AKR1B10, first cloned from liver cancer tissues, has recently been reported to be up-regulated significantly in hepatocellular carcinoma (HCC) tissues, but the relationship between serum level of AKR1B10 and the risk of HCC is not understood.
Methods170 HCC patients and 120 health donors from October 2014 to March 2017 were recruited in the affiliated hospital of Guilin Medical University. Serum AKR1B10 in all cases were detected and in 30 HCC patients were analyzed preoperatively and postoperatively by Time-resolved fluoroimmunoassay.
ResultsThe level of serum AKR1B10 was significantly higher in HCC patients (1800.24±2793.79) than in health donors (129.34±194.129), and downregulation of serum AKR1B10 in HCC patients was observed after hepatectomy. When samples were grouped according to the serum level of AKR1B10 (≥232.7pg/ml), serum AKR1B10 positively correlated to serum AFP (χ2=6.295, P=0.012), ALT (χ2=18.803, P=0.000), AST (χ2=33.421, P=0.000), tumor nodule number (χ2=6.777, P=0.009), cirrhosis (χ2=43.458, P=0.000), and tumor size (χ2=6.042, P=0.014) in the Chi-square test.
ConclusionsDiagnosis of HCC could be improved using the both predictors of serum AKR1B10 and AFP. AKR1B10 was thus considered to be a new serological biomarker for HCC.
Recientemente se ha notificado que el AKR1B10, clonado por primera vez a partir de tejidos hepáticos cancerosos, se encuentra aumentado de forma significativa en tejidos afectados por carcinoma hepatocelular (CHC), aunque no se comprende la relación entre la concentración sérica de AKR1B10 y el riesgo de CHC.
MétodosSe incluyeron 170 pacientes con CHC y 120 donantes sanos desde octubre de 2014 a marzo de 2017 en el hospital afiliado a la Guilin Medical University. Se analizó el AKR1B10 en todos los casos y en 30 pacientes con CHC antes y después de la cirugía, mediante fluoroinmunoensayo a tiempo resuelto.
ResultadosLa concentración sérica de AKR1B10 fue significativamente mayor en los pacientes con CHC (1.800,24±2.793,79) que en los donantes sanos (129,34±194,129), y se observó una reducción del AKR1B10 sérico en los pacientes con CHC tras la hepatectomía. Cuando se agruparon las muestras en función de la concentración sérica de AKR1B10 (≥ 232,7pg/ml), el AKR1B10 sérico se correlacionó positivamente con la AFP sérica (χ2=6,295; p=0,012), la ALT (χ2=18,803; p=0,000), la AST (χ2=33,421; p=0,000), la cifra de nódulos tumorales (χ2=6,777; p=0,009), la presencia de cirrosis (χ2=43,458; p=0,000) y el tamaño tumoral (χ2=6,042; p=0,014) en la prueba de χ2.
ConclusionesPodría mejorarse el diagnóstico del CHC usando los 2 factores pronósticos de AKR1B10 sérico y AFP. Por lo tanto, el AKR1B10 se consideró un nuevo biomarcador serológico del CHC.
Primary hepatocellular carcinoma (PHC) is a serious health problem with 745,000 deaths and 782,500 new cases in 2012, the morbidity and mortality are still increasing worldwide.1,2 Among primary liver cancers, hepatocellular carcinoma (HCC) is the most common neoplasm, accounting for approximately 90% of all cases, following by intra hepatic cholangiocarcinoma (ICC) or combination of HCC and ICC (cHCC-ICC).3 Due to its concealed symptoms, patients diagnosed are usually present at advanced stages with large symptomatic tumors leading to the poor prognosis of HCC. Early screening and differential diagnosis of HCC can significantly improve patients’ survival.4 In addition to imaging examination results, α-fetoprotein (AFP) in serum is a common diagnostic tool in HCC and has been widely used in HCC surveillance.5 Nevertheless, due to its low diagnostic accuracy AFP has recently been excluded from current AASLD HCC surveillance guidelines.6 Thus, finding more accurate diagnostic biomarkers is crucial to promote HCC prognosis.
Aldo-keto reductase 1B10 (AKR1B10), also known as aldose reductase-like-1 (ARL-1), is an enzyme identified from human HCC.7 Recently, studies revealed that AKR1B10 exerts oncogenic roles in carcinogenesis through several mechanisms. AKR1B10 promotes cancer cells survival by regulating metabolites such as α,β-unsaturated carbonyl compounds, retinoic derivatives and lipids.8–11 Besides, it is involved in regulating prenylation and activation of oncogenes like k-ras.12 It is well known that AKR1B10 are up-regulated in many solid tumors, such as hepatocellular carcinoma,7,13 breast cancer,14 lung cancer,15 esophageal adenocarcinoma,16 gastric cancer,17 and pancreatic cancer.18 Our previous study confirmed that AKR1B10 was positively expressed in 83% of PHC tissues significantly higher than 25% of peri-tumor tissues. Meanwhile the mRNA levels of AKR1B10 also increased in HCC tissue.13
Although located in the cytoplasm, AKR1B10 can be secreted mediated by lysosome.14,19 Secreted AKR1B10 has been considered as a new serum marker and potential therapeutic target for breast cancer.14 However, the possibility that AKR1B10 as a new serological biomarker for HCC has not been investigated before our study. Here, we identified serological level of AKR1B10 in HCC from a single center and the relationship between AKR1B10 level and clinical features.
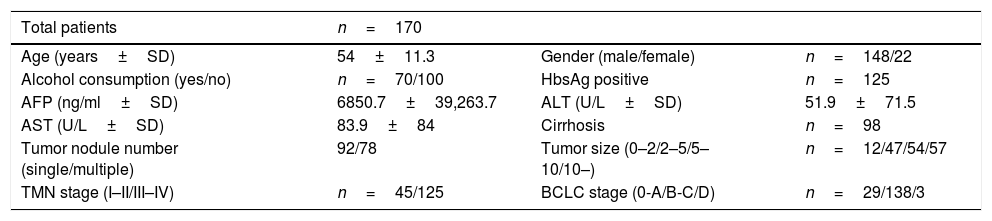
Materials and methodsClinical samples collectionSerum specimens, along with complete clinical and pathological data, were collected from 170 HCC patients treated with surgical tumor resection at the Affiliated Hospital of Guilin Medical University, Guangxi, China, between October 2014 and March 2017. Sera from age-matched healthy donors (n=120) undergoing medically examination without having any mental or drug therapy, operation and any known hepatopathy, collected were used as control. All the HCC patients performed routine assessments before surgery, which included complete hematologic and biochemistry profiles, physical examination, ultrasonography (US), computed tomography (CT) scans, and magnetic resonance imaging (MRI). All of the participants had no other lymphatic system disorders, such as lymphocytic leukemia and lymphoma, thus ensuring that lymphocyte count was normal. Patients with non-hepatocellular carcinoma (non-HCC), acute and chronic gastrointestinal diseases or receiving any anticancer therapy before blood sample collection; pregnant or lactating women; others that are considered not suitable for this study were excluded. Patient baseline and clinical data, including age (years), gender, alcohol consumption, HBsAg, AFP (ng/ml), ALT (U/L), AST (U/L), tumor nodule number, HBV-DNA (copies/ml), cirrhosis, TNM stage, BCLC stage, portal vein tumor thrombus, tumor size (cm), differentiation degree are listed in Tables 1 and 2.
Basic characteristic of HCC patients.
| Total patients | n=170 | ||
|---|---|---|---|
| Age (years±SD) | 54±11.3 | Gender (male/female) | n=148/22 |
| Alcohol consumption (yes/no) | n=70/100 | HbsAg positive | n=125 |
| AFP (ng/ml±SD) | 6850.7±39,263.7 | ALT (U/L±SD) | 51.9±71.5 |
| AST (U/L±SD) | 83.9±84 | Cirrhosis | n=98 |
| Tumor nodule number (single/multiple) | 92/78 | Tumor size (0–2/2–5/5–10/10–) | n=12/47/54/57 |
| TMN stage (I–II/III–IV) | n=45/125 | BCLC stage (0-A/B-C/D) | n=29/138/3 |
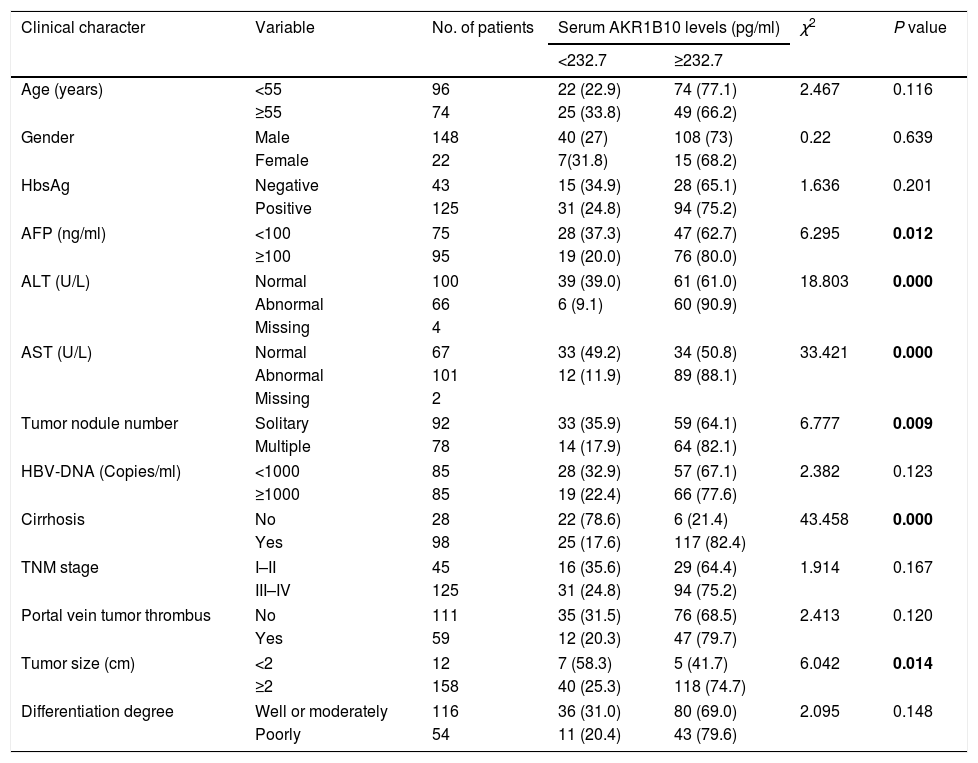
Correlation between serum AKR1B10 levels and the clinicopathologic parameters in HCC.
| Clinical character | Variable | No. of patients | Serum AKR1B10 levels (pg/ml) | χ2 | P value | |
|---|---|---|---|---|---|---|
| <232.7 | ≥232.7 | |||||
| Age (years) | <55 | 96 | 22 (22.9) | 74 (77.1) | 2.467 | 0.116 |
| ≥55 | 74 | 25 (33.8) | 49 (66.2) | |||
| Gender | Male | 148 | 40 (27) | 108 (73) | 0.22 | 0.639 |
| Female | 22 | 7(31.8) | 15 (68.2) | |||
| HbsAg | Negative | 43 | 15 (34.9) | 28 (65.1) | 1.636 | 0.201 |
| Positive | 125 | 31 (24.8) | 94 (75.2) | |||
| AFP (ng/ml) | <100 | 75 | 28 (37.3) | 47 (62.7) | 6.295 | 0.012 |
| ≥100 | 95 | 19 (20.0) | 76 (80.0) | |||
| ALT (U/L) | Normal | 100 | 39 (39.0) | 61 (61.0) | 18.803 | 0.000 |
| Abnormal | 66 | 6 (9.1) | 60 (90.9) | |||
| Missing | 4 | |||||
| AST (U/L) | Normal | 67 | 33 (49.2) | 34 (50.8) | 33.421 | 0.000 |
| Abnormal | 101 | 12 (11.9) | 89 (88.1) | |||
| Missing | 2 | |||||
| Tumor nodule number | Solitary | 92 | 33 (35.9) | 59 (64.1) | 6.777 | 0.009 |
| Multiple | 78 | 14 (17.9) | 64 (82.1) | |||
| HBV-DNA (Copies/ml) | <1000 | 85 | 28 (32.9) | 57 (67.1) | 2.382 | 0.123 |
| ≥1000 | 85 | 19 (22.4) | 66 (77.6) | |||
| Cirrhosis | No | 28 | 22 (78.6) | 6 (21.4) | 43.458 | 0.000 |
| Yes | 98 | 25 (17.6) | 117 (82.4) | |||
| TNM stage | I–II | 45 | 16 (35.6) | 29 (64.4) | 1.914 | 0.167 |
| III–IV | 125 | 31 (24.8) | 94 (75.2) | |||
| Portal vein tumor thrombus | No | 111 | 35 (31.5) | 76 (68.5) | 2.413 | 0.120 |
| Yes | 59 | 12 (20.3) | 47 (79.7) | |||
| Tumor size (cm) | <2 | 12 | 7 (58.3) | 5 (41.7) | 6.042 | 0.014 |
| ≥2 | 158 | 40 (25.3) | 118 (74.7) | |||
| Differentiation degree | Well or moderately | 116 | 36 (31.0) | 80 (69.0) | 2.095 | 0.148 |
| Poorly | 54 | 11 (20.4) | 43 (79.6) | |||
AFP, Alpha-fetoprotein; HBsAg, hepatitis B surface antigen; TNM, tumor-node-metastasis; HBV-DNA, Hepatitis B virus-DNA.
The survey protocol was in accordance with the ethical guidelines of the Declaration of Helsinki. Ethical approval was granted by the Ethical Committee of the Affiliated Hospital of Guilin Medical University, and the written consents were obtained from all patients or their guardians prior to surgery.
Time-resolved fluoroimmunoassayAKR1B10 amounts in serum were measured using a time-resolved fluoroimmunoassay (TRFIA) by the Light of Life Biotechnology, Ltd. (Changsha, China). Briefly, a rabbit AKR1B10 polyclonal antibody was coated to 96-well plate; 100μl samples were added and incubated at room temperature for 60min. After washing for four times, 100μl of biotin-labeled mouse monoclonal AKR1B10 antibody was added and kept at room temperature to gently shake 40min. Then plates were washed for four times again, 100μl of europium-labeled streptavidin was added into each hole, and incubated at room temperature for 30min. After four times washing, 100μl of enhancement solution was added into each hole, the plate was gently shaken for 5min at room temperature, and fluorescence was measured. High-purified recombinant AKR1B10 protein was used as standards.
Selection of cutoff scoreReceiver operating characteristic (ROC) curve analysis was utilized to determine the cutoff score of preoperative serum level of AKR1B10 in patients with HCC. The optimal cutoff value was closest to the point with maximum sensitivity and specificity. To perform ROC curve analysis, we dichotomized the rest of clinicopathological features, and further investigated the clinicopathologic and prognostic significance of preoperative AKR1B10 in patients with HCC.
Statistical analysisThe data are expressed as the mean±S.D. and Student's t test was used to analyze differences between two groups. Correlation between AKR1B10 serum level and clinicopathologic parameters was evaluated using the Chi-square test. Paired-Samples t test analysis was used to analyze the serum level between pre-and postoperative. A value of P<0.05 was designed as statistically significant. All the analyses were performed using the SPSS software (version 19.0, Chicago, IL).
ResultsBaseline clinical characteristics of HCC patients170 HCC patients were enrolled in this study. The mean age of the recruited patients was 54±11.3 years, of which 148 were males and 22 were females. 70 of the patients were with alcohol history and 125 of them were HbsAg positive. The mean serum AFP, ALT, AST was 6850.7±39263.7ng/ml, 51.9±71.5U/L, 83.9±84U/L respectively. Cirrhosis presented in 98 patients. The tumor nodule number in 92 of the patients was single. Tumor size of four categories (0–2/2–5/5–10/10–) were found in 12, 47, 54, 57 patients respectively. 45 patients were in TMN stage I-II, 125 patients were in TMN stage III-IV. 29 patients were in BCLC stage 0-A, 138 patients in stage B-C, 3 patients were in stage D (Table 1).
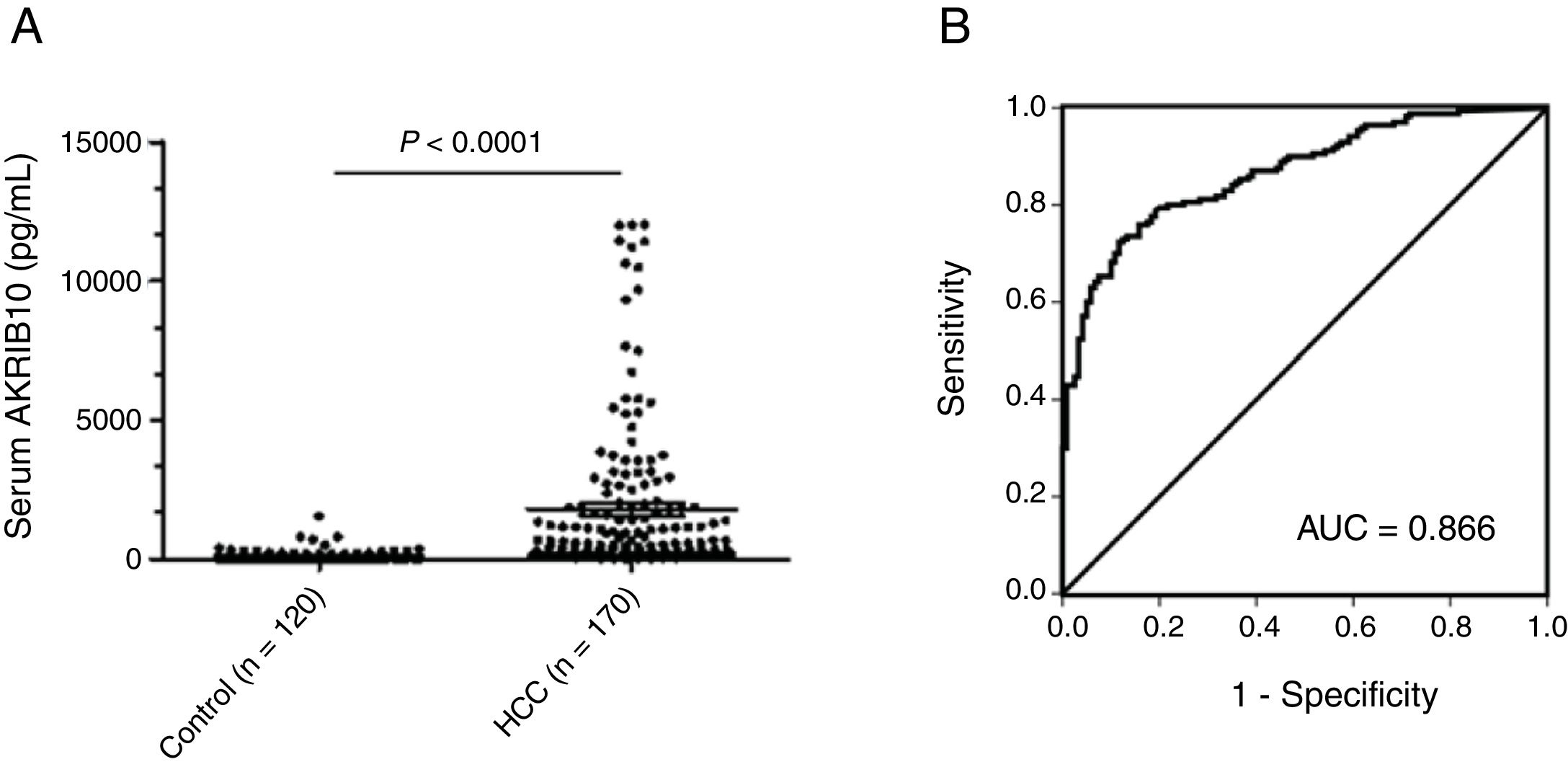
AKR1B10 protein is increased in the serum of HCC patientsAs a non-classical secretory protein, the levels of AKR1B10 in serum were highly correlated with its expression in tumor tissues.14 To evaluate the association between serum AKR1B10 and the risk of HCC with higher expression of AKR1B10 in tumor, levels of AKR1B10 in sera from 170 HCC patients and 120 healthy donors were measured by TRFIA. Results showed that AKR1B10 in sera of HCC patients was increased to 1800.24±2793.79pg/ml (n=170; 95% CI, 1377.25–2223.24), with the highest level up to 12000pg/ml, significantly higher than 129.34±194.129pg/ml (n=120; 95% CI, 94.25–194.43) in healthy donors (t=6.537, P<0.0001) (Fig. 1A). These data suggested AKR1B10 is a new serum marker for human HCC.
Up-regulated serum AKR1B10 in HCC. (A) Mean serum level of AKR1B10 detected by TRFIA was significantly higher in HCC patients than in health donors. P<0.0001. (B) Receiver operating characteristic (ROC) analysis was performed to evaluated the diagnosis value of preoperative serum AKR1B10. The area under the ROC curve value was 0.866.
To identify an optimal cut-off value of preoperative AKR1B10 in serum, an ROC curve was generated to validate diagnostic value of this model (Fig. 1B). The results indicated that optimal diagnostic cut-off for AKR1B10 in serum was 232.7pg/mL with a sensitivity of 72.4%, and a specificity of 88.3%; the area under the curve (AUC) was 0.866 with a 95% CI of 0.826–0.907.
Association between preoperative AKR1B10 and clinicopathological featuresThe relationship between preoperative AKR1B10 and clinicopathological variables of patients with HCC was investigated after the results obtained from the ROC curve analysis. The data showed that preoperative AKR1B10 was correlated with AFP (χ2=6.295, P=0.012), ALT (χ2=18.803, P=0.000), AST (χ2=33.421, P=0.000), tumor nodule number (χ2=6.777, P=0.009), cirrhosis (χ2=43.458, P=0.000), tumor size (χ2=6.042, P=0.014). But, there were no statistical connections between preoperative AKR1B10 and other clinicopathological parameters including age, gender, HBsAg, HBV-DNA,TNM stage, portal vein tumor thrombus, differentiation degree, alcohol consumption and BCLC stage (all P>0.05, partly listed in Table 2).
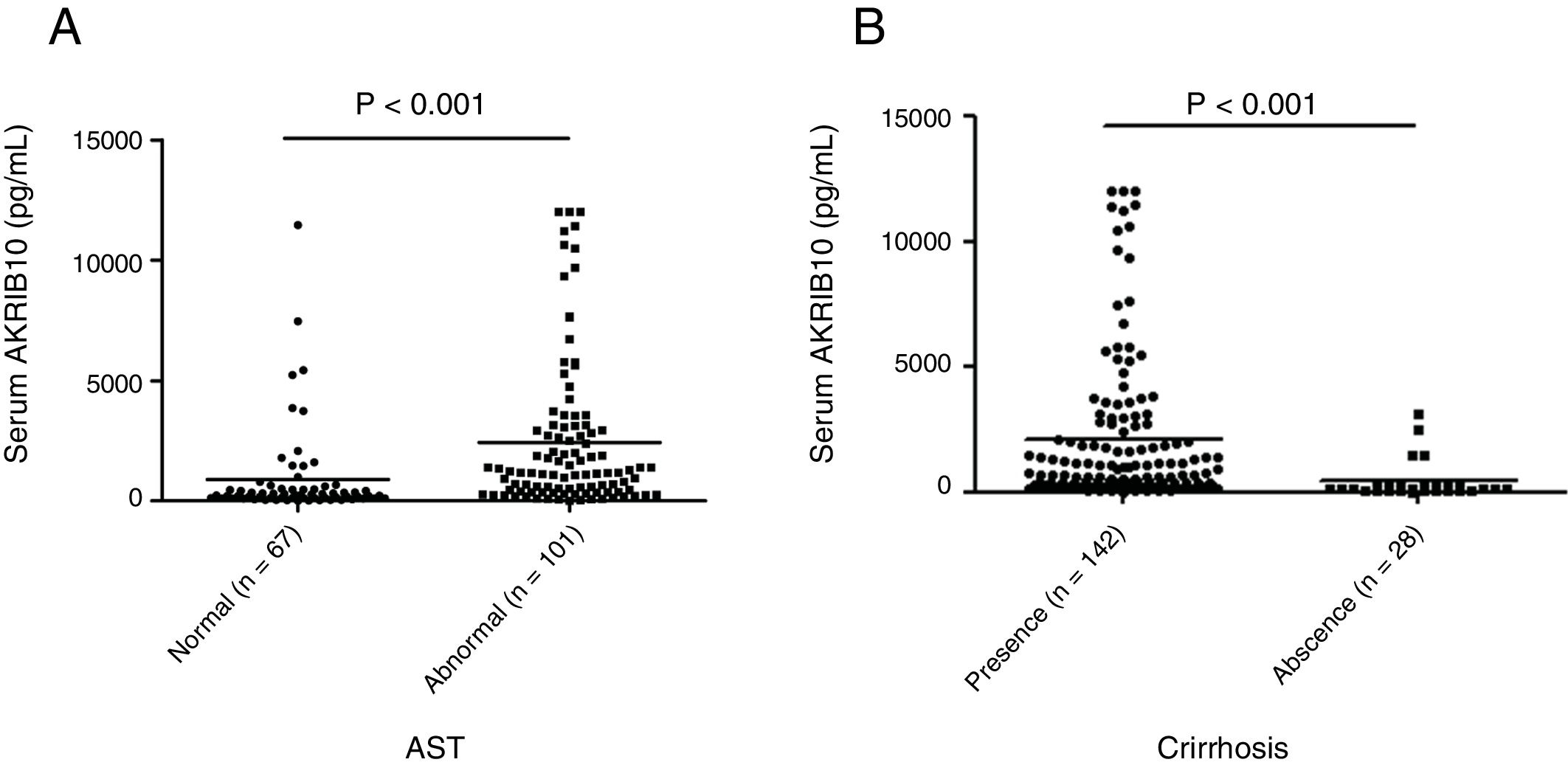
Stratified analysis according to liver parametersPatients were stratified according to AST or cirrhosis in order to compare the AKR1B10 content in two different subgroups. We found that serum AKR1B10 was significantly higher in HCC patients with abnormal AST compared to that in patients with normal AST (892.156±1908.634, 2435.816±3125.687 respectively) (t=−3.971, P<0.001, Fig. 2A). While, serum AKR1B10 in patients with and without cirrhosis were 2074±248.7 and 412.1±146.8pg/ml respectively (t=2.941, P<0.001, Fig. 2B). This result suggested that higher serum AKR1B10 predicted liver cell damage and cirrhosis.
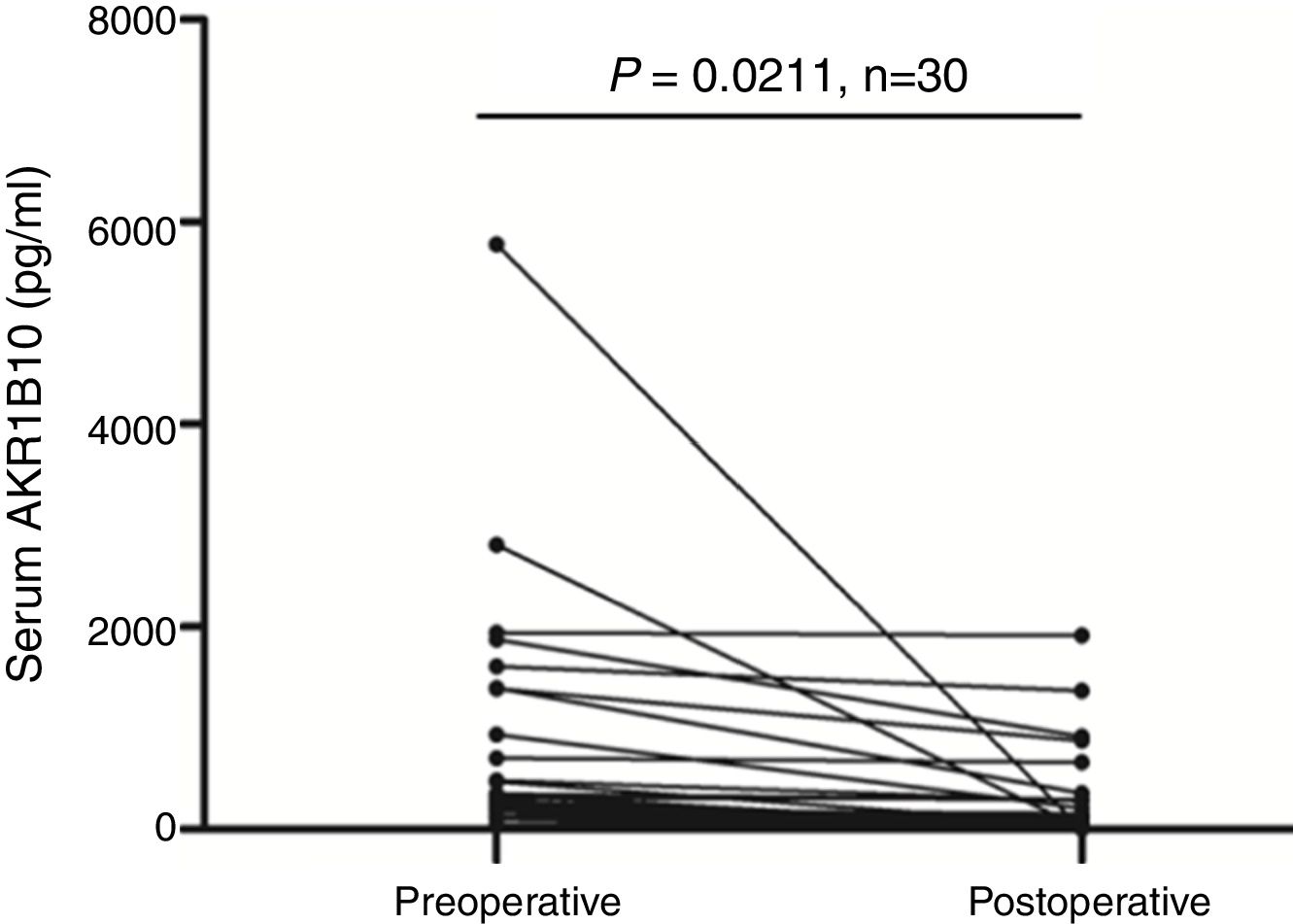
HCC patient's serum AKR1B10 level decreased significantly after hepatectomyTo validate whether serological AKR1B10 in HCC patients was changed after hepatectomy, we examined AKR1B10 in sera from the same patient at different time point including pre-operation and 3–4 days after hepatectomy. Results from 30 patients with HCC revealed that postoperative serological AKR1B10 was 266.85±452.69pg/ml, which was significantly lower than preoperative serological AKR1B10 (771.35±1175.05) (T=2.439, P=0.0211, Fig. 3). Therefore, these data suggested that hepatectomy could decrease serological AKR1B10.
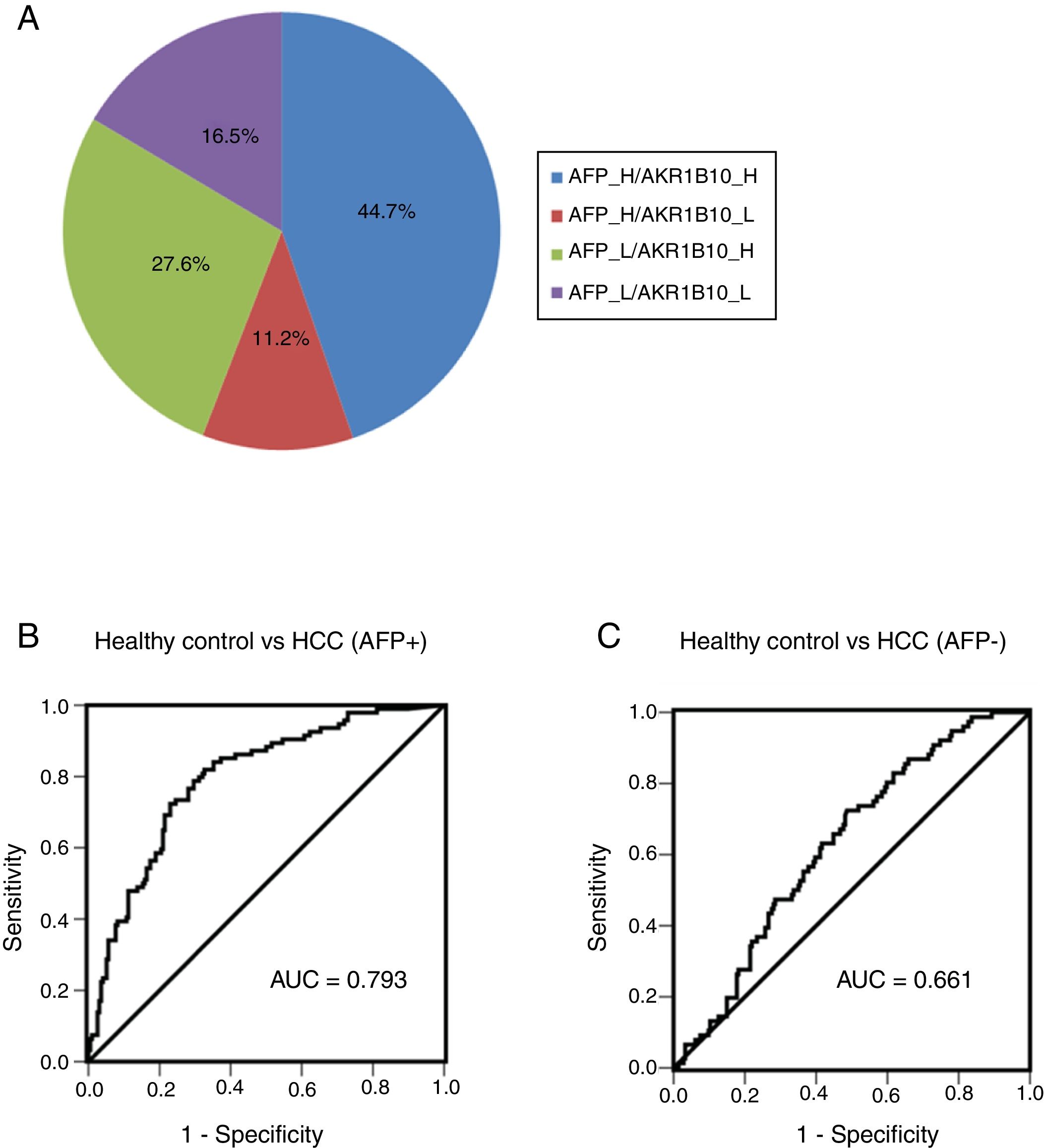
Combination of AKR1B10 and AFP improved the efficiency of HCC diagnosisIn order to further detect diagnostic efficiency of AKR1B10 combined with AFP in HCC, an optimal diagnostic cut-off for AKR1B10 was set at 232.7pg/ml according to diagnostic value from the model generated by ROC curve. Of note, increased AKR1B10 were not completely consistent with increased AFP (≥100ng/ml) in serum from HCC patients. As Fig. 4A showed, both of these markers increased appeared in 44.7% of all HCC patients, only AFP increased showed in 11.2%, and only AKR1B10 increased in 27.6% of all HCC cases. The positive rate of individual AKR1B10 for HCC diagnosis was 72.3%, while AFP was 55.9%. Interestingly, positive rate increased to 83.5% when AKR1B10 and AFP were combined used, which significantly increased efficiency of HCC diagnosis (Fig. 4A).
Combined serum of AKR1B10 and AFP in the HCC diagnosis. The distribution of both serum AKR1B10 (the cut-off value is 232.7pg/ml) and serum AFP (the cut-off value is 100ng/ml) in 170 HCC patients; the numbers in the pie indicated the percentages of serum AKR1B10 and/or AFP whose level is higher (H) or lower (L) than the cut off value.
To detect the sensitivity and specificity of AKR1B10 in AFP positive and negative HCC patients, ROC curves were generated. The results showed that for AFP positive HCC patients the optimal diagnostic cut-off for AKR1B10 in serum was 248pg/ml with a sensitivity of 78.7%, and a specificity of 70.4%; the area under the curve (AUC) was 0.793 with a 95% CI of 0.739 to 0.847 (Fig. 4B). For AFP negative HCC patients the optimal diagnostic cut-off for AKR1B10 in serum was 178.3pg/ml with a sensitivity of 72.4%, and a specificity of 51.4%; the area under the curve (AUC) was 0.661 with a 95% CI of 0.56 to 0.695 (Fig. 4C). These results indicated that the sensitivity of AKR1B10 increased in AFP positive HCC patients.
DiscussionHCC is an extremely malignant tumor, and most of the patients when diagnosed are in the advanced stage. Serum biomarkers are worthy to be investigated in order to improve the early diagnosis of HCC which is essential to increase the 5-year survival rate. Here we identified the association between serum AKR1B10 and the risk of HCC. The level of AKR1B10 in sera from HCC patients was obviously increased and was positively related to the serum level of AFP, tumor nodule number, cirrhosis and tumor size. The biomarker's role of serum AKR1B10 for HCC was thus established.
AFP is still used as a regular indicator for screening and early diagnosis of liver cancer with sensitivity ranging from a mere 18–60% and specificity approximately 85–90%. However, nearly 80% of small HCC nodules do not display increased AFP and the sensitivity of AFP for tumors smaller than 3cm is no larger than 25%.20 In our study, serum AKR1B10 was revealed to be a novel predictor for HCC with a sensitivity of 72.4%, and a specificity of 88.3%. Meanwhile serum AKR1B10 positively correlated to cirrhosis and tumor size (≥2cm) benefited for early diagnosis of HCC. It seems that serum AKR1B10 is a better early diagnosis for HCC than AFP which needs further investigation. Besides, combination of AKR1B10 and AFP has the potential to improve the efficiency of HCC diagnosis. Increased serum AKR1B10 together with AFP would be a promising diagnosis standard in clinic.
Jin et al. demonstrated that AKR1B10 was significantly increased along with stepwise progression of hepatocarcinogenesis (from liver cirrhosis to moderately differentiated HCC).21 Our laboratory also confirmed both the intracellular levels of mRNA and protein of AKR1B10 were significant increased in HCC patients, and a higher AKR1B10 mRNA expression was related to a shorter disease-free survival and an overall survival.13 This study that showed serum AKR1B10 indeed decreased in HCC patients after hepatectomy, together with a previous report that revealed the level of AKR1B10 in serum can indirectly reflect the expression level of this protein in tumor tissues,14 indicated that serum AKR1B10 might also be treated as an independent predictor for prognosis of HCC.
AKR1B10 was originally identified as a gene whose expression was up-regulated in human HCC but was low in normal liver tissues.7 Recently, AKR1B10 has been found to be up-regulated in various solid tumors and is considered as a potential tumor marker for smokers’ non-small cell lung carcinoma,15 breast cancer22 and pancreatic cancer.12 Just like the phenomenon in breast cancer and HCC, serum AKR1B10 is probably increased in patients with other cancers, which needs further investigation to confirm.
In our study, serum AKR1B10 positively correlated to cirrhosis (χ2=43.458, P=0.000) in the Chi-square test. Therefore, cirrhosis is a potential counfounder in the association between AKR1B10 and HCC. Meanwhile, cirrhosis patients without HCC should be included in order to clarify the role of AKR1B10 as a marker of HCC but not a marker of cirrhosis. If cirrhosis is a confounder for the association between AKR1B10 and HCC which warrants further clarification, then it would have a limited applicability in clinical practice.
It has been demonstrated that AKR1B10 is secreted through a HSP90α dependent lysosome-mediated non-classical pathway.19 Overexpression of HSP90α promoted AKR1B10 secretion and inhibition of HSP90α by geldanamycin had a counteractive effect.19 Previous study showed that HSP90α could promote cell survival of HCC, HSP90α inhibition with geldanamycin analogs caused cell death and reduced tumor growth.23 These results are to some degree consistent with an increased AKR1B10 in serum as an indicator of HCC confirmed by our study and also suggest that decreased secretion of AKR1B10 by tumor cells leading serological AKR1B10 reduction might be one of the mechanisms in HCC tumor growth retardation mediated by HSP90α inhibition. Binding analysis revealed that helix10 domain of the AKR1B10C terminal is very important for its interaction with HSP90α.19 The critical amino acid sites involved in AKR1B10 secretion modulation were Lys-233, Glu236 and Lys-240.19 So the increment in serum from patients with HCC might be due to the indicated site mutations present in ARK1B10.
However, the present study has several limitations. Firstly, the quantity of specimens is related small, and secondly patients are recruited from one medical center. So, further exploration using larger quantities of samples from multiple centers is needed. Meanwhile, Caucasian population, other liver diseases and other types of treatments, such as loco-regional therapies should be included in the further study to test the universality and specificity of the AKR1B10 in HCC diagnosis. It's needed to be mentioned here that the role of AKR1B10 in HCC diagnosis from a multiple-center study has just been reported.24 Therefore, this single center study could be interpreted as a hypothesis-generating research.
ConclusionsIn conclusion, AKR1B10 protein is increased significantly in serum from HCC patients; it was expected to act as a new serological biomarker in HCC. The preoperative serum level of AKR1B10 was correlated with AFP, ALT, AST, tumor nodule number, cirrhosis, tumor size. Compared with preoperative level, serological AKR1B10 decreased significantly after hepatectomy, and it can be expected to be a potential indicator of HCC prognosis. Cirrhotic patients and patients submitted to curative or systemic treatments for HCC should be included in the further investigation.
GrantsThe present study was supported in part by the National Natural Science Foundation of China (Nos. 81360309, 81572738), the Natural Science Foundation of Guangxi (No. 2015GXNSFEA139003), “Sphingolipids and Related Diseases” Program for Innovative Research Team of Guilin Medical University, Hundred Talents Program “the Introduction of Overseas High-Level Talents in Colleges and Universities in Guangxi”, the Lijiang Scholar Award in Guilin, and the High Level of Innovation Team and Outstanding Scholars Program in Colleges and Universities in Guangxi.
Conflicts of interestThere are no conflicts of interest from any authors.