To identify and characterise the severe complications of diagnostic confirmation colonoscopies carried out as part of the Colorectal Cancer Screening Programme of the Valencian Community (CCSP-VC).
MethodA retrospective observational study from 2005 to 2012. To identify complications, the CCSP-VC information system was used, as well as Spanish Minimum Basic Data Set hospital discharge summaries and medical records. Cumulative incidence rates were estimated for all complications, immediate complications (occurring the same day as the colonoscopy) and delayed complications (occurring 1–30 days after the colonoscopy) for the 1000 colonoscopies performed. A bivariate analysis using the Chi-square test was performed for the onset of complications, according to gender, age and type of test (guaiac/immunological), as well as for the complication onset time (immediate/delayed) based on the type of colonoscopy (diagnostic/therapeutic) and type of complication (haemorrhage/perforation).
ResultsOf the total 8831 screening colonoscopies performed, 23 severe complications were observed, 13 of which were perforations (56.5%) and 10 haemorrhages (43.5%). No serious vagal syndrome, peritonitis or deaths were recorded. The cumulative incidence rate was 2.60‰; 2.85‰ for the guaiac test and 2.56‰ for the immunological test. The incidence rate was higher in men (2.93‰) than in women (2.16‰), as well as in older groups (3.02‰ versus 1.98‰). Of the total complications, 61% (n=14) were immediate.
ConclusionsThe severe complication rates of screening colonoscopies are a quality indicator for population-based colorectal cancer screening programmes that require extensive research in order to maintain the appropriate risk/benefit ratio of such programmes.
Identificar y caracterizar las complicaciones graves de las colonoscopias de confirmación diagnóstica del Programa de Prevención de Cáncer Colorrectal de la Comunitat Valenciana (PPCCR-CV).
MétodoEstudio observacional retrospectivo (2005-2012). Para identificar las complicaciones se utilizó el sistema de información del PPCCR-CV, las altas hospitalarias del conjunto mínimo básico de datos (CMBD) y la historia clínica. Se estimaron tasas de incidencia acumulada para el total de complicaciones, para inmediatas (mismo día de la colonoscopia) y tardías (1-30 días desde la colonoscopia) por 1.000 colonoscopias. Análisis bivariado con la prueba Chi cuadrado para la aparición de complicación según sexo, edad y tipo de test (guayaco/inmunológico) y para el tiempo de aparición de la complicación (inmediata/tardía) según el tipo de colonoscopia (diagnóstica/terapéutica) y el tipo de complicación (hemorragia/perforación).
ResultadosDe las 8.831 colonoscopias del estudio se detectaron 23 complicaciones graves de las cuales 13 fueron perforaciones (56,5%) y 10 hemorragias (43,5%) y ningún síndrome vagal grave, peritonitis o fallecimiento. La tasa de incidencia acumulada fue del 2,60‰, para el test de guayaco del 2,85‰ y del 2,56‰ para el inmunológico. La tasa de incidencia fue mayor en hombres (2,93‰) que en mujeres (2,16‰) y en grupos de mayor edad (3,02‰ vs. 1,98‰). El 61% (n=14) de las complicaciones fueron inmediatas.
ConclusionesLas tasas de complicaciones de las colonoscopias de cribado graves son un indicador de calidad de los programas poblacionales de cribado de cáncer colorrectal y requieren una investigación exhaustiva para mantener el balance adecuado de beneficios y efectos adversos de estos programas.
Colorectal cancer screening (CRCS) is a secondary prevention strategy recommended by various organisations to reduce the high incidence and associated mortality rates of colorectal cancer.1–4
This organised, population-wide intervention requires continuous assessment of the benefits and adverse effects to ensure an overall benefit for the population, and all the processes that form part of the screening therefore need to be monitored. The CRCS programmes have to keep their own registers in order to be able to provide process evaluation indicators and indicators of the programme's outcomes, and to compare them with the standards of the European CRCS quality guidelines.5
The methods used for the CRCS programmes differ from country to country. The most widely used screening model is the faecal occult blood test (FOBT). People with a positive test result are referred for a colonoscopy, as a diagnostic confirmation test.
Having a colonoscopy, with or without removal of a lesion, is an invasive procedure with a significant risk of complications which can cause people health problems. Severe complications that occur in screening colonoscopies are an adverse effect of the population screening programmes and need to be audited for assessment and control.
The European guidelines for quality assurance in colorectal cancer screening and diagnosis5 report severe complication rates covering a wide range depending on the screening test used; for the Guaiac FOB test (gFOBT) the rates range from 5‰ to 16‰ in people who have a colonoscopy. There are no data for the immunological FOBT (iFOBT). For flexible sigmoidoscopy the range is 0.2–0.3‰ and for screening colonoscopies, it is 0–3‰. The European Society of Gastrointestinal Endoscopy (ESGE) guidelines6 set as a minimum standard complication rates in colonoscopies of ≤5‰ for 7-day readmission. The guidelines of the Asociación Española de Gastroenterología (AEG) [Spanish Association of Gastroenterology] and the Sociedad Española de Endoscopia Digestiva (SEED) [Spanish Society of Gastrointestinal Endoscopy]7 mention an acceptable level for severe complications of screening colonoscopies of <1‰. The implementation of the CRCS programmes in Spain is relatively recent and they have not yet reached full territorial coverage in all of the autonomous regions.8–10 The Programa de Prevención de Cáncer Colorrectal-Comunitat Valenciana (PPCCR-CV) [Colorectal Cancer Prevention Programme-Valencia Region] began in three of the health boards in the Autonomous Region of Valencia in 2005 and has now been extended to all the region's health boards.11 The programmes analysing the complications of colonoscopies report a great variability in the rates of severe complications, ranging from 0% to 28.1‰.12–18 These variations cannot be explained purely by factors related to the screening programme methodology, with the control of a good quality colonoscopy (preparation, sedation, caecal intubation, endoscopic findings, etc.) or characteristics of the people being screened (age, gender, family history, comorbidity, previous treatments, etc.).19,20 They may also be influenced by the thoroughness of the system for identifying complications up to 30 days after an examination which is required of an active search or other methods.
We need to establish a thorough, reliable system capable of obtaining this information in order to accurately determine the extent of the problem of severe complications, identify errors and implement the necessary corrective mechanisms to reduce these figures to acceptable levels for CRCS programmes. The Spanish National Health Service's “minimum basic data set” (MBDS) has been shown to be a valid tool for identifying a number of healthcare processes and health problems.21,22 It can therefore be used as a source of information to identify the complications of colonoscopies and obtain the incidence rate of severe complications, as a quality indicator of the CRCS programmes.
The aim of this study is to identify severe complications of the diagnostic confirmation colonoscopies performed in people with a positive FOBT in the PPCCR-CV from December 2005, when the programme started, to 31 December 2012. We also aim to estimate the incidence rates of severe complications and categorise them as immediate or late, diagnostic or therapeutic, gFOBT or iFOBT and by age and gender.
MethodStudy designThis was a retrospective observational epidemiological study, whose reference population were the participants in the PPCCR-CV with a positive FOBT and an optical colonoscopy performed for diagnostic confirmation, to identify the incidence of severe complications in screening colonoscopies.
Study periodThe study covers from the beginning of the programme, 1 December 2005, to 31 December 2012. The PPCCR-CV is aimed at people aged 50–69. The screening test used is the FOBT, with a 2-year interval between screenings. The gFOBT was used initially, but in 2010 it was replaced by the quantitative iFOBT. Optical colonoscopy is the diagnostic confirmation test following a positive FOBT.11 The programme was progressively implemented across all 24 health boards into which the Valencia Region is divided for provision of health services, achieving 100% coverage of the population in 2014.
During the study period, the PPCCR-CV was being implemented in ten health boards, with a population of 480,331 people aged 50–69 (coverage rate of 45.5%). Three of these health boards were already in the fourth round (every two years the entire population assigned to the health board is invited to participate in the PPCCR-CV), one was in the third round, three in the second round and three in the first round; and for diagnostic confirmation, the endoscopy units of ten Valencian Health Service hospitals were involved in the study.
Sources of information for the studyTo obtain information on possible severe complications of the colonoscopies generated in this period by the PPCCR-CV, three data sources were reviewed:
- -
PPCCR-CV Information System: the endoscopy reports from the programme's own records were analysed for immediate complications.
- -
Hospital Discharge Minimum Basic Data Set (MBDS-HD) Information System: discharge episodes potentially associated with a hospital admission within the 30 days after a PPCCR screening colonoscopy were analysed.
- -
Medical records: To confirm the colonoscopy complications, we carried out an active search of medical records and reviewed episodes of hospital admission associated with screening colonoscopy with diagnostic verification in the hospitals, both public and private, of the 10 health boards involved in the study.
Follow-up of the positive FOBT with indication for colonoscopy from the PPCCR-CV in public and private hospitals: If the patient goes to a private hospital, the hospital is asked for the endoscopy report or the patient is contacted by telephone and asked for the report, in order to enable closure of the diagnostic confirmation process in the programme and to assess whether or not there were any complications resulting from the colonoscopy.
The response variable was the occurrence of a severe complication due to the screening colonoscopy procedure in PPCCR-CV participants with a positive FOBT result.
If the colonoscopy complication occurs at the time of the examination or immediately afterwards, it is recorded in the endoscopy report and is available in the CRCS programme's information system. If the complication occurs later, there is greater difficulty in identifying the episode and associating it with the screening colonoscopy the person underwent.
Colonoscopy complications can be classified as mild or severe. Mild complications include symptoms such as pain or bloating, diarrhoea, constipation, nausea, vomiting, blood in the stool, anal or rectal pain and headaches.
Severe complication was defined according to the definition proposed in the European Guide for the quality of screening and diagnosis of colorectal cancer, “complication which requires hospital admission or causes death due to perforation or haemorrhage which requires transfusion or severe vasovagal syndrome or peritonitis, and occurs within a period of 0 to 30 days from completion of the colonoscopy”.5
A severe complication was considered as an immediate complication when it occurred on the same day as the colonoscopy and as a late complication when it occurred any time from the following day to 30 days after the colonoscopy.
The explanatory variables were:
- -
Period in which colonoscopy performed (in two categories): 1 December 2005 to 31 December 2010 (2005–2010); and 1 January 2011 to 31 December 2012 (2011–2012). There were two separate periods according to the type of FOBT used in the programme: in the first period the gFOBT was used (three consecutive samples and the test being positive if any of the three is positive); and in the second period the quantitative iFOBT was used (one sample and cut-off point for positive test ≥20μg/ml).
- -
Gender: Males and females.
- -
Age (in two categories): (i) people aged 50 to 59 and (ii) people ≥60.
- -
Type of severe complication (in five categories): (i) perforation; (ii) haemorrhage (requiring transfusion); (iii) severe vasovagal syndrome; (iv) peritonitis; and (v) death.
- -
Type of colonoscopy (in two categories): (i) diagnostic and (ii) therapeutic (involving excision of lesions).
In the PPCCR-CV information system, all people who had undergone a colonoscopy for the diagnostic confirmation of colorectal cancer from 2005 to 2012 inclusive were selected. The people who had colonoscopies were definitively identified by their health card number (Sistema de Información Poblacional (SIP) [Population Information System]). Other variables of interest were also obtained, such as date of birth, gender, health board and colonoscopy date.
A registry link-up, based on the SIP number of the selected PPCCR-CV population, was made with the MBDS-HD databases corresponding to the study period and provided by the hospitals of the health boards which formed part of the study. This allowed us to obtain information about episodes of hospital admission in the study period involving people who had had a colonoscopy under the PPCCR-CV.
We selected hospital discharges in the MBDS-HD information system for all episodes of hospital admission involving people who had undergone a colonoscopy under the PPCCR-CV for 30 days starting from the date of the colonoscopy. If there was more than one admission of the same person in the 30 days being considered, we included all individual episodes.
In the episodes identified from the MBDS-HD, we reviewed the main diagnosis and other diagnoses, the main surgical procedure and other procedures, the diagnosis of external cause associated with the main diagnosis, and the morphology codes for neoplasms. The diagnostic codes of the International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]23 considered to select the potential complications of the screening colonoscopy were: 153 –malignant neoplasm of the colon; 154 – malignant neoplasm of the rectum, rectosigmoid junction and anus; 211 – benign neoplasm; 230 – carcinoma in situ of the digestive organs; 235 – neoplasms of uncertain behaviour of digestive and respiratory systems; 239 – neoplasms of unspecified nature; 567 – peritonitis and retroperitoneal infections; 569 – other disorders of intestine; 578 – gastrointestinal haemorrhage; 780 – general symptoms; 785 – symptoms involving cardiovascular system; 998 – other complications of procedures not elsewhere classified; V58 – encounter for other and unspecified procedures and aftercare; V76 – special screening for malignant neoplasms; E870 – accidental cut puncture perforation or haemorrhage during medical care; and 99 – other nonoperative procedures.
We obtained the SIP number, the medical record number, health board, date of admission and date of discharge from the MBDS-HD for the people who had been admitted to hospital and met these conditions.
Once each person was identified through the SIP number, the medical records were reviewed in collaboration with those in charge of the PPCCR-CV for the Public Health Centres and the healthcare professionals in the Gastroenterology Departments and Clinical Documentation Services of the 10 health boards in the study.
The results of the active search in the medical records were verified against the information provided with the MBDS-HD, in order to confirm the true severe complications associated with screening colonoscopy, and other situations, such as mild complications, other colorectal diseases and other causes of admission unrelated to colonoscopy.
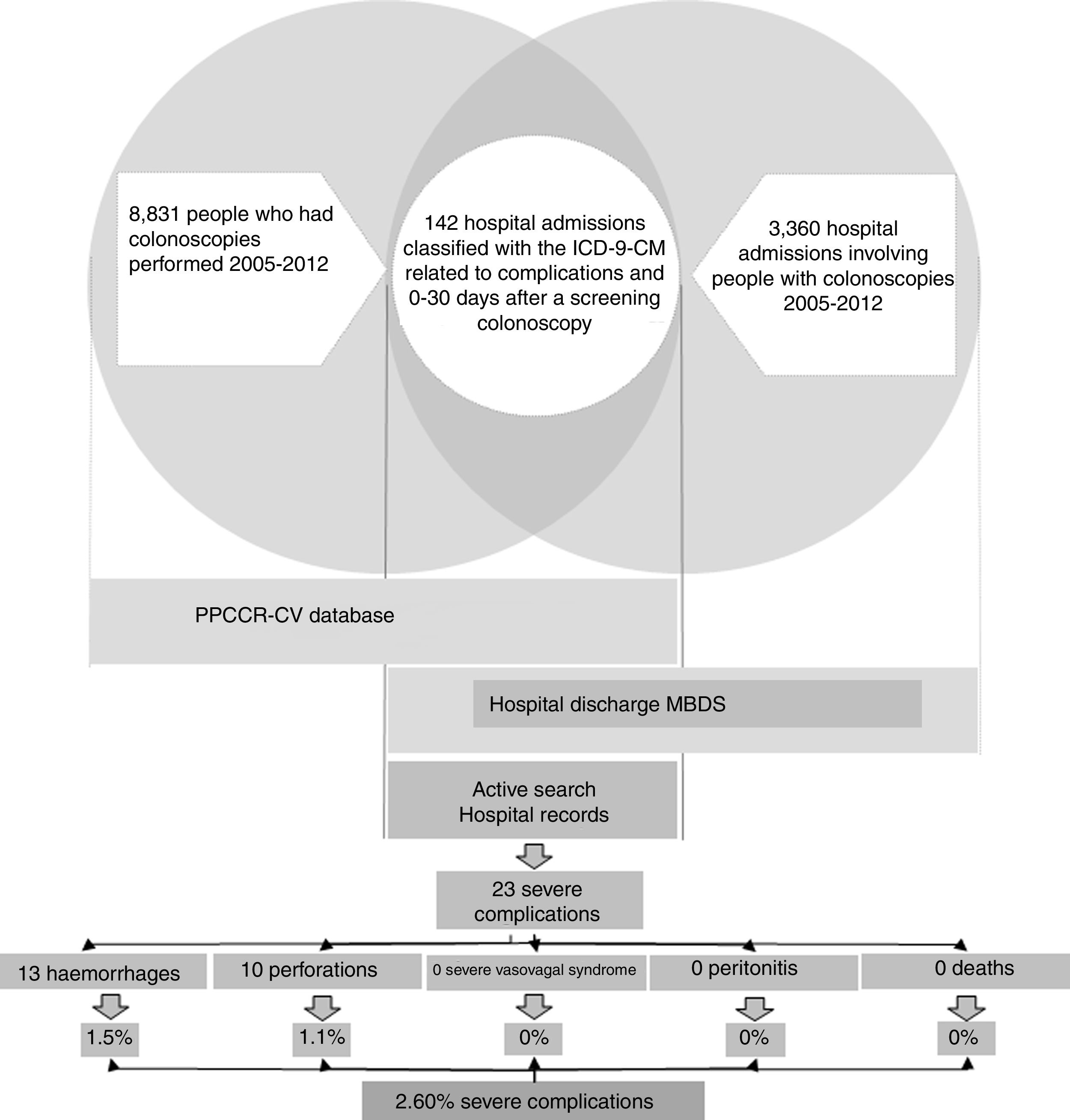
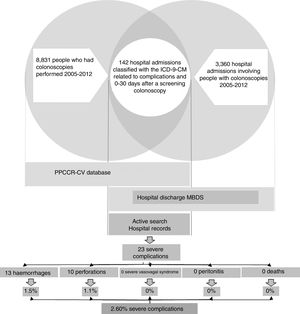
In the case of the true severe colonoscopy-related complications, we obtained information about the process and each person's subsequent health status (Fig. 1).
Statistical analysisWe have described the number of colonoscopies performed and the number of severe complications. For severe complications, we calculated total, immediate and late complication rates per 1000 colonoscopies performed.
Using the Chi-square test we carried out a descriptive bivariate analysis comparing the development of complications (yes/no) according to the year the colonoscopy was performed, gender and age.
We also performed a descriptive analysis using contingency tables to show the relationship between the times of onset (immediate or late), the types of complication (perforation or haemorrhage) and the types of colonoscopy (diagnostic or diagnostic-therapeutic). To compare these variables, the Chi-square test with Yates’ correction for continuity was used.
The analysis was carried out with the statistical programme R.
Ethical considerationsThis study was conducted in the context of evaluating the General Directorate of Public Health's Colorectal Cancer Prevention Programme. The people participating in the PPCCR-CV are informed that the information may be used for research purposes and that they may refuse to provide their consent. The study was conducted in accordance with the principles of the Declaration of Helsinki and Spanish legal requirements on confidentiality.
ResultsDuring the study period, 2005–2012, 242,302 people aged 50–69 and resident in the Autonomous Region of Valencia took part in the PPCCR-CV. Out of that total, 10,158 people had a positive FOBT and 8831 screening colonoscopies were performed.
The registry link based on the SIP number between the PPCCR-CV information system and the MBDS-HD enabled us to identify 202 episodes of hospital admission from the day of the screening colonoscopy to 30 days later. Of these, 142 included among the discharge diagnoses one or more of the ICD-9-CM codes selected as possible causes of complications of the screening colonoscopy (Table 1).
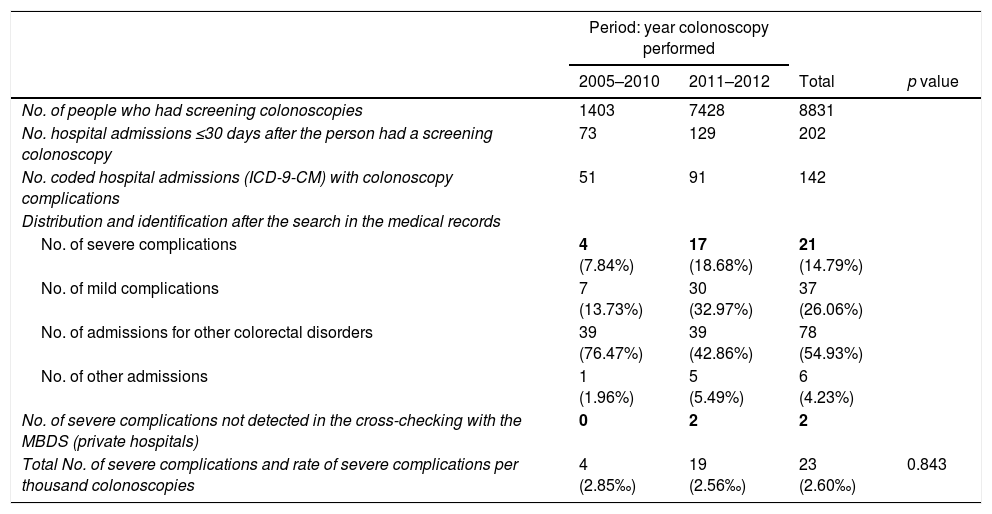
Identification and rate of severe complications of screening colonoscopies performed in the period 2005–2012.
| Period: year colonoscopy performed | ||||
|---|---|---|---|---|
| 2005–2010 | 2011–2012 | Total | p value | |
| No. of people who had screening colonoscopies | 1403 | 7428 | 8831 | |
| No. hospital admissions ≤30 days after the person had a screening colonoscopy | 73 | 129 | 202 | |
| No. coded hospital admissions (ICD-9-CM) with colonoscopy complications | 51 | 91 | 142 | |
| Distribution and identification after the search in the medical records | ||||
| No. of severe complications | 4 (7.84%) | 17 (18.68%) | 21 (14.79%) | |
| No. of mild complications | 7 (13.73%) | 30 (32.97%) | 37 (26.06%) | |
| No. of admissions for other colorectal disorders | 39 (76.47%) | 39 (42.86%) | 78 (54.93%) | |
| No. of other admissions | 1 (1.96%) | 5 (5.49%) | 6 (4.23%) | |
| No. of severe complications not detected in the cross-checking with the MBDS (private hospitals) | 0 | 2 | 2 | |
| Total No. of severe complications and rate of severe complications per thousand colonoscopies | 4 (2.85‰) | 19 (2.56‰) | 23 (2.60‰) | 0.843 |
After the active search and review of the 142 medical records, 78 admissions were identified related to other colorectal diseases (54.93%), 37 for mild complications (26.06%), six due to other hospital admissions (4.23%) and 21 resulting from true severe complications (14.79%). Two further severe complications from private hospitals were detected through the follow-up performed in the PPCCR-CV of people with a positive FOBT who are referred for diagnostic confirmation by colonoscopy. In total, 23 severe complication events associated with screening colonoscopy were identified, with a severe complication rate of 2.60‰.
During the period 2005–2010, when the gFOBT was being used, 1403 colonoscopies were performed and in the active search of 51 sets of medical records, four severe complications were identified (incidence rate 2.85‰).
During the period 2011–2012 in which the iFOBT was used, 7427 colonoscopies were performed. After reviewing 91 sets of medical records, 17 severe complications were identified in public hospitals and two in private hospitals (incidence rate 2.56‰). No statistically significant differences were found between the two periods.
In terms of the timing of the complication, of the 23 severe complications, 14 were immediate (incidence rate 1.59‰) and nine were late (incidence rate 1.02‰).
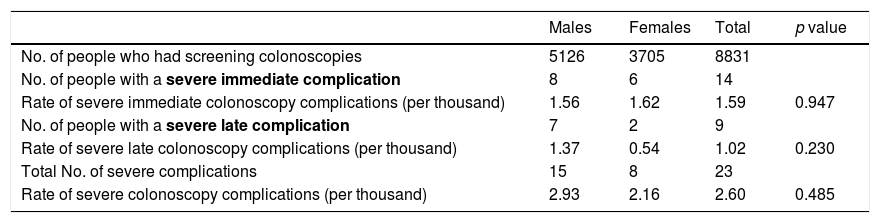
No statistically significant differences were found when stratifying the severe complications associated with screening colonoscopy by timing and gender (Table 2). Out of the 5126 colonoscopies performed in males, 15 severe complications were detected (incidence rate 2.93‰), while eight severe complications were identified out of the 3705 colonoscopies performed in females (incidence rate 2.16‰). In males, the severe complication rates were 1.56‰ for immediate and 1.37‰ for late. In females, the severe complication rates were 1.62‰ for immediate and 0.54‰ for late.
Rate of severe complications by timing and gender.
| Males | Females | Total | p value | |
|---|---|---|---|---|
| No. of people who had screening colonoscopies | 5126 | 3705 | 8831 | |
| No. of people with a severe immediate complication | 8 | 6 | 14 | |
| Rate of severe immediate colonoscopy complications (per thousand) | 1.56 | 1.62 | 1.59 | 0.947 |
| No. of people with a severe late complication | 7 | 2 | 9 | |
| Rate of severe late colonoscopy complications (per thousand) | 1.37 | 0.54 | 1.02 | 0.230 |
| Total No. of severe complications | 15 | 8 | 23 | |
| Rate of severe colonoscopy complications (per thousand) | 2.93 | 2.16 | 2.60 | 0.485 |
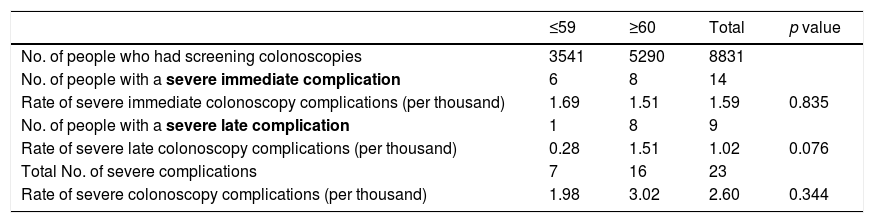
In relation to age (Table 3), the severe complication rate in the ≤59 age group was 1.98‰ (based on 7 events in 3541 screening colonoscopies). In the older age group (≥60), out of the 5290 colonoscopies performed, 16 severe complications were detected (incidence rate 3.02‰). The younger age group (≤59) had severe complication rates of 1.69‰ for immediate and 0.28‰ for late, while in the older age group (≥60) the rate was 1.51‰ for both immediate and late severe complications. No statistically significant differences were found.
Rate of severe complications by timing and age.
| ≤59 | ≥60 | Total | p value | |
|---|---|---|---|---|
| No. of people who had screening colonoscopies | 3541 | 5290 | 8831 | |
| No. of people with a severe immediate complication | 6 | 8 | 14 | |
| Rate of severe immediate colonoscopy complications (per thousand) | 1.69 | 1.51 | 1.59 | 0.835 |
| No. of people with a severe late complication | 1 | 8 | 9 | |
| Rate of severe late colonoscopy complications (per thousand) | 0.28 | 1.51 | 1.02 | 0.076 |
| Total No. of severe complications | 7 | 16 | 23 | |
| Rate of severe colonoscopy complications (per thousand) | 1.98 | 3.02 | 2.60 | 0.344 |
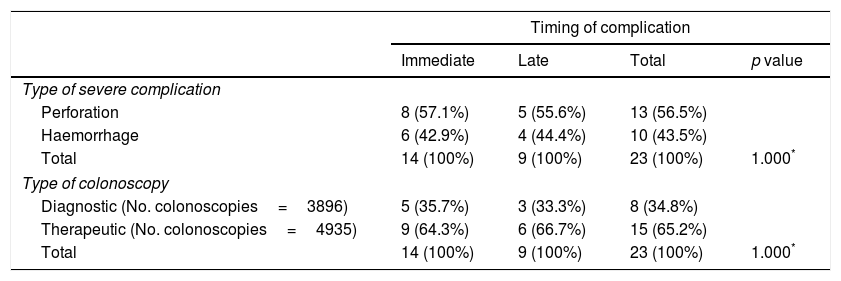
Of the 23 severe complications occurring during the study period (Table 4), 13 involved perforations (56.5%) and 10 were haemorrhages requiring transfusion (43.5%); there were no hospital admissions for severe vasovagal syndrome, peritonitis or death. Of the immediate colonoscopy complications, 57.1% were perforations and 42.9% were haemorrhages. Of the late complications, 55.6% were perforations and 44.4% were haemorrhages.
Severe complications of screening colonoscopies according to timing of complication, type of severe complication (perforation, haemorrhage) and type of colonoscopy (diagnostic or therapeutic).
| Timing of complication | ||||
|---|---|---|---|---|
| Immediate | Late | Total | p value | |
| Type of severe complication | ||||
| Perforation | 8 (57.1%) | 5 (55.6%) | 13 (56.5%) | |
| Haemorrhage | 6 (42.9%) | 4 (44.4%) | 10 (43.5%) | |
| Total | 14 (100%) | 9 (100%) | 23 (100%) | 1.000* |
| Type of colonoscopy | ||||
| Diagnostic (No. colonoscopies=3896) | 5 (35.7%) | 3 (33.3%) | 8 (34.8%) | |
| Therapeutic (No. colonoscopies=4935) | 9 (64.3%) | 6 (66.7%) | 15 (65.2%) | |
| Total | 14 (100%) | 9 (100%) | 23 (100%) | 1.000* |
In terms of type of colonoscopy, 34.8% of the severe complications occurred in a diagnostic colonoscopy and 65.2% when the procedure was therapeutic, i.e. when excision of a polyp and/or cancer was required. Of the immediate severe complications, 35.7% were in diagnostic colonoscopies and 64.3% in therapeutic. Of the late severe complications, 33.3% were in diagnostic colonoscopies and 66.7% in therapeutic. The severe complication rates were 2.05‰ for the diagnostic and 3.04‰ for the therapeutic colonoscopies.
DiscussionIn our study, the incidence rate of severe complications for the colonoscopies performed in the PPCCR-CV during the entire period was 2.60‰, with rates of 1.47‰ for perforations and 1.13‰ for haemorrhages. The incidence rate of severe colonoscopy screening complications during the period in which the gFOBT was used (2.85‰) was slightly higher than during the period in which the iFOBT was used (2.56‰), although the differences were not statistically significant. The rates obtained in our study are below the reference rates for the gFOBT in the European quality assurance guidelines for screening.5
Most of the studies conducted in Spain on colonoscopy complications have obtained higher rates than ours. In 2007, with two rounds of screening using gFOBT, Peris et al.12 detected six severe complications (two bradycardias, three haemorrhages and one perforation) out of 623 colonoscopies performed (a rate of 9.6‰). Brugos-Llamazares et al.14 identified two haemorrhages from 71 colonoscopies performed from 2008 to 2010 with qualitative iFOBT (haemorrhage incidence rate of 28.2‰). For the period 2009–2011, when iFOBT was used, Portillo et al.15 detected 56 severe complications (29 haemorrhages, 15 perforations and 12 vasovagal syndromes) in 9277 colonoscopies. However, only 29 required hospitalisations (rate of 3.1‰). In a time series of five rounds of screening (2000–2012), Binefa et al.17 described rates of 9.0–11.1‰ for the gFOBT and 8.7‰ for iFOBT. However, in the randomised, controlled clinical trial conducted in Spain (ColonPrev), an incidence rate of 1 in 1000 severe complications was estimated in the group screened by iFOBT followed by diagnostic colonoscopy,24 somewhat below that observed in our study. The European guidelines on quality assurance in screening5 report an incidence of severe complications of 5–16 per 1000 people with colonoscopy performed in the CRCS programmes with gFOBT as the screening test.
The rates for late severe complications in the population-based screenings also vary according to the study, with rates in the range of 0.79–8.4 per 1000 colonoscopies in the Centres for Disease Control and Prevention (CDC) programme.25 The immediate complication rate in CRCS programmes in the United States (CORI database) is estimated to be 12.9 per 1000 colonoscopies.26 According to Kaminski et al.6 over half of all complications are late complications. In our study, the severe complication rates were 1.59‰ for immediate and 1.02‰ for late, meaning that 61% of complications were immediate.
Perforation was more common among the immediate complications (57.1% vs. 55.6%), while haemorrhages were slightly more common in late complications (44.4% vs. 42.9%). In Germany, in a context more similar to that of our programme, although with the population screening data based on colonoscopies as a screening test, among other adverse effects, complication rates due to perforation and haemorrhages were estimated to be 0.8 and 0.5 per 1000 colonoscopies.27 These values are somewhat lower than those found in our programme (1.1‰ and 1.5‰, respectively).
As we have seen, the estimations of these rates vary considerably. However, much of the variation can be attributed to the recent implementation of screening programmes, differences in populations, the duration of the study period, different organisational models for population programmes, type of FOBT, the number and experience of endoscopists involved in the screening colonoscopies19 and the method used to identify complications. It is important to identify and record the rate of severe complications in CRCS programmes. In our study, the method used with the MBDS-HD information system enabled us to simply and comprehensively identify severe complications by investigating hospital discharge reports. This method meant it was not necessary to review all the colonoscopies performed, delimiting the population and directing us towards a small number of people likely to have had a severe complication deriving from the CRCS programme.
Few of the available studies describe the complication rates by age and gender. We found a higher rate of complications among males (2.93‰) than among females (2.16‰) and in older age groups (3.02‰ for those ≥60 and 1.98‰ for those ≤59). Late severe complications in particular were greater in males than in females (1.37‰ vs. 0.54‰) although no statistically significant differences were found.
There is more likelihood of complications if the investigation involves excision of polyps or cancerous lesions.7,19 In our study, we found that complications occurred more frequently in therapeutic colonoscopies (65.2%) than in diagnostic colonoscopies (34.8%). There were also more late than immediate complications (66.7% vs. 64.3%). However, in the complications related to diagnostic colonoscopies, immediate complications were more common than late complications (35.7% vs. 33.3%).
Severe complications appear to be more likely to occur in males, at older ages, and in colonoscopies with excision of lesions. Specific epidemiological studies are needed to identify the risk factors behind the characteristics determining a greater disposition to complications, in order to add to those we are already aware of, such as the quality of the preparation, anaesthesia, the experience of the endoscopist and the location of the lesion.
One of the limitations of our study is that the results were not significant. Although our sample corresponded to the entire period studied in the programme, it may have been insufficient. The results should therefore be interpreted with caution. In terms of the methodology used, the greatest limitation is the difficulty obtaining information from private hospitals, because all the information is not available in the MBDS-HD. The active search for information in the medical records is therefore essential for identifying severe complications of colonoscopies.
ConclusionsThe PPCCR-CV has a severe complication rate of 2.60 per 1000 colonoscopies, lower than most of the studies in our region, although figures vary greatly. All the people with severe complications recovered from the complication. The severe complication rates were higher with the gFOBT (2.85‰), in males (2.93‰), in people aged ≥60 (3.02‰) and in therapeutic colonoscopies (3.04‰).
Severe complication rates for screening colonoscopies in colorectal cancer prevention programmes are an indicator of quality. A significant number of late complications of screening colonoscopies end up not being registered unless an active search is performed. Severe colonoscopy complications can be identified by investigating hospital discharge reports. It is essential for complications to be properly investigated, in order to maintain an acceptable balance between the benefits and adverse effects of CRCS.
FundingThis research did not receive specific financial assistance from public sector agencies, the commercial sector or non-profit organisations.
Conflicts of interestNone declared.
This study was conducted in the context of evaluating the General Directorate of Public Health's Colorectal Cancer Prevention Programme. The authors thank the professionals of the Valencian Health Service who participate in the programme for their contribution to early diagnosis and to improving the population's health.
Carmen Fernández García, María José Valverde Roig, Josefa Ibáñez Cabanell, Dolores Salas Trejo (DGSP); M.a José Ripoll Toledo (CSP Alcoi); Carmen López-Quiñones Llamas y María Plasencia Dolz (CSP Alicante); Asensio García Machi y Xavi Torremocha Vendrell (CSP Alzira); M.a Teresa Pedrosa Roca (CSP Benicarló); Isabel Sáez Lloret (CSP Benidorm); Gloria Teruel Teruel y M.a José Pérez Martínez (CSP Castellón); María Gironés Gil y Cristóbal Llorens Ivorra (CSP Denia); Juana Llompart Vidal y Sara Montoya Jiménez (CSP Elx); Gerardo Arroyo Fernández (CSP Elda); Rebeca Cremades Pallas y Ruth Risueño Albuixec (CSP Gandía); Vicente Carrasco Arroyo y Mercedes Andrés Martínez (CSP Manises); Rosa María Navarro González y Patricia López Medina (CSP Orihuela); Isabel Villena Blázquez y Teresa Tasa Zapater (CSP Torrent); Diana Patricia Botella de Maglia y Rubén Muñoz Aznar (CSP Utiel); Susana Castán Cameo, Inmaculada González Serrano, Amparo Lluch Esteve, Patricia Escobar Flores, Etel Trullenque Molina, Begoña Medina Cortés y Silvia Gimeno Martos (CSP Valencia); Tamara Sendra Barbosay Óscar Plá Femenía (CSP Xàtiva). Dirección General deSalud Pública (DGSP) y Centros de Salud Pública (CSP) del Sistema Valenciano de Salud. Conselleria de Sanidad Universal y Salud Pública. Generalitat Valenciana.
Please cite this article as: Ibáñez J, Vanaclocha-Espí M, Pérez-Sanz E, Valverde MJ, Sáez-Lloret I, Molina-Barceló A, et al. Complicaciones graves en las colonoscopias de cribado del cáncer colorrectal en la Comunidad Valenciana. Gastroenterol Hepatol. 2018;41:553–561.
The names of the components of the Working Group of the Colorectal Cancer Prevention Program of the Comunitat Valenciana (Spain) are listed in Annex 1.