The resistance of Helicobacter pylori to antibiotics is a growing problem in Spain and eradication rates must be improved. The new Spanish consensus considers quadruple therapy with bismuth as first- or second-line therapy. This study evaluated the use of Pylera® in real-life clinical practice.
Patients and methodsA cross-sectional descriptive study was conducted to evaluate the eradication rate of H. pylori in patients treated with Pylera® between March and September 2016. Patients (naïve or with previous treatment failure) were treated for 10 days. Eradication was confirmed using a breath test with urea 30 days or more after treatment. In addition, demographic, clinical–analytical and treatment-related data were collected.
ResultsA total of 185 patients were included (51.6±16.19 years); 63.8% were women and 9.2% had a family history of gastric cancer. The most frequent indication was dyspepsia (55.1%). Approximately 57.8% received Pylera® as first-line therapy, while 95.7% received Pylera® in combination with omeprazole. A first-line eradication rate of 78.15% was observed in the intention-to-treat population (86.6% per protocol). There were no statistically significant differences between naïve patients and those previously treated. Nine patients abandoned the treatment (4.9%), 7 due to mild side effects and 2 due to incorrect dosing.
ConclusionsPylera® has acceptable eradication rates in first- and second-line therapy and shows a suitable safety profile.
La resistencia de Helicobacter pylori a los antibióticos es un problema creciente en nuestro medio y es necesario mejorar las tasas de erradicación. La cuádruple terapia con bismuto se ha considerado el tratamiento de primera o segunda línea en el nuevo consenso. Este estudio evalúa el uso de Pylera® en un escenario clínico real.
Pacientes y métodosSe llevó a cabo un estudio descriptivo transversal, entre marzo y septiembre de 2016, para evaluar los porcentajes de erradicación de Helicobacter pylori en pacientes tratados con Pylera®. Los pacientes (naïve o con fallo a terapias previas) fueron tratados durante 10 días. La erradicación fue confirmada usando un test del aliento con urea al menos 30 días después de la finalización del tratamiento. Además se recogieron datos demográficos, clínico-analíticos y relacionados con el tratamiento.
ResultadosFueron incluidos 185 pacientes (51,6±16,19 años); el 63,8% fueron mujeres y el 9,2% tenían historia familiar de cáncer gástrico. La indicación más frecuente fue la dispepsia (55,1%). El 57,8% recibieron Pylera® como primera línea de tratamiento. El 95,7% asociaron omeprazol. Se detectó una tasa de erradicación en primera línea de tratamiento del 78,15% por intención de tratar (86,6% por protocolo). No hubo diferencias estadísticamente significativas entre pacientes naïve y los tratados previamente. Nueve pacientes abandonaron el tratamiento (4,9%), 7 debido a efectos secundarios leves y 2 por toma incorrecta.
ConclusionesPylera® tiene unas aceptables tasas de erradicación como primera y segunda línea de tratamiento y muestra un adecuado perfil de seguridad.
Helicobacter pylori is one of the most common bacterial infections in the world and affects 50% of the Spanish population. It is known to be associated with chronic gastritis, stomach ulcer and stomach cancer.1 In previous Spanish consensus documents,2–4 a H. pylori eradication rate of at least 80% was established as acceptable for the different antibiotic regimens. However, the most recent Spanish consensus document increases that rate to 90%.1 Moreover, triple therapy has been ruled out as the first line of treatment as its effectiveness has been reduced by increasing resistance, with the eradication rate reported to have fallen to around 70%.5,6
Bismuth-containing quadruple therapy has been proposed as a potential initial alternative, once its efficacy has been confirmed here in Spain.1 There is no evidence to suggest that H. pylori is resistant to bismuth salts, and resistance to tetracyclines has only very rarely been reported.7 A new dosage form of the bismuth quadruple therapy (Pylera®) has recently been marketed, consisting of three antibiotics within the same capsule: 140mg bismuth subcitrate, 125mg tetracycline hydrochloride and 125mg metronidazole.8 This formula is more convenient for patients and could therefore improve adherence to treatment. A European multicentre trial shows a 90% eradication rate with this formulation9 and, in 2014, Delchier et al. reported eradication rates of 93.2–93.8% by intention-to-treat (ITT) analysis in patients with prior treatment failure.10
The aim of this study was to provide a true picture of use of the bismuth quadruple therapy with the new formulation (Pylera®), used as first- and second-line treatment in Spain. Few studies have been published to date on the use of this drug in actual clinical practice in Spain.
Our primary endpoint was to determine the eradication rate in our area in a heterogeneous population treated with Pylera®. We also analysed the characteristics of patients with H. pylori infection treated with Pylera® in Spain and attempted to determine the rate of intolerance to the drug, as well as the variables predictive of treatment response.
Patients and methodsStudy design and patient screeningWe conducted a descriptive, cross-sectional study in the Gastroenterology Department of the tertiary Hospital Rey Juan Carlos in Madrid, Spain from March to September 2016 to determine the H. pylori eradication rate in actual clinical practice.
We used the following inclusion criteria: (1) adult patients (age≥18); (2) diagnosed with H. pylori infection; (3) had not received previous treatment or had prior failed treatment; and (4) were treated with Pylera®.
H. pylori infection was detected by using the urea breath test (which requires the following preparation: no antibiotic treatment for at least the previous month, no treatment with proton pump inhibitors (PPI) in the previous two weeks and no smoking or brushing teeth on the morning of the test) or by histological analysis of gastric mucosa biopsies. Antibiotic resistance was not studied by sample culture. All patients with a positive result were administered Pylera® in the form of three capsules every 6h for a total of ten days. We added a PPI to the treatment, as instructed in the Pylera® summary of product characteristics. Eradication was measured at least 30 days after the end of the treatment by urea breath test.
Statistical analysisThe following information was obtained for each patient included in the study: demographic data (age, stratified into under or over 65; gender, male or female; and whether or not they had a family history of stomach cancer), clinical and analytical variables (H. pylori detection method: gastroscopy with biopsies or urea breath test; endoscopic findings; and whether or not a peptic ulcer was present, metaplasia in pathology samples, iron deficiency anaemia, thrombocytopenia or vitamin B12 deficiency) and variables related to eradication treatment (line of treatment; treatment duration; dosage frequency; type and dose of PPI added; addition of probiotics to treatment; and eradication rates). For the patients taking Pylera® in second and third line, we reviewed their previously tried eradication treatments.
Quantitative variables are expressed with measures of central tendency and dispersion (mean, standard deviation and intervals) and qualitative variables, using frequencies and percentages. Qualitative variables were compared using the Chi-square statistical test (or Fisher's nonparametric test).
Risk was estimated by determining the odds ratio and the confidence interval in the univariate analysis. Last of all, we used logistic regression for a multivariate analysis to determine which variables were associated with a higher H. pylori eradication rate. All analyses were performed using the SPSS v. 21.0 statistics package.
Ethical considerationsThe study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Before the research began, the study was approved by our institution's Ethics Committee.
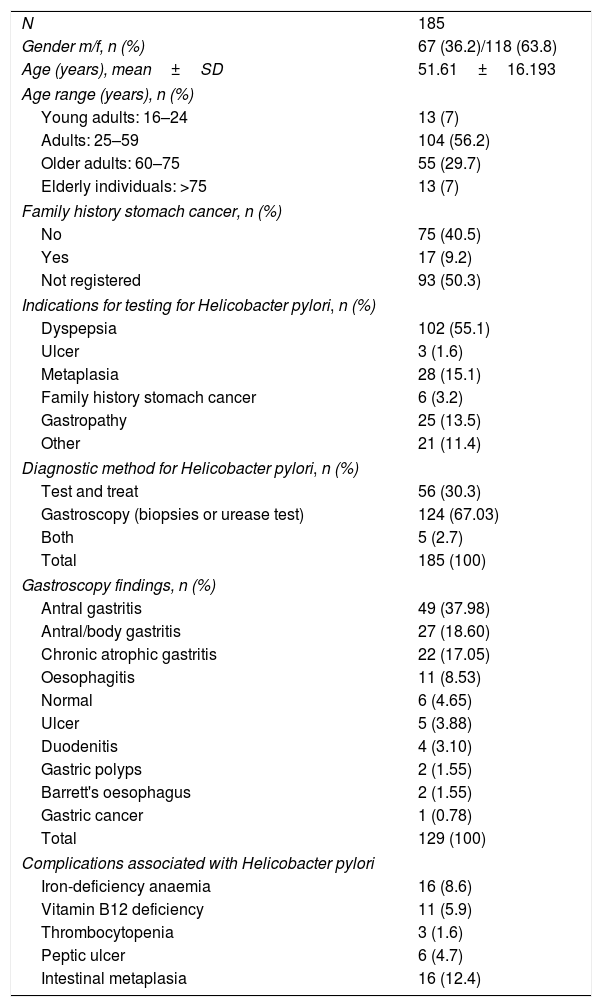
ResultsA total of 185 patients were included, with a mean age of 51.6 (SD 16.19). They were predominantly female (n=118, 63.8%) and 9.2% (n=17) had a family history of stomach cancer. The characteristics of our sample are shown in Table 1. The most common indication was dyspepsia (55.1%). The H. pylori infection diagnosis was made by gastroscopy in the majority of cases (n=129, 69.7%), but the urea breath test was also used (n=50, 30.3%) and, in some cases, both methods (n=5, 2.7%) were used. The most common endoscopic findings were antral gastritis (37.98%), antral/body gastritis (18.60%) and chronic atrophic gastritis (17.05%). Sixteen patients had intestinal metaplasia (12.4%) and gastric cancer was detected in one patient (0.7%). As far as complications of H. pylori infection were concerned, three patients had thrombocytopenia (1.6%), 11 had vitamin B12 deficiency (5.9%), 16 iron deficiency anaemia (8.6%) and six had a gastric ulcer (4.7% of those who had an endoscopy).
General characteristics of the sample.
| N | 185 |
| Gender m/f, n (%) | 67 (36.2)/118 (63.8) |
| Age (years), mean±SD | 51.61±16.193 |
| Age range (years), n (%) | |
| Young adults: 16–24 | 13 (7) |
| Adults: 25–59 | 104 (56.2) |
| Older adults: 60–75 | 55 (29.7) |
| Elderly individuals: >75 | 13 (7) |
| Family history stomach cancer, n (%) | |
| No | 75 (40.5) |
| Yes | 17 (9.2) |
| Not registered | 93 (50.3) |
| Indications for testing for Helicobacter pylori, n (%) | |
| Dyspepsia | 102 (55.1) |
| Ulcer | 3 (1.6) |
| Metaplasia | 28 (15.1) |
| Family history stomach cancer | 6 (3.2) |
| Gastropathy | 25 (13.5) |
| Other | 21 (11.4) |
| Diagnostic method for Helicobacter pylori, n (%) | |
| Test and treat | 56 (30.3) |
| Gastroscopy (biopsies or urease test) | 124 (67.03) |
| Both | 5 (2.7) |
| Total | 185 (100) |
| Gastroscopy findings, n (%) | |
| Antral gastritis | 49 (37.98) |
| Antral/body gastritis | 27 (18.60) |
| Chronic atrophic gastritis | 22 (17.05) |
| Oesophagitis | 11 (8.53) |
| Normal | 6 (4.65) |
| Ulcer | 5 (3.88) |
| Duodenitis | 4 (3.10) |
| Gastric polyps | 2 (1.55) |
| Barrett's oesophagus | 2 (1.55) |
| Gastric cancer | 1 (0.78) |
| Total | 129 (100) |
| Complications associated with Helicobacter pylori | |
| Iron-deficiency anaemia | 16 (8.6) |
| Vitamin B12 deficiency | 11 (5.9) |
| Thrombocytopenia | 3 (1.6) |
| Peptic ulcer | 6 (4.7) |
| Intestinal metaplasia | 16 (12.4) |
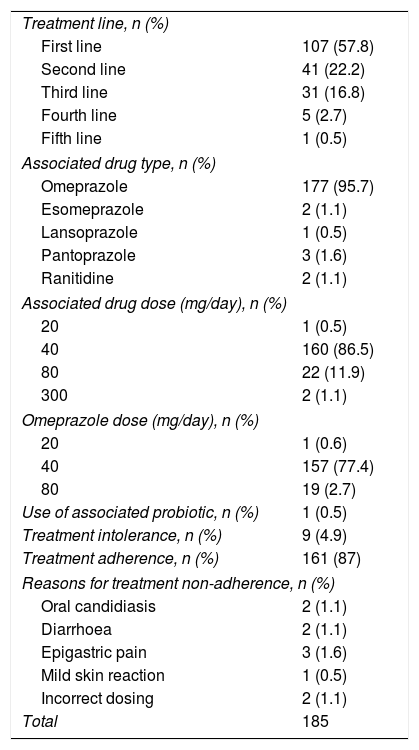
Data on the characteristics of the treatment are shown in Table 2. All patients took Pylera®, three capsules every 6h for 10 days; it was used as first-line treatment in the majority of cases (57.8%). The most common PPI to be added to the treatment was omeprazole (n=177, 95.7%), at 20mg every 12h (86.5%). We investigated the use of associated probiotics, but it was not possible to determine their influence on eradication rates, as only one patient was taking them. Nine patients did not complete the treatment (4.9%), seven of them due to side effects and two due to incorrect treatment. The side effects were mild in all seven affected patients (oral candidiasis in two, diarrhoea in two, stomach pain in another two and a mild skin reaction in one); none of the side effects were serious.
General characteristics of the treatment.
| Treatment line, n (%) | |
| First line | 107 (57.8) |
| Second line | 41 (22.2) |
| Third line | 31 (16.8) |
| Fourth line | 5 (2.7) |
| Fifth line | 1 (0.5) |
| Associated drug type, n (%) | |
| Omeprazole | 177 (95.7) |
| Esomeprazole | 2 (1.1) |
| Lansoprazole | 1 (0.5) |
| Pantoprazole | 3 (1.6) |
| Ranitidine | 2 (1.1) |
| Associated drug dose (mg/day), n (%) | |
| 20 | 1 (0.5) |
| 40 | 160 (86.5) |
| 80 | 22 (11.9) |
| 300 | 2 (1.1) |
| Omeprazole dose (mg/day), n (%) | |
| 20 | 1 (0.6) |
| 40 | 157 (77.4) |
| 80 | 19 (2.7) |
| Use of associated probiotic, n (%) | 1 (0.5) |
| Treatment intolerance, n (%) | 9 (4.9) |
| Treatment adherence, n (%) | 161 (87) |
| Reasons for treatment non-adherence, n (%) | |
| Oral candidiasis | 2 (1.1) |
| Diarrhoea | 2 (1.1) |
| Epigastric pain | 3 (1.6) |
| Mild skin reaction | 1 (0.5) |
| Incorrect dosing | 2 (1.1) |
| Total | 185 |
For the patients taking Pylera® as second- and third-line therapy, we reviewed the eradication treatments they had tried previously.
Of the patients who took bismuth-containing quadruple therapy as second-line (n=41), 30 were found to have previously taken omeprazole–clarithromycin–amoxicillin for 10 days; one, omeprazole–clarithromycin–amoxicillin for seven days; one, omeprazole–clarithromycin–amoxicillin for 14 days; one, omeprazole–clarithromycin–metronidazole for 10 days; one, omeprazole–levofloxacin–amoxicillin for 10 days; and five, omeprazole–clarithromycin–amoxicillin–metronidazole for 14 days.
The patients who took bismuth-containing quadruple therapy as third-line (n=31) were found to have taken the following first-line treatments: 26 patients, omeprazole-clarithromycin-amoxicillin for 10 days; one, pantoprazole–clarithromycin–metronidazole for 10 days; one, esomeprazole–amoxicillin–clarithromycin–bismuth for 14 days; and the first-line treatment could not be determined in two. They were also found to have taken the following second-line treatments: 18 patients, omeprazole–levofloxacin–amoxicillin for 10 days; three, esomeprazole–levofloxacin–amoxicillin for 10 days; one, omeprazole–clarithromycin–amoxicillin for 10 days; three, esomeprazole–levofloxacin–amoxicillin–bismuth for 14 days; one, omeprazole–clarithromycin–levofloxacin for 10 days; one, pantoprazole–levofloxacin–metronidazole for 10 days; and the second-line treatment could not be determined in three.
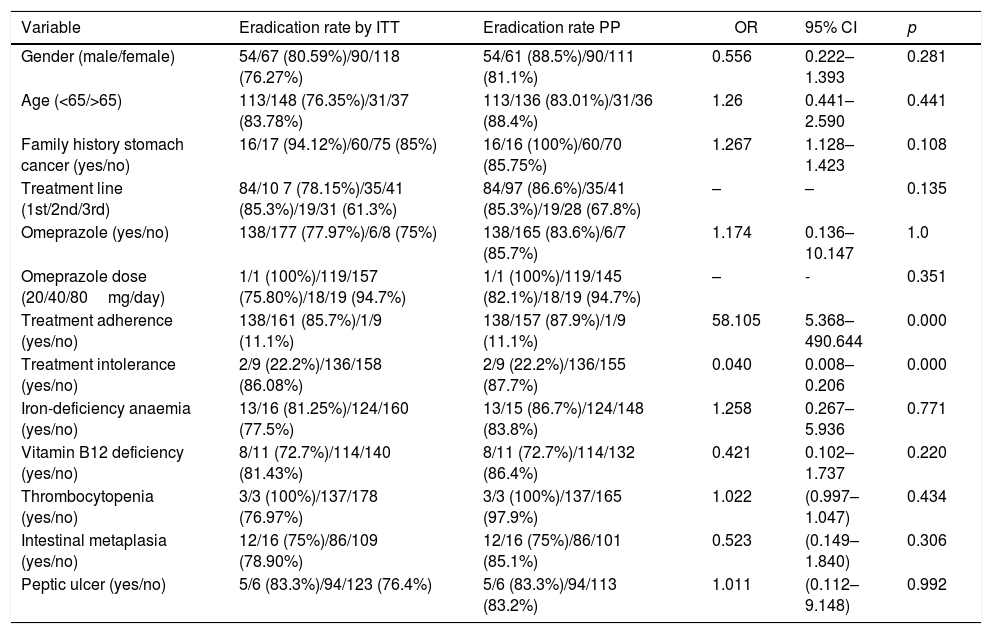
In the univariate analysis (Table 3), a higher eradication rate was found among males (n=54/67, 80.6% by ITT/n=54/61, 88.5% per protocol [PP]), among patients aged over 65 (n=31/37, 83.8% by ITT/n=31/36, 88.4% PP) and among patients with a family history of stomach cancer (n=16/17, 83.78% by ITT/n=16/16, 100% PP), although none of these variables showed statistical significance. A higher eradication rate was found with second-line treatment (n=35/41, 85.3% by ITT and PP) than with first-line (n=84/107, 78.15% by ITT and n=84/97, 86.6% PP) or third-line (n=19/31, 61.3% by ITT and n=19/28, 67.9% PP), but the difference was not statistically significant (p=0.135). As the majority of patients were treated with omeprazole, we were unable to carry out an adequate comparative study between different PPI. However, we were able to compare the treatment response rates with the different doses of omeprazole used. No statistically significant differences were found (p=0.351), but there was a trend towards higher eradication rates the greater the degree of acid inhibition: with 20mg every 12h, there was 75.8% success by ITT and 82.1% PP; and with 40mg every 12h, there was 94.7% success by ITT and PP. Statistical significance was found in the eradication rate in patients with adequate therapeutic compliance (85.7% by ITT and 87.9% PP, p=0.000) and in those without treatment intolerance (86.8% by ITT and 87.7% PP, p=0.000).
Univariate analysis.
| Variable | Eradication rate by ITT | Eradication rate PP | OR | 95% CI | p |
|---|---|---|---|---|---|
| Gender (male/female) | 54/67 (80.59%)/90/118 (76.27%) | 54/61 (88.5%)/90/111 (81.1%) | 0.556 | 0.222–1.393 | 0.281 |
| Age (<65/>65) | 113/148 (76.35%)/31/37 (83.78%) | 113/136 (83.01%)/31/36 (88.4%) | 1.26 | 0.441–2.590 | 0.441 |
| Family history stomach cancer (yes/no) | 16/17 (94.12%)/60/75 (85%) | 16/16 (100%)/60/70 (85.75%) | 1.267 | 1.128–1.423 | 0.108 |
| Treatment line (1st/2nd/3rd) | 84/10 7 (78.15%)/35/41 (85.3%)/19/31 (61.3%) | 84/97 (86.6%)/35/41 (85.3%)/19/28 (67.8%) | – | – | 0.135 |
| Omeprazole (yes/no) | 138/177 (77.97%)/6/8 (75%) | 138/165 (83.6%)/6/7 (85.7%) | 1.174 | 0.136–10.147 | 1.0 |
| Omeprazole dose (20/40/80mg/day) | 1/1 (100%)/119/157 (75.80%)/18/19 (94.7%) | 1/1 (100%)/119/145 (82.1%)/18/19 (94.7%) | – | - | 0.351 |
| Treatment adherence (yes/no) | 138/161 (85.7%)/1/9 (11.1%) | 138/157 (87.9%)/1/9 (11.1%) | 58.105 | 5.368–490.644 | 0.000 |
| Treatment intolerance (yes/no) | 2/9 (22.2%)/136/158 (86.08%) | 2/9 (22.2%)/136/155 (87.7%) | 0.040 | 0.008–0.206 | 0.000 |
| Iron-deficiency anaemia (yes/no) | 13/16 (81.25%)/124/160 (77.5%) | 13/15 (86.7%)/124/148 (83.8%) | 1.258 | 0.267–5.936 | 0.771 |
| Vitamin B12 deficiency (yes/no) | 8/11 (72.7%)/114/140 (81.43%) | 8/11 (72.7%)/114/132 (86.4%) | 0.421 | 0.102–1.737 | 0.220 |
| Thrombocytopenia (yes/no) | 3/3 (100%)/137/178 (76.97%) | 3/3 (100%)/137/165 (97.9%) | 1.022 | (0.997–1.047) | 0.434 |
| Intestinal metaplasia (yes/no) | 12/16 (75%)/86/109 (78.90%) | 12/16 (75%)/86/101 (85.1%) | 0.523 | (0.149–1.840) | 0.306 |
| Peptic ulcer (yes/no) | 5/6 (83.3%)/94/123 (76.4%) | 5/6 (83.3%)/94/113 (83.2%) | 1.011 | (0.112–9.148) | 0.992 |
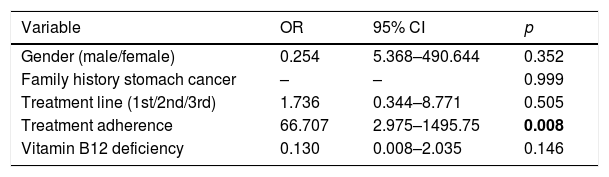
In the multivariate analysis (Table 4) statistical significance was found in the eradication rate for patients who completed the treatment (OR 66.7, 95% CI 2.98–1495.75, p=0.008).
DiscussionThe latest Spanish consensus document for the treatment of H. pylori infection reports that it is an ever-increasing health problem with an estimated worldwide prevalence of up to 50%.1 Eradication therapies used in the past provided suboptimal eradication rates, largely due to the increase in resistance to antibiotics, such as clarithromycin, used in previous treatments. Resistance here in Spain reached 14% in 200910 and 18% in 2013,5 and, in western-central and southern parts of Europe, it reached over 20%.11
The treatment algorithm in Spain has been modified in the last year through the creation of a new consensus document1 based on the latest available evidence. The new algorithm introduces bismuth-containing quadruple therapy as a treatment that should be considered from the moment H. pylori infection is diagnosed. Pylera® is included in the treatment algorithm as second-line therapy for patients who are not allergic to penicillin, and may be considered as first-line once its efficacy is confirmed here in Spain.
The single dosage form Pylera® has only recently been marketed in Spain. This is a positive step and should be more convenient for patients, possibly leading to an improvement in treatment adherence. In terms of efficacy, 90% eradication success was demonstrated in a multicentre European clinical trial in 20119 and in a multicentre study in 2014.10 Recently published data on a sample of 131 patients in routine clinical practice in Italy show an overall efficacy of 94.7% by ITT.12 All the above data make Pylera® very attractive as an alternative treatment option.
Our results show that first-line treatment with Pylera®, 3 tablets every 6h for 10 days, achieved a lower eradication rate than those described in the aforementioned studies (78.15% by ITT and 86.6% PP). Three different meta-analyses previously found similar eradication rates with bismuth-containing quadruple therapy (81%, 78% and 77%), there being no benefit compared to triple therapy. However, these were seven-day treatments and did not use the single dosage form, so they cannot be considered fully comparable with our study.13–15 A multicentre phase III European clinical trial demonstrated efficacy of 93% PP with the use of Pylera®,9 and a US multicentre study, an efficacy of 88% by ITT.16 It is possible that the significant difference with our study is a consequence of the high rates of metronidazole resistance, as data published in 2013 point to an average resistance rate of 41% to this antibiotic in Spain.5 However, we did not perform cultures for the detection of antibiotic resistance in our study.
In short, in our sample, naïve patients treated with Pylera® achieved an optimal eradication rate of 86.6% PP (78% by ITT), which could support the use of such treatment in first-line. The previously treated patients who received Pylera® as second-line also achieved an acceptable efficacy rate of 85.3% by ITT and PP. The use of Pylera® can therefore be considered in both first- and second-line treatment. However, its use may be limited in third-line treatment, as much lower eradication rates were obtained (61.3% by ITT and 67.9% PP).
The data from the European registry on the management of H. pylori infection, published in 2018 as an abstract at the annual meeting of the Spanish Association of Gastroenterology,17–19 showed somewhat better results in terms of the effectiveness of Pylera® treatment, mainly in first- and third-line. In first-line, an efficacy of 92% was reported by ITT (n=738 patients) and 95% PP (n=706 patients).17 In second-line, they reported an efficacy closer to that of our study, 76% by ITT (n=169 patients) and 91% PP.18 Lastly, in third-line, 81% efficacy was reported by ITT (n=110 patients) and 82% PP.19 With data from this same registry, another abstract20 was published with 1258 patients treated with Pylera® where the analysed data showed: in first-line, efficacy of 92% by ITT (n=666) and 95% PP (n=633); in second-line, efficacy of 89% by ITT (n=273) and 92% PP (n=260); and, in third-line, efficacy of 82% by ITT (n=151) and 85% PP (n=142). The better efficacy data in that study can possibly be explained by a much larger sample than in our study and because it is a European registry, which includes other populations with different antibiotic-resistance profiles.
Previous efficacy studies with Pylera®,9 as instructed in the product's summary of product characteristics,8 combined treatment with standard doses of omeprazole every 12h. However, in the recent preliminary results published as abstracts at the annual meeting of the Spanish Association of Gastroenterology in 2017,21,22 the treatment combined with esomeprazole at double dose every 12h shows higher eradication rates: up to 91.4% PP in first-line and 85.9% in third-line treatment. In our study, the majority of patients were treated with omeprazole, with very few on other PPI. However, we found in our analysis that 20mg of omeprazole every 12h had an efficacy of 75.8% for ITT and 82.1% PP, and 40mg every 12h improved the eradication rate to 94.7% for both ITT and PP. Although these differences were not statistically significant, it seems reasonable to assume that the more potent the acid inhibition, the better the efficacy of the treatment.
Pylera® would seem to have an adequate safety profile. In our sample, only mild side effects were recorded in seven patients (3.8%) and none were serious. In view of the results from other series, however, such as the one recently published in Italy12 reporting 26.7% affected by mild side effects, and the European multicentre trial that reports a rate of 47%, our sample may have had a significant bias in the detection of side effects, perhaps because they were considered of little relevance and not properly reflected by the gastroenterologists in the medical records; they were only registered for patients who did not complete the treatment.
In our series, treatment adherence was achieved in 87% of patients, and was the only factor to have a statistically significant effect on the improvement of eradication rates. Our results showed low side effect rates associated with high rates of treatment adherence. Last of all, no significant correlation was found between any other variable and eradication rates, enabling us to consider treatment with Pylera® for a broad spectrum of patients.
The main limitations of this study are related to the sample size, which may have restricted our conclusions. However, we present the first actual clinical practice study in Spain, providing an initial evaluation of Pylera® treatment in our area.
To sum up, we conclude that treatment with Pylera® has acceptable eradication rates for first- and second-line therapy in Spain. The eradication rates can be further improved if we combine it with higher doses of omeprazole than those stated in the summary of product characteristics. This treatment has an adequate safety profile and a high treatment adherence rate. We therefore believe Pylera® can be considered for H. pylori infection in a broad spectrum of patients. However, more clinical studies with larger sample sizes are needed to confirm these results.
AuthorshipDr Sandra Agudo-Fernández designed the project, participated in the collection of data and the analysis and interpretation of the results, and wrote this article.
Dr Ana González Blanco participated in the collection of data and the analysis of the results, and carried out a critical review of the study.
Conflicts of interestThe authors of this study declare that they have no conflicts of interest and that this research did not receive any specific grants from public sector or commercial sector agencies or non-profit organisations.
Please cite this article as: Agudo-Fernández S, González Blanco A. Análisis retrospectivo del uso de la cuádruple terapia con bismuto (Pylera®) en la práctica clínica real en España. Gastroenterol Hepatol. 2018;41:483–489.