Eradication of the hepatitis C virus with interferon-free therapies (DAAs) has modified the course of the disease, as the rate of patients with compensated cirrhosis who achieve a sustained virological response exceeds 95%. However, the impact on development of hepatocellular carcinoma (HCC) is currently in dispute. This argument could be divided into three key points: the impact of DAA on rate of HCC recurrence, the temporal link between starting DAAs and HCC recurrence, and finally, the aggressive pattern of HCC. Therefore, the aim of this review is to analyse the available results in this population of patients from a clinical perspective where the risks and benefits of HCV eradication with DAA therapies are evaluated in patients with complete response of HCC.
La erradicación del virus de la hepatitis C con esquemas libres de interferón (AAD) han modificado la evolución de la enfermedad ya que más del 95% de los pacientes con cirrosis compensada logran la respuesta virológica sostenida. Sin embargo, el impacto de la erradicación del VHC sobre el desarrollo del carcinoma hepatocelular (CHC) es controvertido. Dicha controversia podría dividirse en tres aspectos fundamentales: el impacto del AAD en la tasa de recurrencia del CHC, la asociación temporal entre el inicio de AAD y el desarrollo de la recurrencia del CHC y finalmente la agresividad de los CHC. Es por ello que esta revisión tiene por objetivo analizar los resultados disponibles en esta población de pacientes desde una perspectiva clínica donde se valoran los riesgos/beneficios de erradicar el VHC con AAD en el contexto de pacientes con CHC en respuesta completa.
Patients with chronic liver disease in the cirrhosis stage are at risk of developing complications and liver decompensation regardless of the aetiology. It is for this reason that the main objective of all physicians working to manage these patients is to eliminate the aetiological agent (if possible) or to prevent the development of complications using prophylactic measures. In the case of patients with hepatitis C virus (HCV), the approval of the interferon-free treatments known internationally as direct-acting antivirals (DAAs) allows HCV to be eradicated and a sustained virologic response (SVR) to be achieved in some 90% of patients who are compensated (Child-Pugh A) and in 81.6% of patients who are decompensated (Child-Pugh B and C) at the time of starting DAAs.1–3
The inclusion of DAAs has enabled the medical community to consider HCV eradication as an adjuvant treatment option to lower the rate of recurrence of hepatocellular carcinoma (HCC). Nevertheless, in 2016 a clinical observation and subsequent confirmation in a multicentre/retrospective study led by ourselves from the Hospital Clínic de Barcelona’s Hepatic Oncology and Viral Hepatitis Units alerted the scientific community to the possible time-related association (time between start of treatment with DAAs and development of HCC recurrence), as well as the presence of a rate of recurrence above that usually observed in patients with a documented complete response (CR).4–12 Simultaneously, another Italian cohort with similar characteristics described near-identical results.13
More than two years have gone by since these publications and researchers around the world have echoed them, assessing the impact of interferon-free treatments in retrospective data cohorts designed with other objectives or launching prospective cohorts with the aim of analysing the development of HCC recurrence.
The objective of this review was therefore to critically analyse the available results rather than simply transcribing the conclusions of the published manuscripts. In so doing, the reader will be able to identify that there are some interpretations of the original results that do not precisely match the authors’ conclusions. This allows us to review the literature from a clinical perspective, assessing the risks/benefits of eradicating HCV in the context of a patient with hepatocellular carcinoma in CR.
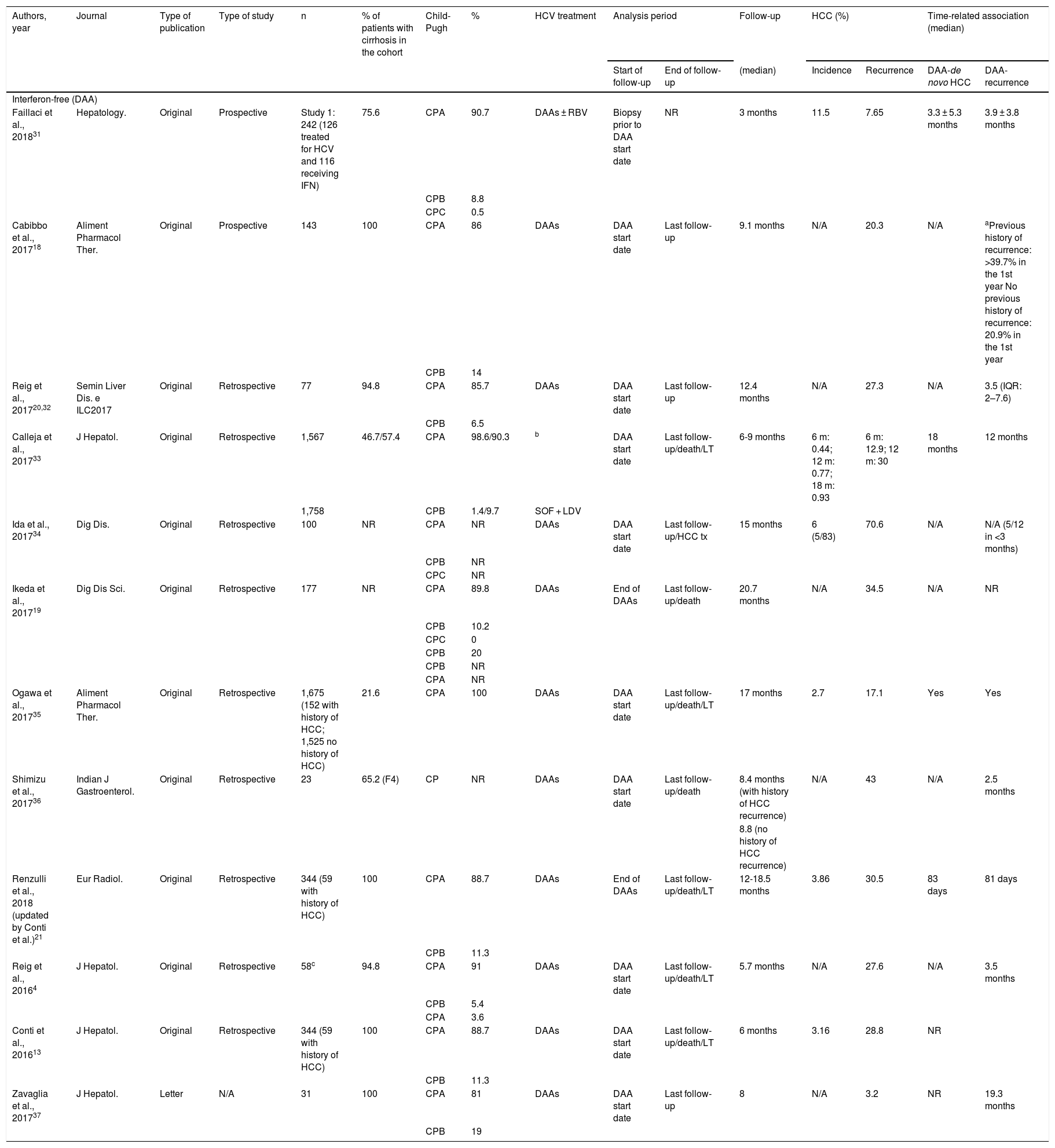
Table 1 describes the characteristics of all publications in relation to HCC recurrence, the methodology used and the results observed.
Publications that assess the incidence of recurrence of hepatocellular carcinoma in patients treated with regimens that include interferon-free treatments (DAAs) and those including interferon.
| Authors, year | Journal | Type of publication | Type of study | n | % of patients with cirrhosis in the cohort | Child-Pugh | % | HCV treatment | Analysis period | Follow-up | HCC (%) | Time-related association (median) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Start of follow-up | End of follow-up | (median) | Incidence | Recurrence | DAA-de novo HCC | DAA-recurrence | |||||||||
| Interferon-free (DAA) | |||||||||||||||
| Faillaci et al., 201831 | Hepatology. | Original | Prospective | Study 1: 242 (126 treated for HCV and 116 receiving IFN) | 75.6 | CPA | 90.7 | DAAs ± RBV | Biopsy prior to DAA start date | NR | 3 months | 11.5 | 7.65 | 3.3 ± 5.3 months | 3.9 ± 3.8 months |
| CPB | 8.8 | ||||||||||||||
| CPC | 0.5 | ||||||||||||||
| Cabibbo et al., 201718 | Aliment Pharmacol Ther. | Original | Prospective | 143 | 100 | CPA | 86 | DAAs | DAA start date | Last follow-up | 9.1 months | N/A | 20.3 | N/A | aPrevious history of recurrence: >39.7% in the 1st year No previous history of recurrence: 20.9% in the 1st year |
| CPB | 14 | ||||||||||||||
| Reig et al., 201720,32 | Semin Liver Dis. e ILC2017 | Original | Retrospective | 77 | 94.8 | CPA | 85.7 | DAAs | DAA start date | Last follow-up | 12.4 months | N/A | 27.3 | N/A | 3.5 (IQR: 2–7.6) |
| CPB | 6.5 | ||||||||||||||
| Calleja et al., 201733 | J Hepatol. | Original | Retrospective | 1,567 | 46.7/57.4 | CPA | 98.6/90.3 | b | DAA start date | Last follow-up/death/LT | 6-9 months | 6 m: 0.44; 12 m: 0.77; 18 m: 0.93 | 6 m: 12.9; 12 m: 30 | 18 months | 12 months |
| 1,758 | CPB | 1.4/9.7 | SOF + LDV | ||||||||||||
| Ida et al., 201734 | Dig Dis. | Original | Retrospective | 100 | NR | CPA | NR | DAAs | DAA start date | Last follow-up/HCC tx | 15 months | 6 (5/83) | 70.6 | N/A | N/A (5/12 in <3 months) |
| CPB | NR | ||||||||||||||
| CPC | NR | ||||||||||||||
| Ikeda et al., 201719 | Dig Dis Sci. | Original | Retrospective | 177 | NR | CPA | 89.8 | DAAs | End of DAAs | Last follow-up/death | 20.7 months | N/A | 34.5 | N/A | NR |
| CPB | 10.2 | ||||||||||||||
| CPC | 0 | ||||||||||||||
| CPB | 20 | ||||||||||||||
| CPB | NR | ||||||||||||||
| CPA | NR | ||||||||||||||
| Ogawa et al., 201735 | Aliment Pharmacol Ther. | Original | Retrospective | 1,675 (152 with history of HCC; 1,525 no history of HCC) | 21.6 | CPA | 100 | DAAs | DAA start date | Last follow-up/death/LT | 17 months | 2.7 | 17.1 | Yes | Yes |
| Shimizu et al., 201736 | Indian J Gastroenterol. | Original | Retrospective | 23 | 65.2 (F4) | CP | NR | DAAs | DAA start date | Last follow-up/death | 8.4 months (with history of HCC recurrence) | N/A | 43 | N/A | 2.5 months |
| 8.8 (no history of HCC recurrence) | |||||||||||||||
| Renzulli et al., 2018 (updated by Conti et al.)21 | Eur Radiol. | Original | Retrospective | 344 (59 with history of HCC) | 100 | CPA | 88.7 | DAAs | End of DAAs | Last follow-up/death/LT | 12-18.5 months | 3.86 | 30.5 | 83 days | 81 days |
| CPB | 11.3 | ||||||||||||||
| Reig et al., 20164 | J Hepatol. | Original | Retrospective | 58c | 94.8 | CPA | 91 | DAAs | DAA start date | Last follow-up/death/LT | 5.7 months | N/A | 27.6 | N/A | 3.5 months |
| CPB | 5.4 | ||||||||||||||
| CPA | 3.6 | ||||||||||||||
| Conti et al., 201613 | J Hepatol. | Original | Retrospective | 344 (59 with history of HCC) | 100 | CPA | 88.7 | DAAs | DAA start date | Last follow-up/death/LT | 6 months | 3.16 | 28.8 | NR | |
| CPB | 11.3 | ||||||||||||||
| Zavaglia et al., 201737 | J Hepatol. | Letter | N/A | 31 | 100 | CPA | 81 | DAAs | DAA start date | Last follow-up | 8 | N/A | 3.2 | NR | 19.3 months |
| CPB | 19 | ||||||||||||||
| Treatment with interferon or interferon-free treatments (DAAs) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adhoute et al., 201828 | Eur J Gastroenterol Hepatol. | Original | Retrospective (propensity score) | 22 | 100 | CPA | 82 | DAAs | DAA start date | Last follow-up/death | 19 months | N/A | 41 | N/A | 7 months |
| 49 (controls) | CPB | 18 | No HCV treatment | 19 months | 35 | ||||||||||
| Mashiba et al., 201838 | PloS One | Original | Retrospective (propensity score) | 148 | N/A (FIB score) | CPA | N/A | IFN | DAA end date | Last follow-up/death | 25.5 months | N/A | NR | N/A | NR |
| 368 | CPB | N/A | DAAs | 7.7 months | NR | ||||||||||
| Merchante et al., 201839 | AIDS. | Original | Retrospective/Prospective | 322 (HIV-HCV) | 100 | CPA | 53 | Tx start date | NR | 10 months | N/A | 25% on IFN; 8 with a previous history of HCC | N/A | 22 months | |
| CPB | 32 | IFN | 21% on DAAs; 31 with a previous history of HCC | ||||||||||||
| CPC | 15 | DAAs | |||||||||||||
| Bielen et al., 201740 | J Viral Hepat. | Original | Retrospective | 567 | IFN + DAAs: 46.8 | CPA | 100 | IFN + DAAs | Tx end date | Last follow-up/death | 6 months | 1.7 | 0 | NR | 24 months |
| DAAs: 67.1 | DAAs | 1.1 | 15 | ||||||||||||
| Virlogeux et al., 201741 | Liv Int. | Original | Retrospective | 68 (only 34% treated) | 100 | CP | NR | DAAs (23) | Date of HCC remission | Last follow-up | Treated: 35.7 months. Not treated: 15.4 months | N/A | 47.8 | N/A | 13 months |
| No HCV Tx (45) | N/A | 73.3 | |||||||||||||
| El Kassas et al., 201822 | J Viral Hepat. | Original | Prospective | 116 | 100 | CPA | 89.7 | DAAs (53) | Date of complete radiological response | Last follow-up/death | 16 | N/A | 37.7 | N/A | NR |
| CPB | 10.3 | N/A | |||||||||||||
| CPC | 0 | 23 | N/A | 25.4 | |||||||||||
| Nagata et al., 201742 | J Hepatol. | Original | Retrospective (propensity score) | 1897 | NR | CP | NR | IFN (1145) | Date of curative tx for initial HCC | HCC recurrence/last follow-up | 7.5 years | 2.5 | 53 | N/A | N/A |
| DAAs (752) | 1.1 | 29 | |||||||||||||
| Warzyszyńska et al., 201743 | Clin Exp Hepatol. | Letter | N/A | 51 | NR | CP | NR | DAAs 19 | NR | NR | NR | NR | DAAs 42 | NR | Days post-resection: 265 on DAAs |
| No DAAs 32 (control) | No DAAs 65 | 532 on no DAAs | |||||||||||||
| Zanetto et al., 201744 | Liver Transpl. | Original | Retrospective (case control) | 46 | 100 | CPA | 60.9 | DAAs 23 | Tx start date | Last follow-up/death | 10 months | NR | DAAs 12.5 | NR | NR |
| CPB | 23.9 | NO DAAs 23 (control) | 7 months | NO DAAs 8.3 | |||||||||||
| CPC | 15.2 | ||||||||||||||
| Tsai et al., 201745 | J Hepatol. | Letter | N/A | 105 | NR | NR | NR | pegIFN + RBV | NR | NR | 6 months post-treatment | N/A | 22.9 | NR | NR |
| ANRS cohorts, 201624 | J Hepatol. | Original | Prospective/retrospectived | HEPATHER (n = 267): DAAs = 189d | 77.5 | CPC | N/A | DAA+/DAA- | NR | Last follow-up | DAA+: 20.2 months, DAA-: 26.1 months | N/A | HR 1.21 (95% CI [0.62–2.34], p = 0.5782) | N/A | NR |
| CIRVIR (n = 79): DAAs = 13 | 100 | DAA+/DAA- | HCC tx | Last follow-up | 21.3 months | HR 0.41 (95% CI [0.05-3.08], p = 0.386) | NR | ||||||||
| CUPILT (n = 314) DAAs = 314d | 15.6 | DAA+/DAA- | LT | Last follow-up | NR | 2.2e | 7 ± 3 months | ||||||||
| Petta et al., 201746 | Aliment Pharmacol Ther. | Post-hoc analysis of information from other publications | Retrospective | 58 | 100 | CPA | 91 | DAAs | HCC tx | Last follow-up | 18 months | N/A | 27.6 | N/A | NR |
| CPB | 5 | ||||||||||||||
| CPC | 4 | ||||||||||||||
| 57 | 100 | CPA | 90 | IFN | 34 months | 38.6 | NR | ||||||||
| CPB | 10 | ||||||||||||||
| CPC | (−) | ||||||||||||||
| Torres et al., 201615 | J Hepatol. | Letter | N/A | 8 | 87.5 | CPA | 42.9 | DAAs | Use of DAAs | NR | 12 months | N/A | 0 | N/A | |
| Yang et al., 201647 | J Hepatol. | Letter | N/A | 63 (no DAAs) | NR | NR | NR | No DAAs | LT | NR | NR | NR | 9.6 | N/A | NR |
| 18 (DAAs) | DAAs | NT | 4/5 patients in the first 6 months post-LT | 27.8 | |||||||||||
| 578 (IFN) | IFN | 0.28–0.035f | |||||||||||||
| Minami et al., 201648 | J Hepatol. | Letter | N/A | 27 | 100 | CPA | 100 | DAAs | NR | Last follow-up | 1.3 years | N/A | 29.6 | N/A | 5.8 months |
| 38 | 100 | CPA | 100 | IFN | NR | 68.4 | 5.4 months | ||||||||
| 861 | 100 | CPA | 74 | No HCV treatment | 64.2 | NR | |||||||||
CPA: Child-Pugh A score; CPB: Child-Pugh B score; CPC: Child-Pugh C score; DAAs: direct-acting antivirals; HCC: hepatocellular carcinoma; HCV: hepatitis C virus; IFN: interferon; LT: liver transplant; N/A: not applicable; NR: not reported; RBV: ribavirin; Tx: treatment.
Cohort updated in ILCA 2016 (77 patients; median follow-up: 8.2 months; 27.3% HCC recurrence; 14.3% evolution to terminal HCC; 47.6% transarterial chemoembolisation, radioembolisation or regorafenib after recurrence; 38.1% candidates for resection, percutaneous treatment or liver transplant after recurrence.
Following the publication of the original works,4,13 letters to the editor with similar results and others with conflicting results were published, suggesting that starting antiviral treatment during the first six months from CR being documented was the cause of a higher rate of recurrence or that the inclusion of patients treated with chemoembolisation (CE) may have led to this association (due to CE being considered a treatment without curative intent).14,15 In this sense, Cammà et al.14 performed a post-hoc analysis of our study’s results,4 concluding that early DAA indication (during the first six months from CR being documented) was associated with an increase in HCC recurrence. Based on this analysis, the authors recommended starting antiviral treatment after the first six months from CR being documented with the aim of thereby preventing a false increase in HCC recurrence by ruling out those patients whose cancer treatment had failed.
Taking the interpretation of Cammà et al. as a rational basis, other authors concluded their work with the same recommendation.16,17 However, in a prospective study by the same Italian group18 that enrolled patients similar to Reig et al.,4 including 143 patients with CR and no evidence of uncharacterised nodes (86% Child-Pugh A, 100% BCLC [Barcelona clinic liver cancer] 0/A), they observed that the predictive factors for HCC recurrence were a history of prior HCC recurrence and HCC size.18 Nevertheless, they did not identify the time elapsed between CR and the start of DAAs as a predictive factor of recurrence. This finding suggests that the initial recommendation to start treatment with DAAs six months after documenting CR to avoid or reduce the risk of recurrence was merely an over-interpretation of the Reig et al. data.4 In fact, recurrence in the first year of DAA treatment in BCLC 0/A patients was 40% in patients without a history of prior HCC recurrence and more than 60% in those who did have a history of prior recurrence. For this reason, the fact that they did not observe that recurrence is related to the time at which the antiviral treatment is started, and that the rates of recurrence described in their study are similar to those originally described in our study, validates the data reported in the original studies.4,13 The impact of prior history of recurrence (a factor that is directly associated with the risk of recurrence and that has been widely studied) was also analysed by Ikeda et al.19 and, like Cabibbo,18 they observed that patients with a history of HCC recurrence at the time of starting DAAs had higher rates of recurrence. Nevertheless, the same authors19 conducted a case-control study in which they compared patients treated with DAAs after receiving their first treatment for HCC versus those patients who did not receive antiviral treatment (no DAAs), but the number of prior treatments for HCC was not taken into account. This factor that was omitted from patient selection is a predictive factor for HCC recurrence and, therefore, introduces selection bias. The authors compare patients starting from a different risk profile and, as a result, invalidate any type of interpretation. It is because of this that, in spite of the work having been presented as a case-control study adjusted for age and BCLC, the conclusion, with regard to the lower rate of recurrence in patients treated with DAAs compared to untreated patients, is not valid.
Moreover, other works report HCC recurrences that vary between 23.6% and more than 60%18–22 and that validate the initial data reported by our multicentre study and the Italian group in Bologna.4,13
Publications with results that conflict with the original publications and their interpretationHowever, cohorts with a larger number of patients and prospective cohorts in which the development of HCC was not one of the primary endpoints also assessed the development of HCC recurrence23,24 and reached different conclusions, as they did not observe a change in the rate of recurrence. How can this discrepancy be explained? Is sample size the factor that determines the presence of conflicting results? In general, it can be considered that having larger cohorts is associated with more reliable results. Nevertheless, having a large sample but not studying the event of interest brings in new bias to the matter, and this bias cannot be minimised with statistical tools. In this sense, part of the medical community considers works that have a large sample size to have robust data and uses them to refute the hypotheses posed in 2016 regarding a change in the rate of recurrence. However, in order to study the impact of any factor in the development of HCC, we must have studies that consider HCC development as a primary endpoint as it is an event that is only identified by imaging tests. The absence of pre-established and regulated images makes it impossible to observe the event. In this sense, most of the cohorts that study the evolution of patients with HCV perform blood tests at the start of treatment, at points of interest during antiviral treatment, at the end of treatment and when establishing SVR attainment. However, follow-up is often limited to SVR assessment and ultrasound screening for HCC is not part of the studies. All of this may explain why, in spite of observing a time-related association and HCC in more advanced stages, they did not observe a change in the rate of recurrence. For all of these reasons, sample size is not a sine qua non condition to consider a study more robust when analysing/comparing the results with a study that has a smaller sample size but is designed to study HCC development. This interpretation is applicable to works analysing both the incidence and recurrence of HCC.
Other authors opted to perform a meta-analysis25 to analyse the impact of DAAs in the context of HCC recurrence, but the high degree of heterogeneity (I; 89%) observed in said study invalidates the authors’ conclusions. The heterogeneity described is a consequence of the characteristics of the studies included (different populations, follow-ups and analysis points, as well as the evidence level of the information selected) and cannot be minimised even with statistical tools designed for this purpose (meta-regression). For these reasons, given the limitations of the works analysed and the impossibility of carrying out prospective, randomised studies, the debate continues regarding the impact of DAAs in terms of rate of recurrence. Nevertheless, what it has been possible to demonstrate is that HCV eradication with interferon-free regimens does not eliminate the risk of HCC recurrence. From a practical point of view and in the context of patients with compensated cirrhosis of the liver in whom the objective is not only to eradicate HCV for epidemiological reasons, but also to avoid the risk of decompensation (including HCC recurrence), their indication is controversial. The reason for this controversy is defined by the risk-benefit ratio presented by each patient prior to starting antiviral treatment. This risk is not established and will depend on whether or not SVR is attained, as it has been demonstrated that patients who do not achieve SVR have a higher risk of developing HCC. On the other hand, the benefit of eradicating HCV (95%) is associated with a lower number of liver disease complications, although the probability of preventing HCC development has not been established. Unfortunately, the benefit of the treatment is not known prior to starting it, as in patients with a history of HCC it is variable and depends on baseline characteristics such as the degree of portal hypertension and its evolution after starting DAAs, the presence or absence of CR and the stage of HCC. In relation to this subject, if we take as a basis the data of Cabibbo et al.,26 the expected risk of recurrence of HCC after documenting CR increases over time from around 21% at one year up to 64% at five years (41.5% and 61% at two, three and four years, respectively). The same is true of the risk of decompensation, which increases from just 10% at one year up to 44% at five years in patients with compensated HCV not receiving HCV treatment (21.3% and 36% at two, three and four years, respectively), who presented early-stage HCC and achieved CR. Because of this, according to said work (which reflects the natural evolution of patients with untreated HCV), the risk of decompensation is always lower than the risk of recurrence. If we add to these data the controversy regarding the rate of HCC recurrence, the decision to treat these patients must be personalised. For this reason, at Hospital Clínic de Barcelona, patients with a history of HCC are informed of the risk of recurrence and risk of decompensation and, pending results that would invalidate the initial warning published in 2016, treatment with interferon-free regimens is not recommended. In this regard, the recent work by Huang et al.27 suggests that patients treated with DAAs do not have a higher risk of recurrence than untreated patients. Nevertheless, this assertion lacks robustness for the following reasons: a) the risk of recurrence was greater in non-DAA patients [the non-DAA patients were mostly patients without CR at the time of analysis (67.8% vs. 98.4% in the DAA group; p < 0.001) and patients with a higher degree of decompensation without the option of treatment while on a waiting list in comparison with patients who received DAAs (50.5% Child-Pugh B/C in the non-DAA group vs. 33.8% in the DAA group; p = 0.006)]; b) the result of the propensity score (n = 59 vs. n = 61 patients) did not allow it to be concluded that DAA patients have the same risk as non-DAA patients because the confidence intervals included 1 [univariate (HR, 1.02; 95% CI, 0.65–1.60; p = 0.93) and multivariate (HR, 0.91; 95% CI, 0.58–1.42; p = 0.67)]. The proper interpretation of these results is that similar outcomes cannot be ruled out as, according to the multivariate result, the risk of recurrence in the DAA group may be up to 1.42 times higher than in the non-DAA group.
Time-related association between the start of antiviral treatment with interferon-free regimens and the development of hepatocellular carcinoma recurrenceIn order to be able to adequately assess the impact of the rate of recurrence and reach an unequivocal conclusion, we would need randomised works. However, studies of this type are not indispensable to study the time-related association. The time-related association is a “time-based pattern between a certain factor and an event of interest” and, as with any other pattern, it is validated by its repetition.
Surprisingly, the titles and conclusions of most of the publications suggest that a time-related association between starting DAAs and the development of HCC recurrence was not observed and/or that DAA treatment reduces or does not alter HCC recurrence. Nevertheless, the detailed analysis of these publications reflects a clear time-related association in the majority of the studies, as can be seen in Table 1 of this review and graphically in Figure 4 by Adhoute et al.28 In this regard, Colombo and Boccaccio,16 in an expert opinion letter called “News & Views”, conclude that the time between the CR and the development of HCC recurrence is shorter in patients treated with DAAs than in untreated patients or patients treated with regimens that include interferon. Although the way this time-related association is expressed differs, how could a shortening of the period between CR and the development of HCC recurrence be explained without a time-related association?
Form of presentation and speed of progression of hepatocellular carcinoma recurrence in the context of interferon-free treatmentThe form of presentation and speed of progression of HCC recurrence are simplified as the term “tumour aggressiveness”. The aggressiveness of HCC in the context of treatment with DAAs is currently another area of controversy in the medical community. Tumour aggressiveness can be demonstrated by the clinical picture the patient presents, the impact the HCC has on liver function and/or the tumour load at the time of the HCC diagnosis. The BCLC classification29 considers the prognostic factors and classifies patients in stages from BCLC 0 (single HCC ≤ 2 cm), which is the earliest stage, to patients in the terminal stage (BCLC-D) in which, regardless of tumour load, patients develop tumour-related symptoms (ECOG-PS ≥ 2) and/or liver decompensation (Child-Pugh B-C) without the option of a liver transplant. Nevertheless, it is not only the presentation of HCC, but also patients’ evolution as described above, that determines the degree of aggressiveness. In this sense, the updating of our original cohort20 to include a greater number of patients and a longer follow-up time (n = 77, median follow-up: 12.4 months) reveals that both the onset pattern and the evolution of the recurrences are aggressive. In said analysis, some 32.4% of patients developed HCC recurrence in spite of having presented CR with no evidence of uncharacterised nodes when starting DAAs, and 6.5% died due to HCC progression within the first year after starting treatment with DAAs. In addition, HCC recurrence presented in stage BCLC B/C in 28.6% of patients and stage BCLC-D in 14.3%. Nevertheless, the 33% of patients who could be offered specific treatment (resection, percutaneous treatment, locoregional or systemic treatments; n = 19) developed HCC recurrence or progressed within the first six months of DAA treatment (only one patient had the option to receive a liver transplant as a treatment for HCC recurrence).
These disheartening results (in spite of the painstaking selection of patients to minimise the overvaluation of recurrence and considering only those patients with CR but without uncharacterised nodes) led part of the medical community to consider that the published results must have been affected by the inclusion of patients treated with CE. Nevertheless, none of the patients treated with CE in our cohort developed HCC recurrence or progression during follow-up.20 However, because the inclusion of patients treated with CE could be considered a selection bias, and, in view of the criticism received in this regard when updating our cohort, we performed that same analysis as in the original cohort, excluding patients treated with CE and only considering those patients in stage BCLC 0-A who started treatment with DAAs within four months of CR being documented (n = 20). In this analysis, we observed that nine patients developed HCC recurrence (45%) and four died due to HCC progression within the first 16 months of receiving treatment with DAAs (at 9, 10, 15 and 16 months after starting DAA treatment). Subsequently, this pattern of aggressiveness was described by other authors18,19,21,22,30; although they do not mention it as a time-related association, they do describe a short follow-up time and mention the event, hence the recurrence occurred within said period. Another point to consider is the presence of greater microvascular invasion described by Renzulli et al.21
It is because of this that, although we cannot draw a direct comparison between the cohorts that study this phenomenon, the work of Cabibbo et al.26 describes that 21% of patients with HCV and early-stage HCC who achieved CR after receiving treatments with curative intent and who did not receive treatment for HCV developed HCC recurrence within the first year of CR being documented. This percentage of patients (21%) is clearly lower than that described in our cohort (32.4%) or other subsequently studied cohorts in which the rates of recurrence vary from 23% to more than 60% depending on the prior history of recurrence.19,22,26
Another indirect factor of aggressiveness is the overall survival of patients, and, based on the cohort of patients not treated for HCV published by Cabibbo et al.,26 some 3% of patients died within the first year of CR of the HCC being documented, a figure that again is markedly lower than that observed in our cohort (6.5%)4 or in the cohort of patients treated with DAAs by Cabibbo et al.’s group (4.2%).18 It is worth noting that the baseline characteristics of the two cohorts published by Cabibbo et al.18,26 are similar but not identical. In the cohort of patients treated with DAAs, all patients with uncharacterised nodes were excluded.18 However, these patients were not excluded from the cohort of patients not treated for HCV, so it can be anticipated that these patients would have a higher rate of recurrence and lower survival than patients without uncharacterised nodes.26 Thus, the differences could be even greater than those observed in a simple direct comparison between both cohorts.
Even now, there are authors who believe that the pattern of aggressiveness should not be considered a characteristic of patients receiving DAAs. Nevertheless, most of them do not rule out this hypothesis and suggest that it needs to be studied.16
ConclusionsFor this reason, we can conclude that, two years from the initial warning, the debate surrounding the time-related association (start of interferon-free antiviral treatment and development of HCC recurrence) and the unexpected rate of recurrence remains open. The initial scepticism has evolved to: a) acceptance of the fact that the risk of HCC recurrence is not eliminated despite the HCV being eradicated with DAAs; b) observation of a shortening of the time between CR and HCC development in patients treated with DAAs; c) lack of denial of the pattern of aggressiveness being a key factor in the evolution of these patients.
Due to all of this, the challenge in coming years will be to answer the question: how can we explain a shortening of the time between CR and HCC development in patients treated with DAAs without accepting the presence of a time-related association, a change in the rate of recurrence and the presence of more aggressive HCC?
Progress in HCV treatment has led to an unequivocal impact on HCV eradication, but has in turn given rise to a new area of research that challenges us to study and understand the yet-unknown mechanisms relating to hepatocarcinogenesis. However, given that the follow-up of patients treated with DAAs is still very short, the long-term impact cannot be known. Because of this, we do not currently have data that would enable us to assess the risk-benefit ratio of the impact of DAAs in relation to long-term recurrence as this will depend on whether or not SVR is achieved.
FundingThis work was funded by the Instituto de Salud Carlos III [Institute of Health Carlos III] (grant number CM15/00050 and PI15/00145).
Conflicts of interestTania Hernáez Alsina: travel costs paid by Bayer.
Berta Caballol-Oliva: travel costs paid by Bayer.
Álvaro Díaz-González: speaker and travel costs paid by Bayer. BTG travel grants.
Cassia Regina Guedes-Leal: travel costs paid by Bayer.
María Reig: consultancy for Bayer, BMS, Roche, Ipsen, AstraZeneca and Lilly. Conference costs paid by Bayer, BMS, Gilead and Lilly. Research grants from Bayer and Ipsen.