand, rapid healing of ulcers has been linked to potential formation of strictures, and some studies have even found that TNF inhibitors carry an increased long-term risk of causing strictures.13,14
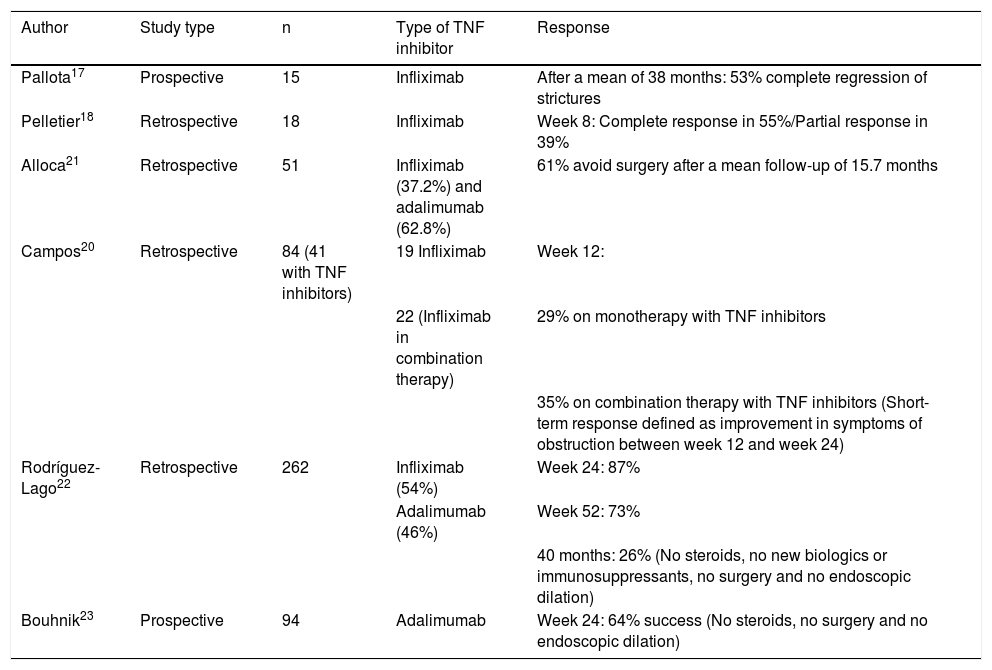
The TREAT registry and the ACCENT study did not find treatment with infliximab to be associated with a higher risk of bowel obstruction in patients with a stricturing pattern in inclusion.15,16 More specifically, data from some studies have shown that TNF inhibitors are useful in this scenario and could even reverse stricturing lesions, although most of these studies have been retrospective and have included limited numbers of patients.17–21 The GETECCU recently published a retrospective multicentre study on stricturing CD. Treatment with TNF inhibitors demonstrated short-term effectiveness in a high percentage of patients.22 Very similar data were obtained in another prospective multicentre observational cohort study (CREOLE) on the effects of induction and maintenance therapy with adalimumab.23 There is no evidence on the use of immune-modulating agents, vedolizumab or other biologics for such complications. Table 1 summarises the clinical studies that have evaluated treatment with TNF inhibitors in stricturing CD.
Clinical studies that have evaluated treatment with TNF inhibitors in strictures in CD.
| Author | Study type | n | Type of TNF inhibitor | Response |
|---|---|---|---|---|
| Pallota17 | Prospective | 15 | Infliximab | After a mean of 38 months: 53% complete regression of strictures |
| Pelletier18 | Retrospective | 18 | Infliximab | Week 8: Complete response in 55%/Partial response in 39% |
| Alloca21 | Retrospective | 51 | Infliximab (37.2%) and adalimumab (62.8%) | 61% avoid surgery after a mean follow-up of 15.7 months |
| Campos20 | Retrospective | 84 (41 with TNF inhibitors) | 19 Infliximab | Week 12: |
| 22 (Infliximab in combination therapy) | 29% on monotherapy with TNF inhibitors | |||
| 35% on combination therapy with TNF inhibitors (Short-term response defined as improvement in symptoms of obstruction between week 12 and week 24) | ||||
| Rodríguez-Lago22 | Retrospective | 262 | Infliximab (54%) | Week 24: 87% |
| Adalimumab (46%) | Week 52: 73% | |||
| 40 months: 26% (No steroids, no new biologics or immunosuppressants, no surgery and no endoscopic dilation) | ||||
| Bouhnik23 | Prospective | 94 | Adalimumab | Week 24: 64% success (No steroids, no surgery and no endoscopic dilation) |
TNF inhibitors: tumour necrosis factor inhibitors.
At present, research is being conducted on new treatment targets focused on inhibiting fibrosis based on advances in other diseases such as pulmonary, renal and hepatic fibrosis and scleroderma.4 However, today, fibrosis remains an unsolved clinical problem in CD, and an effective treatment for bowel fibrosis remains unavailable.
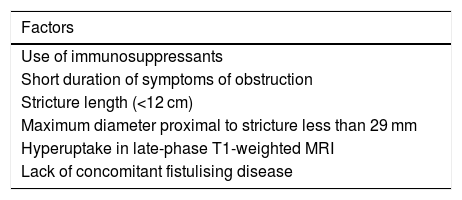
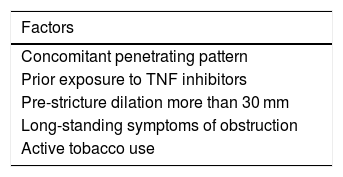
In which patients with stricturing CD should medical treatment be proposed?Based on the results of the French CREOLE study, an attempt was made to design a scoring system to aid in predicting the efficacy of treatment with TNF inhibitors in stricturing CD (Table 2). All variables were assigned one point, except pre-stricture dilation less than or equal to 29 mm, which was assigned two points. The likelihood of adalimumab being effective was 88% in subjects with more than four points, and just 6% in subjects with fewer than two points.23 While it is not yet validated, pending its validation and simplification, some of these criteria could be taken into account towards more objective decision-making between medical treatment and surgical/endoscopic treatment.
Factors associated with treatment efficacy with TNF inhibitors in a small-bowel strictures in CD.
| Factors |
|---|
| Use of immunosuppressants |
| Short duration of symptoms of obstruction |
| Stricture length (<12 cm) |
| Maximum diameter proximal to stricture less than 29 mm |
| Hyperuptake in late-phase T1-weighted MRI |
| Lack of concomitant fistulising disease |
MRI: magnetic resonance imaging.
Moreover, the results of some studies that have evaluated which factors are associated with surgery in patients with stricturing CD may also be helpful. Symptoms of obstruction, a Crohn's Disease Activity Index (CDAI) greater than 220, being an active smoker and having a disease duration of fewer than three years at the time of stricture diagnosis are factors associated with a higher risk of surgery. Having more than three factors is associated with a risk of surgery greater than 73%, and having more than four factors is associated with a risk of surgery greater than 100%.24 In this same vein, more recently, a study retrospectively evaluated patients with an ileal stricture according to radiological criteria on MRI. Factors associated with surgery were identified and a risk model called BACARDI was created (Table 3). Each of these variables was scored with one point, apart from pre-stricture dilation, which was scored with two points, with a high risk of surgery with more than four points. As in the CREOLE study, this model remains unvalidated.25
Does stricture location influence medical treatment efficacy?Although most studies that have evaluated the efficacy of TNF inhibitors in the treatment of patients with CD and strictures have included patients with ileal stricture in particular,22,25a priori medical treatment should be attempted in all small-bowel strictures, regardless of location. The mixed nature of the studies and the lower prevalence of high strictures have precluded the making of any firm recommendations based on specific location in the small bowel. Colon strictures, however, occur at higher rates than small-bowel strictures and are associated with lower response to medical treatment.22 In addition, in colon strictures, the risk of dysplasia or cancer must not be overlooked; hence, each case must be personalised and an endoscopic or surgical approach must be pursued, in particular in cases in which the stricture cannot be passed.
Anastomotic strictures exhibit different morphological and radiological characteristics due to postoperative changes and the possibility of a chronically dilated bowel which may not return to normal after resection.26 The CREOLE study included patients with ileocolic anastomotic strictures; such strictures were not seen to be a risk factor for medical treatment failure.23 Despite this, no extensive series have specifically evaluated medical treatment efficacy in anastomotic strictures. In addition, their accessibility and typically short length make them very good candidates for endoscopic versus medical treatment.
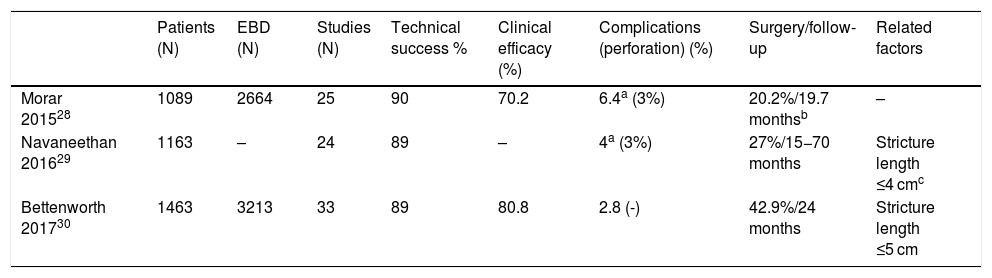
Endoscopic treatment of strictures in Crohn’s diseaseBalloon dilationAt present, endoscopic balloon dilation (EBD) is the endoscopic treatment of choice in IBD. The latest published meta-analyses, all based on uncontrolled observational studies, have shown that EBD in selected patients is a safe and effective alternative to surgery, with an overall success rate of 58%–80.8% and a rate of complications of 2.8%–6.4%.27–30 To date, these studies have had several limitations due to heterogeneity in the endoscopic technique used and differences in factors that could influence the safety and efficacy of the procedure, with few studies including more than 100 patients.31–34 At present, there is available information from a clinical trial (PROTDILAT) comparing this endoscopic technique to another endoscopic alternative (stent) and contributing sounder evidence on its efficacy and safety in CD and on the factors that contribute to its success.9 Nevertheless, there is a lack of well-designed clinical trials comparing EBD to other endoscopic alternatives and conducted in particular in situations that are less favourable to dilation, such as longer strictures. In addition, it is important to note that, although most available data come from studies conducted at IBD referral centres, at present, EBD is known to be a safe and effective technique, regardless of the level of healthcare complexity at the centre at which it is performed.34 In this regard, it differs markedly from surgery, in which the outcomes achieved at tertiary hospitals are significantly better.35 Still, even more studies are needed to compare EBD to surgery in terms of not only efficacy and safety but also patient quality of life.
Factors related to treatment success in EBDTable 4 summarises the results obtained in the four recently published meta-analyses and the factors related to treatment success that have been reported in each. When speaking of EBD, there are two important concepts to bear in mind: 1). Technical success is defined as the ability to pass the endoscope through the stricture once EBD has been performed. 2). Treatment success is defined as the long-term resolution of symptoms of obstruction, established in some guidelines as being surgery-free after a year of follow-up.36
Summary of the latest published meta-analyses on endoscopic balloon dilation.
| Patients (N) | EBD (N) | Studies (N) | Technical success % | Clinical efficacy (%) | Complications (perforation) (%) | Surgery/follow-up | Related factors | |
|---|---|---|---|---|---|---|---|---|
| Morar 201528 | 1089 | 2664 | 25 | 90 | 70.2 | 6.4a (3%) | 20.2%/19.7 monthsb | – |
| Navaneethan 201629 | 1163 | – | 24 | 89 | – | 4a (3%) | 27%/15−70 months | Stricture length ≤4 cmc |
| Bettenworth 201730 | 1463 | 3213 | 33 | 89 | 80.8 | 2.8 (-) | 42.9%/24 months | Stricture length ≤5 cm |
EBD: endoscopic balloon dilation.
Stricture length is the most determining factor of EBD success.27,29,30,34 Most published studies have attempted to define a stricture length after which it can be determined whether EBD will succeed or not, almost always 4 cm.27,29 What is important is not establishing a specific length, but knowing that the shorter the stricture, the greater the success of dilation, such that dilation of very short strictures (<2–2.5 cm) has an efficacy close to 100%.9 In fact, the probability of surgery has been seen to increase by 8% for every additional centimetre of stricture length.30 It is also important to be aware that the length reported in an imaging test is often significantly greater than that visualised in endoscopy. Therefore, the option of dilating longer strictures should not be ruled out, as analysis of the various published studies reveals that EBD success rates in strictures >4 cm are around 60%–70%,34 higher than the highest success rates of most other endoscopic techniques.
Is there any difference between anastomotic stricture and de novo stricture?There is disagreement in the literature as to whether stricture type (anastomotic versus de novo) represents a determining factor in EBD success or failure. At present, there is enough evidence to suggest that the true determining factor in this case is once again stricture length. Most anastomotic strictures are very short; this probably accounted for bias in most prior studies (almost all retrospective), which suggested that dilating anastomotic strictures was better than dilating de novo ones. The only randomised clinical trial published to date, as well as the latest meta-analysis published, corroborated the fact that EBD is equally effective, regardless of stricture type (anastomotic versus de novo), and that the true determining factor is the length thereof.9,30
What other factors have been linked to EBD success?Other factors have been reported to be related to EBD success but not consistently corroborated across most published studies. These factors include: being an active smoker,37 not receiving treatment with TNF inhibitors at the time of dilation,34 CD duration, CRP levels,38 use of combination treatment with immunosuppressants and biologics,39 pre-stricture dilation40 and severity of symptoms of obstruction.9,40 All seem to be different expressions of a single phenomenon and to translate to more severe or advanced disease. Optimisation of medical treatment prior to EBD may improve the outcomes thereof and reduce or avoid the need for subsequent dilation.39 In addition, although inflammation in the stricture area is not a contraindication to EBD, significant inflammation could increase the risk of serious adverse events.34,41 Therefore, accelerated intensification of medical treatment should be pursued with a view to optimising EBD outcomes if necessary.
Achieving technical success in EBD is also important in achieving therapeutic success.30,34 The rate of technical success is linked to the use of larger-diameter balloons (>12 mm).34,42 The choice of balloon size should also be weighed against patient safety, since equal clinical response rates can be achieved with smaller-diameter balloons.34
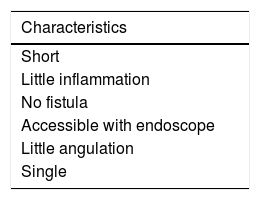
Table 5 summarises the ideal characteristics of a stricture for performing EBD.
Should strictures be dilated in asymptomatic patients?Although it is a safe technique, it may lead to potentially serious complications such as perforation. The recently published 2020 Global Interventional Inflammatory Bowel Disease Group international consensus recommends incidental dilation of asymptomatic strictures in the course of follow-up endoscopy. This statement is based on a single retrospective study that found that patients who had pre-stricture dilation in imaging studies were more likely to end up undergoing surgery after EBD.40 In this same study, symptoms of obstruction were also linked to higher odds of EBD failure, but imaging studies were only performed in patients with more serious disease, and therefore patients who had symptoms of obstruction. Others scenarios in which dilation in asymptomatic patients could be proposed would be those in which examining the rest of the bowel to assess disease activity is important to establishing medical treatment. As a general summary, we recommend only dilating strictures that are symptomatic, but not delaying EBD once the patient starts to show symptoms of obstruction.
Is EBD a safe technique? At which hospitals can or should it be performed?EBD is a safe technique. Perforation and clinically significant bleeding or bleeding requiring endoscopic treatment for its resolution are considered major complications. The rate of major complications in the set of published studies ranges from 2% to 3%27–30; in most cases, these complications are perforations. The probability of perforation has been linked to the use of larger-diameter balloons32,43 and significant inflammation at the stricture site.34,41
Selection of balloon diameter, as mentioned above, must weigh achievement of technical success against patient safety. EBD can be repeated; in fact, in most studies, treatment success is not achieved with one-time EBD.32,34 Therefore, dilation success need not be achieved in a single session. Despite the lack of firm scientific evidence showing that sequential and progressive dilation decreases the risk of complications, it seems prudent and logical to do it in this way, thus putting safety before immediate efficacy.
In addition, despite being reported in few studies, it seems that significant inflammation at the stricture site could increase the risk of perforation.34,41 Many studies have not commented on the level of stricture inflammation at the time of dilation; therefore, this factor could be underappreciated. The latest published meta-analysis did not find inflammation at the stricture site to be linked to an increase in perforation rate.30
Safety in patients on concomitant treatment with corticosteroids is a matter of debate. Just one study linked active treatment with systemic corticosteroids to an increased likelihood of perforation,44 and recent consensus documents on endoscopic treatment of strictures in IBD have discouraged their use.36 This single study found only six serious adverse events, four of which were perforations; these patients were on active treatment with corticosteroids and had significant inflammation at the stricture site.44 It is difficult to draw conclusions based on only four patients; moreover, inflammation at the stricture site could have had an impact on the risk of perforation, as reported by other studies.34,41 Therefore, we believe it cannot be affirmed that corticosteroids alone represent a risk factor for perforation; what is distinctly advisable is treating the underlying inflammation and intensifying medical treatment before performing EBD.
There is little scientific evidence to be able to determine the type of hospital at which EBD can or should be performed. Most published studies have been conducted at IBD referral centres. In 2019, a GETECCU-backed study conducted in Spain, with the participation of 19 hospitals of varying levels of healthcare complexity distributed across the country, that analysed nearly 200 patients and more than 400 EBD procedures found no differences in terms of efficacy or safety between tertiary and secondary hospitals.34 These data were also corroborated in a comparative clinical trial on endoscopic treatment of strictures in CD by the same group of researchers who conducted the previous study (PROTDILAT).9 These studies showed that EBD is a highly reproducible technique in a routine clinical practice setting and, even more importantly, is clearly distinct from surgery in that there are differences in not only efficacy but also complications and mortality between tertiary and lower-complexity hospitals.35,45
Does EBD work equally well in upper gastrointestinal tract stricture?Involvement of the upper gastrointestinal tract by CD is seldom reported, and the development of strictures in this segment of the digestive tract is estimated at approximately 4%,46 although it is believed that the true incidence could be much higher, approximately 19%, if upper gastrointestinal endoscopies were routinely performed in patients with CD.47 The most common phenotype in this location is stricturing and in most cases presents in the form of a single stricture, although the proportion of patients with more than one stricture may be as high as 30% in some series.46 Currently, very few studies have evaluated the efficacy of EBD in upper gastrointestinal tract strictures. A recently published meta-analysis found an efficacy of EBD of 70% over the course of a short follow-up period (median of 23 months) and an overall rate of major complications of 3%, with similar results to all other locations.48
Is dilation with balloon-assisted enteroscopy safe and effective in small-bowel strictures?Strictures located beyond the ileocaecal valve that are not reachable with a conventional colonoscope could be candidates for undergoing endoscopic treatment with balloon-assisted enteroscopy. The double balloon-assisted enteroscope was initially developed and subsequently changed to a single balloon enteroscope;49,50 recently, the spiral enteroscope emerged.51 A recent systematic review with a meta-analysis found the use of a double balloon-assisted enteroscope to be predominant.52 A high rate of technical success (around 90%–94.9%) was achieved, with a short-term rate of clinical efficacy of 82.3%. A rate of major complications of 5.3% and a rate of symptom recurrence of 48.3% were seen. Therefore, it can be concluded that EBD using a balloon-assisted enteroscope is an effective and safe tool in the treatment of small-bowel strictures, but, as with all other types of EBD, a non-negligible percentage of patients will require repeat dilation and surgery.
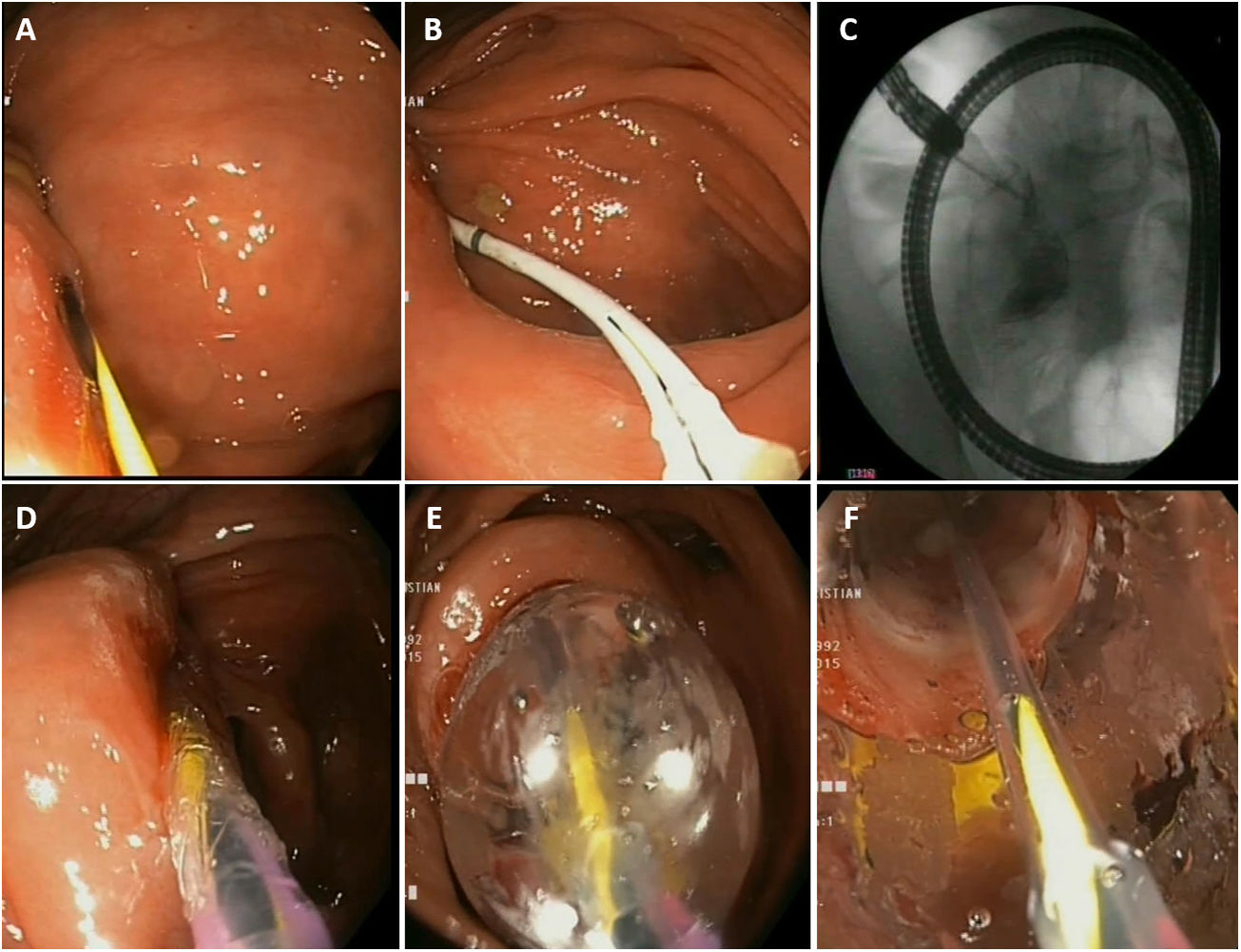
What are the practical aspects of the EBD procedure? (Fig. 1)Is fluoroscopy necessary?It is not essential in performing EBD, but it is advisable. It must be borne in mind that strictures in CD may be complex and located in bowel segments with major incurvations. Fluoroscopy facilitates stricture examination, ensures that the guide passes properly and aids in detecting potential immediate complications.
Practical considerations in endoscopic balloon dilation. A) Passage of the guide through the stricture. B and C) Insertion of a Fogarty balloon and injection of contrast. D and E) Insertion and progressive inflation of the pneumatic balloon. F) Visualisation of stricture dilation through the pneumatic balloon.
Most commercial dilation balloons in Spain come with a rigid guide; it is recommended that this guide be removed and that a long, hydrophilic, guide with a soft tip be used and inserted first before passing the balloon. Cases of perforation with the guide in malignant colon disease prior to metal stent placement have been reported.53
Insertion of a catheter or a Fogarty balloon over the guide and injection of contrast to better characterise the stricture (see Fig. 1, photo B and C)Injection of contrast through a catheter inserted over the guide can aid in estimating, at the time of EBD, the actual length of the stricture, and provides information on the characteristics thereof (angulation, tortuosity, fistulas, etc.).
Sequential and progressive dilation (see Fig. 1, photos D and E)The choice of balloon diameter to be used will depend on stricture diameter, and therefore in very narrow strictures, smaller-diameter balloons should be used initially and the desired diameter will probably not be achieved within a single session. A standard colonoscope has a diameter of around 12 mm; therefore, normally, technical success is achieved with balloons larger than 12 mm.
Attempting to visualise the stricture through the balloon during dilation (see Fig. 1, photo F)This is achieved by advancing the balloon to the tip of the endoscope and gently supporting it, taking care not to displace it. This will aid in having better control of the procedure and detecting potential complications early.
Use the balloon partially inflated at the tip of the endoscope to pass the stricture once EBD has been performed.
This is useful for preventing damage to the stricture mucosa given that the balloon surface is less likely to cause trauma than the tip of the endoscope.
What post-procedure considerations should be taken into account?After EBD, the patient should be kept under observation in a post-endoscopy recovery area for at least one to two hours in order to detect potential early complications. In the event of suspected perforation, antibiotic treatment should be started early and an imaging test (CT) should be performed to confirm it.
Once EBD has been performed, it is recommended that the patient be on a low-fibre diet with plenty of hydration. It is important to monitor symptoms in the days subsequent to the procedure to be able to detect late complications and reassess obstructive signs and symptoms in case further endoscopic treatment be needed.
Endoscopic dilation with a balloon versus surgeryFew comparison studies have directly evaluated endoscopic treatment of strictures to surgery; all have been retrospective.54–57 In general, for both de novo and anastomotic strictures, surgical resection yields better outcomes in the form of longer intervals of surgery-free time than EBD.55–57 One of these studies assessed adverse effects and found no differences in rates of major complications between the two treatments.56 By contrast, in another study, the rate of adverse effects was significantly higher for surgery versus dilation (32.2% versus 4.7%).57 No studies have compared the effects of the two treatments on patient quality of life.
There are also few studies directly comparing surgical strictureplasty to EBD and they too are retrospective studies.54 As with surgical resection, strictureplasty achieves longer further surgery-free intervals compared to EBD. In a meta-analysis published in 2010 that analysed a large number of studies (40 on strictureplasty and 23 on EBD), the median rates of major complications between the two groups were 11% and 3%, respectively, and rates of recurrence were similar, but with less time to onset for EBD.58
Endoscopic treatment with stent placementTechnological advances in stents in recent years along with the clear effectiveness thereof in the treatment of malignant strictures of the gastrointestinal tract have enabled their use in benign disease of any origin and location, including CD. Initially, uncovered self-expanding metal stents (SEMS) were designed for use in malignant colon disease as a palliative treatment with no intention of removing them. Given their effectiveness in this indication, fully covered SEMS were subsequently created to be removed and therefore used in benign bowel disease. Today, there is a wide variety of available stents in terms of size, shape, material and covering (fully covered, partially covered, big-cup, asymmetrical, biodegradable, plastic, metal, etc.). Some were specifically designed for benign bowel strictures and can be placed through the working channel (so-called through-the-scope [TTS] stents).
What scientific evidence is there on the use of self-expanding metal stents in CD?Until not long ago, information on the safety and efficacy of SEMS in the treatment of strictures in CD has been limited and reported in very recent publications.8,9,59–65 The first experiences found in the literature on the use of SEMS in CD were published in the form of case reports.59 Since a wide variety of patient types were included, a wide variety of stent types were used, and SEMS act as a bridge to surgical treatment in half of cases, it is difficult to draw firm conclusions.
Table 6 shows the most important series of patients treated with SEMS.
Summary of the most important series of patients with Crohn's disease reported in the literature treated with self-expanding metal stents.
| Author/Year | No. of patients | Location | Length | Prior treatment | Stent type | Technical/clinical success (rate) | Stent placement time | Course |
|---|---|---|---|---|---|---|---|---|
| Levin 201260 | 5 | IC anastomosis | <6 cm | EBD (2 patients) | UCSEMS | Yes (100%)/Yes (80%) | 3 weeks-9 years | Asymptomatic in 4/5 patients |
| Attar 20128 | 11 | IC anastomosis (9 patients)/ileoterminal anastomosis (2 patients) | 1−4 cm | EBD (9 patients) | FCSEMS | Yes (90%)/Yes (36%) | 1−28 days (SM of 8 stents) | Asymptomatic in 4/11 patients ≥1 year. 2 complications |
| Branche 201261 | 7 | IC anastomosis | <5 cm | EBD | PCSEMS | Yes (100%)/Yes (71.4%) | 1 week | Asymptomatic in 5/7 patients, mean follow-up 10 months |
| Loras 201262 | 17 | IC anastomosis (10 patients)/colon (7 patients) | <8 cm | EBD (14 patients) | PCSEMS (4)/FCSEMS (21) | Yes (92%)/Yes (64.7%) | Mean 28 days (1−112) (SM of 13 stents) | Asymptomatic in 11/17 patients, mean follow-up 67 weeks. 1 complication |
| Das 202064 | 21 | Anastomotic (19 patients)/de novo (2 patients) | ≤6 cm | – | PCSEMS | Yes (95.8%)/Yes (81% ITT/54% PP) | 1 week (SM of 3 stents) | Asymptomatic in 13/16 patients, follow-up 3−50 months |
| Andújar 20209 | 39 | Anastomotic (16 patients)/de novo (23 patients) | ≤9 cm (mean 4 cm) | – | FCSEMS | Yes (92%)/Yes (51%) | Mean 2 days (SM of 38 stents) | Asymptomatic in 20/39 patients, follow-up 12 months, 1 complication |
| Attar 202065 | 46 | Anastomotic (34 patients)/de novo (12 patients) | <5 cm | EBD (36 patients) | PCSEMS | Yes (100%)/Yes (58.7%) | 1 week (5 days in 2 patients; proximal SM of 3 stents) | Asymptomatic in 27/46 patients, mean follow-up 26 months, 5 complications |
EBD: endoscopic balloon dilation; FCSEMS: fully covered self-expanding metal stent; IC: ileocolonic; ITT: intention to treat; PCSEMS: partially covered self-expanding metal stent; PP: per protocol; SM: spontaneous migration; UCSEMS: uncovered self-expanding metal stent.
In 2012, the first brief case series were published. Notable among these series is one by Attar et al., with 11 patients treated with fully covered SEMS.8 A low rate of clinical success was achieved, and spontaneous migration of the stent was the general norm. Given the poor outcomes achieved in terms of stent migration, these same authors subsequently published their initial experience with partially covered SEMS specifically designed for CD (Hanarostent stent; M.I. Tech, Seoul, Korea HRC-20-080-230). A high percentage of patients remained asymptomatic; no cases of stent migration or adhesion were seen.61 In that same year, the then-longest reported case series of patients treated with SEMS found high rates of clinical success despite the fact that most of the patients were refractory to EBD.62
In 2020, two retrospective studies64,65 using the same above-mentioned stent type designed specifically for CD (partially covered SEMS) were published.61 The two studies achieved similar success rates, around 54%–58%, with no significant complications and a substantial reduction in migration rates.
To date, just one study, the PROTDILAT study (pending publication), has been conducted in the form of a randomised clinical trial comparing the efficacy of stents (TaeWoong© fully covered SEMS, 20 mm in diameter, and Niti-S™ S Enteral Colonic Stents, 6−10 cm, Gimpo-si, South Korea) and balloon dilation in 80 patients.9 The results confirmed that the two procedures are safe and effective in the treatment of strictures (both postoperative and de novo), with EBD clearly superior to SEMS as a treatment (80.5% versus 51.3%; remission of symptoms of obstruction after a year of follow-up). However, in the subgroup analysis of patients with longer strictures (>3 cm), the differences between the two procedures disappeared (EBD 66.7% versus SEMS 63.6%). In addition, this study found EBD to be significantly more cost-effective than stent placement (EBD euro1,212.41 versus SEMS euro3,615.07).
What scientific evidence exists on the use of biodegradable stents in CD?In general, biodegradable stents possess low force for reversing strictures and also do not appear to offer clear advantages for use in oesophageal and colonic strictures.66,67 Furthermore, outcomes in CD do not seem to be very promising. A total of two cases and two brief case series have been reported.68–71 The first included a total of 11 patients with short strictures in different locations.68 Despite the difficulty of calculating the overall efficacy of the procedure based on the data reported in the article, it is possible to estimate an overall efficacy of around 50% of cases with a short follow-up period. In addition to these results, technical difficulties in accessing more proximal strictures should be taken into account; these required the creation of a balloon overtube system for stent insertion using radiology. In the second series, with a total of six patients, just one patient (20%) achieved treatment success.70
What role can stents play in the treatment of strictures in CD?SEMS are the only stents that appear to play some sort of demonstrated role in the treatment algorithm for strictures in CD, in opposition to what has been reported with biodegradable stents. As a general summary, in light of the results of the most recent studies, it is clear that stents should not be considered a first-line option in the treatment of strictures in CD. In this context, EBD has a very high success rate and furthermore is highly cost-effective. In light of the current scientific evidence, stents could play a role in cases refractory to prior endoscopic treatment with EBD, in cases in which EBD was not possible and in longer strictures, thus avoiding or delaying future surgery.
The future clearly lies in improving the design of stents tailored specifically to IBD or for benign strictures to improve radial force and also prevent early migration thereof.
Endoscopic stricturotomyEndoscopic stricturotomy (ES) consists of making an incision with a scalpel at the level of the circumference of the stricture. Incisions with this technique in the upper gastrointestinal tract were initially used in biliary cannulation and in the treatment of oesophageal strictures.72,73 At present its widespread use is recognised in strictures of the oesophagus, pylorus, duodenum, distal small bowel, colon, anus and rectum, as well as the ileostomy and the ileal reservoir.74
What is the right indication for performing stricturotomy?Patients with refractory short strictures with a previous poor or partial response to dilation are considered candidates for ES alone or ES combined with EBD. No extensive series have compared or yielded concrete indications in each situation. Each patient must receive personalised care depending on their stricture type and location so that the most suitable option may be pursued. There are no prospective, randomised data in this regard; there are only retrospective studies in which the method of endoscopic treatment used was chosen solely at the discretion of the treating physician.75,76
How is the procedure performed?ES incisions are usually made using tools such as a wire-guided needle-knife scalpel, normally to make "pre-cuts" in endoscopic retrograde cholangiopancreatography procedures, or a wire-guided scalpel with insulated ceramic tip protection (an insulated tip [IT] knife) or a hook-knife scalpel, among others. Cuts can be made in a radial configuration at several points around the circumference of the stricture or in a circumferential arrangement, thus sectioning the circumference into segments. Some authors routinely place haemostatic clips on radial cuts to keep the treated incision open and thus prevent retraction during the healing process (in these cases, the procedure is called a strictureplasty).75,76 It is recommended that it be performed by experienced endoscopists accustomed to handling scalpels, making it significantly different from EBD.
Is stricturotomy effective? What are its advantages? Is it preferable to endoscopic dilation?Whereas with EBD force is applied equally at all points of the circumference of the stricture, with incision the cut-off point and the depth thereof can be chosen, thus minimising the risk of perforation. This aspect is especially useful in ileoanal strictures as it reduces the risk of damaging the anterior wall or the anal sphincter, as could occur with pneumatic dilation.77 On the other hand, it requires great skill and experience with this type of instrument, rendering it clearly less reproducible across centres.
There is just one published retrospective study comparing it to EBD, and it is difficult to draw conclusions from it due to its design and its shorter follow-up time for patients treated with ES.76
Given the limited scientific evidence, the technical difficulty of the procedure and the lack of studies comparing it to EBD, ES cannot be considered a first-line technique in cases of short strictures in CD.
Is endoscopic stricturotomy safe? What are the most common complications?According to reports, ES would seem to carry a lower risk of perforation than EBD as the point and depth of the lesion are monitored at all times. On the other hand, rates of postoperative bleeding requiring admission or transfusion are indeed higher than in EBD. In a total of 272 procedures in 85 patients, 0.4% presented perforation that required surgery and 3.4% presented significant bleeding.75 This bleeding is usually late bleeding four days after the procedure as a result of ulcerations created by electro-incision.
ES could be a safer option compared to surgery in patients with strictures refractory to EBD.5
Intralesional injection of drugsWith a view to improving the long-term efficacy of EBD, techniques of local injection of substances have been studied in an attempt to ameliorate the natural course of stricture healing following dilation.
CorticosteroidsInjection of corticosteroids in healing processes started in dermatology and yielded good outcomes.78 For some time, gastroenterology has used it to treat refractory oesophageal strictures of various aetiologies with beneficial outcomes.79,80 In the past 20 years, different experiences with response to intralesional injection of corticosteroids (IIC) to treat strictures in patients with CD have been accumulated.
What is the mechanism of action of corticosteroids on the stricture?Their capacity appears to be based on their interference with collagen synthesis, fibrosis and chronic healing processes. In the same way, they act by decreasing the fibrotic scarring that occurs after dilation.81 It has also been suggested that triamcinolone prevents collagen reticulation resulting in scar retraction, such that, if the scar distends and corticosteroids are injected into it, retraction of the healing process will presumably not occur.82
Technique, type and doseThe most commonly used and cited corticosteroid is triamcinolone, due to its rapid onset of action and its prolonged effects, lasting around three to four weeks.83–86 Preparations of betamethasone and dexamethasone at different concentrations have also been used.85
The total dose of triamcinolone administered in each session ranges from 40 mg to 100 mg in different concentrations. A standard regimen would be to dilute the 40-mg vial suspension (Trigon Depot®, 40 mg/mL) in a saline solution of 2−5 ml and divide it into 0.5−1–ml aliquots to be administered at each injection site.
A deep injection is administered with a 5-mm sclerotherapy needle and usually the aliquots are administered in the four quadrants, at the anal edge of the stricture, then four to six more injections are administered following dilation along the stricture, if technically possible, depending on its length.
Does injection of corticosteroids improve EBD outcomes?Just two randomised, placebo-controlled trials with limited numbers of patients have been conducted. One, conducted in a paediatric population at a single site, found that intralesional use of triamcinolone achieved a reduction in repeat dilation and operation time versus placebo.87 The other study, conducted in adults at different centres, was prematurely suspended as a complication developed and worse outcomes were seen in the corticosteroid group.83 Despite the limited sample size in the latter study, it has been considered a benchmark for most international guidelines and consensuses, influencing the American College of Gastroenterology and the British Society of Gastroenterology in their decision to discourage routine use of intralesional injection of steroids.36,88,89
All other available studies are retrospective, uncontrolled studies in which it would seem that IIC might yield improved outcomes in combination with EBD.85,90
Therefore, in summary, there is no solid evidence backing the use of ICC in IBD.
TNF inhibitorsThe long-term anti-inflammatory effects of infliximab may be effective if the drug is administered locally as indicated in a study, albeit a study with a limited number of patients, that found intralesional injection of infliximab to be effective in perianal fistulas.91,92
Just three studies published in the literature used infliximab in the treatment of strictures in CD.93–95 The total number of patients was very limited (n = 11); in addition, the dose of infliximab administered varied (from 30 mg to 120 mg), as did the outcomes achieved (from 100% success after 12 months with a need for repeat treatment to 0% after four months). Thus it is difficult to make firm recommendations on the use thereof in this context. There is just one as-yet-unpublished randomised controlled trial comparing EBD alone versus EBD combined with injection of adalimumab (CSAI study).96 Given the characteristics of the study, the required number of patients was not achieved and, therefore, the results thereof are difficult to assess; however, it would seem that injection of this drug also would not bring any benefits to dilation.96
Fig. 2 shows a treatment algorithm for endoscopic management of strictures in CD.
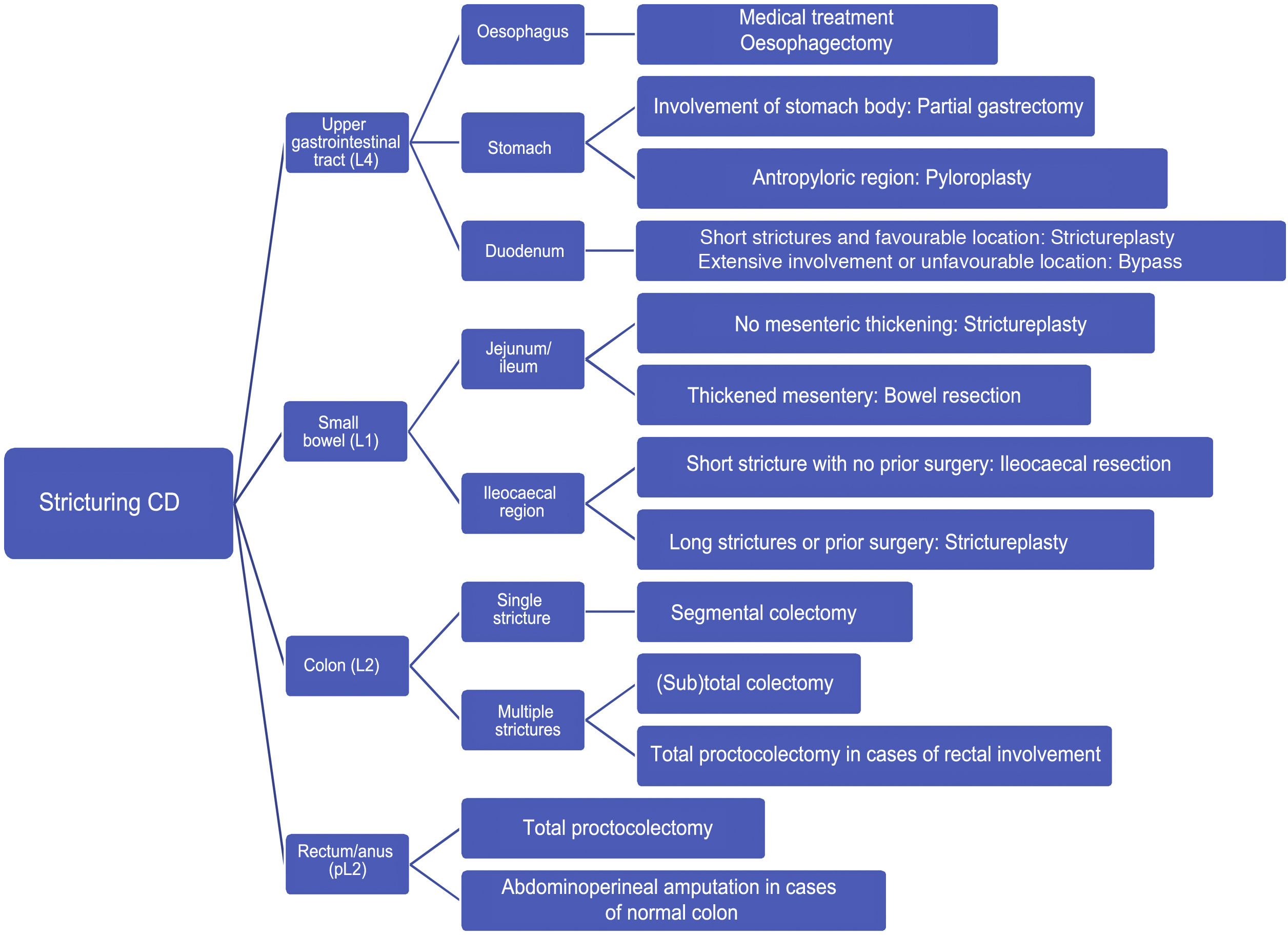
Surgical management of stricturing Crohn’s diseaseThe primary purpose of surgery in stricturing CD is to restore health-related quality of life (pain suppression, recovery of oral tolerance and recovery of occupational and social activities) as of the immediate postoperative period by restoring bowel continuity with minimal postoperative morbidity. The secondary purpose should be to prevent postoperative recurrence and minimise potential future resections.
Under what circumstances should a patient with a stricture due to CD undergo surgery?Some 75% of patients with a stricturing pattern will require surgery at some point in life.97 Some 6%–16% of cases will present with an acute complication requiring emergency surgical treatment.98,99 Medical treatment is effective in strictures with a predominantly inflammatory component,100 and therefore surgical treatment should not be pursued in such strictures unless complications develop and do not respond to medical management (lower gastrointestinal bleeding, perforation, fistulisation, adhesion, etc.).101 Symptomatic fibrocicatricial strictures with conservative medical or endoscopic treatment failure (stricture with nutritional repercussions, pre-stricture dilation >30 mm or otherwise unexplained associated anaemia) and asymptomatic strictures with suspicion or risk of malignant transformation will be candidates for surgical treatment.101
The location of the stricture will not change the indication for surgery.
When should emergency surgery be performed and when should planned surgery be performed in a bowel obstruction?The most common form of presentation consists of subtle subocclusive signs and symptoms, as a result of progressive medical treatment failure, with limited systemic repercussions, allowing for planning of elective surgery. An acute bowel obstruction should be initially treated with conservative measures (fasting, intravenous hydration and a nasogastric tube). As mentioned above, corticosteroid use should be considered depending on potential surgery risk.99 In cases of partial bowel obstruction that do not respond to medical treatment, surgery can generally be planned (delayed emergency surgery) after the patient's situation is optimised (in terms of nutrition, immunosuppression and treatment for sepsis, as applicable).99,101,102
Emergency surgery is indicated in rare cases of complete bowel obstruction or suspicion of bowel ischaemia or peritonitis.103
Surgery is the preferred option in patients with localised ileocaecal CD (short strictures not eligible for endoscopic treatment) with symptoms of obstruction and no significant evidence of active inflammation.102
It is very important to balance the benefits of medical treatment and the risks of delaying surgery. Decisions should be made by a multidisciplinary team, since both medical and surgical treatment are equally valid, as shown in the LIRIC study.104
In a stricture due to CD, when should resection be performed and when should surgical strictureplasty be performed?- -
Resection: this is the most commonly used surgical technique (55% ileocaecal and 48% small-bowel); it is indicated in short strictures (in the ileocaecal region, small bowel and colon); in surgery-naïve patients; and in the presence or suspected presence of neoplastic transformation, dysplasia that cannot be resected endoscopically (colon), bleeding, anastomotic relapses and situations in which another surgical technique cannot be performed due to local conditions (very thickened mesentery, significant adhesion syndrome or involvement of other viscera). It is preferable for the resection to include the mesentery, provided that it is affected (thickened). The problem arises when multiple bowel resections must be performed over the course of the disease (relapses) or when major resections must be performed, resulting in loss of bowel function and ultimately a risk of short bowel syndrome. For this reason, resections should be limited and only the bowel segment that presents the complication, in this case the stricture, should be resected.12 To avoid massive resections, they can be combined with strictureplasties.
Bowel transit reconstruction following resection can be done with hand-sewn or mechanical, end-to-end or side-to-side anastomoses. These should be extensive and facilitate (diagnostic/therapeutic) endoscopic examination. Side-to-side and mechanical anastomoses have less suture dehiscence than end-to-end and hand-sewn ones.102 Recently published studies have pointed at performing a Kono-S antimesenteric functional end-to-end anastomosis.105 The main outcomes of resections are: 15% morbidity, 0%–9% further surgery, 0.9% mortality, 0.2% laparotomy,106 34%–40% clinical recurrence, 70%–100% endoscopic recurrence, 28%–45% surgical recurrence and 25%–35% overall repeat resections.107
In an anastomotic relapse, resection will be performed in cases in which strictureplasty cannot be done.108
- -
Strictureplasty: this is the surgical technique by which the diameter of the bowel lumen is increased, with no need for any resection. It is a safe, effective alternative to bowel resection, with similar rates of morbidity and mortality and lower rates of recurrence. It is indicated when technically feasible, in the following situations: (a) symptomatic strictures with no perforation, fistulas or suspicion of malignancy, (b) diffuse small-bowel involvement with multiple strictures (multifocal involvement or extensive involvement of a long bowel segment), (c) strictures in patients who have undergone prior small-bowel resection or who have short bowel syndrome, (d) rapid stricture recurrence, (e) anastomotic relapses and (f) undernutrition. Strictureplasties in the colon are not recommended due to the risk of malignant transformation at the level of the stricture. It may be technically difficult to perform in severe mesenteric or small-bowel inflammation.
The length of the stricture will determine the type of strictureplasty to be performed109 (Table 7).
Strictureplasty type by stricture length.
| Strictureplasty type | Stricture length |
|---|---|
| Conventional Heineke–Mikulicz (HM): HM, Judd, Moskel–Walske–Neumayer, Double HM, Ileocaecal HM | <10 cm |
| Intermediate procedures: Finney, Jaboulay, HM–Finney, Selvaggi, ileocolic Finney | 10−25 cm |
| Enteroenterostomies: Michelassi strictureplasty | >25 cm |
| Poggioli strictureplasty | |
| Sasaki strictureplasty | |
| Hotokezaka strictureplasty | |
| side-to-side isoperistaltic ileocolic strictureplasty |
The outcomes achieved with respect to strictureplasties were: 5%–23% morbidity, 28%–45% overall recurrence, 23%–52% surgical recurrence, 30% further surgery after five years and 75% further surgery after 10 years.108
In an anastomotic relapse it will be performed as the procedure of choice, whether in the small bowel or in the colon, provided that malignancy is not suspected.108
In colon strictures, is segmental resection enough, or is it imperative to perform more extensive resections?The available evidence indicates that segmental colectomy and total abdominal colectomy are comparable in terms of risk of recurrence and permanent stoma, but with a shorter time to recurrence for segmental resections.110,111
- -
Segmental resection: this is indicated in single short strictures (<20 cm) in an otherwise normal colon. This type of resection is preferable in proximal locations (ascending and transverse) with no distal involvement (rectoanal).102 An attempt to include the mesocolon in the resection should be made. It should not be performed in the presence of dysplasia, since dysplasia generally tends to be multifocal.
- -
One- or two-stage (sub)total colectomy with ileorectal anastomosis (TC-IRA): technique indicated in involvement of multiple colon segments or involvement of the associated distal part of the colon (descending or sigmoid colon).102 The rectum and anus should be spared and this occurs in 25%–50% of patients with colonic involvement. The patient should not present faecal incontinence prior to the operation.
- -
Total proctocolectomy with terminal ileostomy: This will be the technique of choice in multifocal involvement of the rectosigmoid colon and/or perianal disease.
Laparoscopy, whenever possible, should be the approach of choice in surgery for CD. It has been shown to reduce morbidity and decrease hospital stay length, adhesions and incisional hernias, thus improving cosmetic outcomes.101 The surgeon will decide upon the approach in each patient. Minimally invasive surgery has not been shown to reduce relapse rates compared to conventional surgery.112
Does intestinal bypass play a role in stricturing CD?A bypass is a surgical procedure that creates a diversion or alternative conduit that functions as a bridge between two parts. This technique creates a blind loop, which could carry an increased risk of bacterial overgrowth and disease worsening. Disease non-resection carries risks of progression, bleeding and perforation, as well as malignancy. Therefore, this technique should be used as a resource in conditions of the small bowel and colon. In upper gastrointestinal tract involvement, the most common reason for an indication for surgery is strictures (83%).113 Gastrojejunostomy with vagotomy114 (which is most commonly performed) is indicated in gastric (antral) and duodenal involvement. It has low rates of morbidity, with high rates of dumping syndrome, delayed gastric emptying and long-term marginal ulceration, as well as repeat obstruction.115 Vagotomy is not mandatory, must be personalised in each case and should be avoided in patients with chronic diarrhoea, a short bowel or a history of ileocaecal valve resection.115 Ileocolic bypass is performed as salvage surgery in patients with extensive bowel involvement that precludes limited resection and in patients in whom a stoma would lead to high output resulting in dehydration and undernutrition problems, thus enabling improvement of the patient's general condition and initiation of effective treatment to improve the disease.116 It can also act as a bridge to surgery, to improve the patient's general condition.
Fig. 3 shows a treatment algorithm for surgical management of strictures in CD.
GETECCU recommendations on the treatment of strictures in CDMedical treatment
- 1
Before any treatment in a stricture is proposed, it is important to conduct a full assessment of the patient's disease and stricture, including laboratory testing with inflammatory parameters, imaging to assess disease and stricture extent and a scale of symptoms of obstruction.
- 2
It is essential to maintain proper patient nutrition and even to assess the need for enteral nutrition and recommend a no-fibre diet or a low-fibre diet (insoluble fibre) with plenty of fluid intake.
- 3
In acute bowel obstruction, if a significant inflammatory component at the level of a stricture is confirmed, medical treatment that decreases oedema and improves symptoms of obstruction should be added. This treatment may initially involve steroids or TNF inhibitors.
- 4
In asymptomatic patients with a small-bowel stricture and patients with episodes of pain suggestive of intestinal subocclusion, medical treatment with TNF inhibitors may be proposed, provided that certain clinical criteria predictive of a good response to medical treatment are met:
- -
Short duration of symptoms of obstruction
- -
Stenosis length <12 cm
- -
Dilation proximal to stricture less than 29 mm
- -
Presence of hyperuptake in late-phase T1-weighted MRI
- -
Lack of fistulising disease
- -
- 5
The presence of dilation prior to a stricture greater than 30 mm decreases the probability of response to medical treatment.
- 6
If colon strictures cannot be passed with an endoscope, given the risk of dysplasia, they would be eligible for endoscopic or surgical treatment.
- 7
If an anastomotic stricture is short and accessible, endoscopic treatment is preferable to medical treatment.
- 1
EBD is the endoscopic technique of choice for short strictures in CD. The shorter the stricture, the better the EBD outcome.
- 2
There is no difference between dilating an anastomotic stricture and dilating a de novo stricture. The true determining factor of dilation success is stricture length.
- 3
It is advisable not to delay EBD once the patient begins to show symptoms of obstruction; it is also advisable to engage in accelerated intensification of medical treatment, since patients with more serious and/or advanced disease have worse outcomes.
- 4
Achievement of technical success in EBD predicts treatment success, which is directly tied to the diameter of the balloon used.
- 5
EBD is a safe technique, but safety must be balanced against the objective of achieving technical success, especially when choosing the diameter of the balloons to be used.
- 6
It is advisable to treat underlying inflammation at the stricture site before EBD in order to optimise the outcome thereof and prevent potential complications.
- 7
EBD is a reproducible technique in a routine clinical practice setting; unlike surgery, it appears to show no differences in terms of safety or efficacy across hospitals of varying healthcare complexity.
- 8
Although there is little evidence, EBD in upper gastrointestinal tract strictures has moderate efficacy and a good safety profile, and it may represent an alternative to surgery.
- 9
EBD by means of balloon-assisted enteroscopy is a safe and effective tool in the treatment of small-bowel strictures.
- 10
On a technical level, when EBD is performed, it is advisable to use a fluoroscope, make use of a long guide with a soft tip, insert a catheter or a Fogarty balloon over the guide through the stricture, inject contrast, perform sequential and progressive dilation, visualise the stricture through the balloon during EBD, and pass the stricture with the balloon partially inflated at the tip of the endoscope.
- 11
Surgery (both resection and strictureplasty) has a longer further surgery-free interval than EBD. EBD has a better safety profile than surgery, especially in evaluation in a routine clinical practice setting.
- 12
SEMS are the only ones that appear to have some usefulness in opposition to biodegradable stents.
- 13
SEMS should not be considered a first-line option in endoscopic treatment of strictures in CD.
- 14
SEMS could play a role in cases refractory to prior endoscopic treatment with EBD, in cases in which EBD was not possible and in longer strictures.
- 15
Given the limited scientific evidence on ES, the technical difficulty of the procedure and the absence of studies comparing ES to EBD, ES is not considered a first-line technique in cases of strictures in CD.
- 16
Patients with refractory short strictures who have exhibited a poor or partial response to dilation are considered eligible for ES alone or in combination with EBD.
- 17
ES should be performed by endoscopists with expertise in scalpel use, which limits the widespread use thereof at most hospitals.
- 18
The scientific evidence on injecting substances into strictures in CD, be they corticosteroids or TNF inhibitors, is very limited such that no firm recommendation may be made in this regard, although it does not appear to provide any clear prior benefits.
- 1
Symptomatic fibrocicatricial strictures with conservative medical or endoscopic treatment failure (strictures with nutritional repercussions, pre-stricture dilation >30 mm or otherwise unexplained associated anaemia) and asymptomatic strictures with suspicion of malignant transformation will be candidates for surgical treatment.
- 2
An acute bowel obstruction should be initially treated with conservative measures (fasting, intravenous hydration and a nasogastric tube).
- 3
Emergency surgery is indicated in rare cases of complete bowel obstruction or suspicion of bowel ischaemia or peritonitis.
- 4
Resections should be limited and only the bowel segment that presents strictures should be resected.
- 5
Resection is the most commonly used surgical technique and is indicated in short strictures (in the ileocaecal region, small bowel and colon); in surgery-naïve patients; and in the presence or suspected presence of neoplastic transformation, dysplasia that cannot be resected endoscopically (colon), bleeding, anastomotic relapses and situations in which another surgical technique cannot be performed due to local conditions.
- 6
Bowel transit reconstruction following resection can be done with hand-sewn or mechanical, end-to-end or side-to-side anastomoses.
- 7
It is recommended that an antimesenteric functional end-to-end hand-sewn anastomosis or a side-to-side mechanical anastomosis be performed and that all other configurations be avoided.
- 8
Surgical strictureplasty is the procedure of choice in long strictures in a surgery-naïve patient, in short strictures in patients with a history of bowel resection, in strictures of any length in patients at risk of a short bowel and in multifocal small-bowel disease. It is also indicated in strictureplasty relapses and in an anastomotic relapse either in the small bowel or in the colon, provided that malignancy is not suspected.
- 9
Segmental colectomy (single strictures) and (sub)total colectomy (multiple strictures) for the treatment of Crohn's disease in the colon are comparable in terms of risk of recurrence and permanent stoma.
- 10
Laparoscopy, whenever possible, should be the approach of choice in surgery for CD.
No funding was received for the preparation of this position statement.
Conflicts of interestCarme Loras has been a speaker or has received research funding from Boston Scientific.
Miriam Mañosa has been a speaker and consultant and has received research funding from MSD, AbbVie, Janssen, Kern Pharma, Takeda, Gillead, Pfizer, Ferring, Faes Farma, Shire Pharmaceuticals, Dr Falk Pharma, Chiesi and Adacyte.
Xavier Andújar has no conflicts of interest.
Vicente Sánchiz has no conflicts of interest.
Marc Martí-Gallostra has been a speaker for AbbVie and Takeda. Instructor, Master of Colorectal Nursing, Coloplast.
Yamile Zabana has been a speaker or has received research funding from AbbVie, MSD, Ferring, Amgen, Janssen, Pfizer, Dr Falk Pharma, Tillots and Galapagos.
Ana Gutiérrez has been a speaker or has received research funding from AbbVie, MSD, Kern, Ferring, FAES, Amgen, Roche, Sandoz, Janssen, Pfizer, Dr Falk Pharma, Tillots and Galapagos.
Manuel Barreiro-de Acosta has been a speaker and consultant and has received research funding from MSD, AbbVie, Janssen, Kern Pharma, Celltrion, Takeda, Gillead, Celgene, Pfizer, Ferring, Faes Farma, Shire Pharmaceuticals, Dr Falk Pharma, Chiesi, Gebro Pharma, Adacyte and Vifor Pharma.
Please cite this article as: Loras C, Mañosa M, Andújar X, Sánchiz V, Martí-Gallostra M, Zaban Y, et al., Documento de posicionamiento. Recomendaciones del grupo español de trabajo en enfermedad de Crohn y colitis ulcerosa (GETECCU) sobre el tratamiento de la estenosis en la enfermedad de Crohn. Gastroenterol Hepatol. 2022;45:315–334.