Autoimmune hepatitis (AIH) with characteristics of primary biliary cholangitis (PBC) is known as overlap syndrome. Its prevalence and prognosis have not yet been determined comparatively with AIH.
MethodsA retrospective cohort study was conducted comparing patients diagnosed with AIH and AIH-PBC overlap syndrome, followed-up for seven years in a university hospital in Colombia, until 31 December 2016.
ResultsA total of 210 patients were included (195 women, mean age 48.5 years). Of these, 32 (15.2%) had AIH-PBC overlap syndrome. At diagnosis, no significant differences were found by demographic profile, positive autoantibodies (ANA, ASMA), except AMA (81.2% vs 3.9%, p<0.001), and histological grade of fibrosis. The most frequent clinical presentations were nonspecific symptoms in AIH-PBC and acute hepatitis in AIH. Although there were no significant differences, AIH showed a greater biochemical response to immunosuppressive management (87.3% vs 74.2%, p=0.061) and a greater number of relapses in those who achieved partial or complete remission during treatment (12.4% vs 7.63%; p=0.727). Patients with AIH-PBC had greater progression to cirrhosis (22.2% vs 13.1%, p=0.038), even in those who achieved partial or complete biochemical remission without relapse, with greater indication of orthotopic liver transplantation (p=0.009), but not retransplantation (p=0.183); there were no differences in mortality.
ConclusionsAIH-PBC overlap syndrome accounts for a significant proportion of patients with AIH, with greater progression to cirrhosis, indication of liver transplantation and possibly retransplantation. This higher risk of adverse outcomes suggests closer monitoring, probably with follow-up until confirmed histopathological remission.
La hepatitis autoinmune (HAI) con características de colangitis biliar primaria (CBP) es conocida como síndrome de superposición. Su prevalencia y pronóstico aún no han sido determinados comparativamente con aquellos con HAI.
MétodosSe realizó un estudio de cohorte retrospectiva comparando pacientes con diagnóstico de HAI y síndrome de superposición por HAI-CBP, seguidos por 7años en un hospital universitario de Colombia, hasta el 31 de diciembre de 2016.
ResultadosSe incluyeron 210 pacientes (195 mujeres, edad media 48,5años), de los cuales 32 (15,2%) tenían síndrome de superposición HAI-CBP. Al diagnóstico no se hallaron diferencias significativas por perfil demográfico, autoanticuerpos positivos (ANA, ASMA) excepto AMA (81,2% vs 3,9%; p<0,001) y grado histológico de fibrosis. La presentación clínica más frecuente en HAI-CBP fueron síntomas inespecíficos y en HAI, hepatitis aguda. Aunque con diferencias no significativas, en HAI se presentó mayor respuesta bioquímica al manejo inmunosupresor (87,3% vs 74,2%; p=0,061) y mayor número de recaídas en quienes lograron remisión parcial o completa durante tratamiento (12,4% vs 7,63%; p=0,727). Los pacientes con HAI-CBP tuvieron mayor progresión a cirrosis (22,2% vs 13,1%; p=0,038), incluso quienes lograron remisión bioquímica parcial o completa sin recaída, mayor indicación de TOH (p=0,009), pero no retrasplante (p=0,183); no hubo diferencias en la mortalidad.
ConclusiónEl síndrome de superposición HAI-CBP constituye una proporción no despreciable entre aquellos con HAI, con mayor progresión a cirrosis, indicación de trasplante hepático y posiblemente retrasplante. Este mayor riesgo de desenlaces adversos sugiere seguimiento más estricto, probablemente con seguimiento hasta remisión histopatológica confirmada.
Aside from the classic autoimmune liver diseases such as autoimmune hepatitis (AIH), primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC) with their own diagnostic criteria,1 the simultaneous coexistence of two disorders which cannot be assimilated into conventional diagnostic categories is known as overlap syndrome.
The concurrent or consecutive development of PBC with AIH, although recognised, is still subject to debate in terms of its pathogenesis and whether or not it actually represents a variant AIH or PBC.2 Moreover, the diagnostic criteria, which include applying the simplified criteria for AIH3 to patients with overlap syndrome, are not yet standardised. The most accepted approach to diagnosis up to now has been the presence of two out of three of the main characteristics of each disorder proposed by Chazouillères et al.4 These criteria have been incorporated into the guidelines of the European Association for the Study of the Liver (EASL).5
After only small series of AIH-PBC overlap having been reported6–8 since the 1970s, it was considered to be a rare syndrome and even suggested that the name overlap syndrome be abandoned.9 However, subsequent series described in different parts of the world,9–11 some comparing the clinical and prognostic characteristics of PBC and AIH-PBC overlap syndrome, report a worse prognosis than in PBC due to a greater degree of fibrosis at diagnosis, a worse biochemical response to ursodeoxycholic acid (UDCA), and worse progression to fibrosis and related mortality rates. Despite those findings, there are no large series comparing patients with AIH and AIH-PBC overlap syndrome, and it is therefore unclear whether or not the outcomes of these two disorders differ.
In this study, we re-assessed patients characterised in a previous AIH study12 with confirmed diagnosis of AIH and AIH-PBC overlap syndrome, with the aim of determining the prevalence of AIH-PBC overlap syndrome in classic AIH and the differential characteristics, both clinical at the time of diagnosis and disease course and prognosis with the treatment.
MethodsDesign and sampleThis study is a retrospective analytical cohort, with convenience sampling based on patients diagnosed with AIH according to the International Classification of Diseases (ICD-10) in electronic medical records at Hospital Pablo Tobón Uribe (HPTU) in the city of Medellín (Colombia) from January 2010 to 31 December 2016.
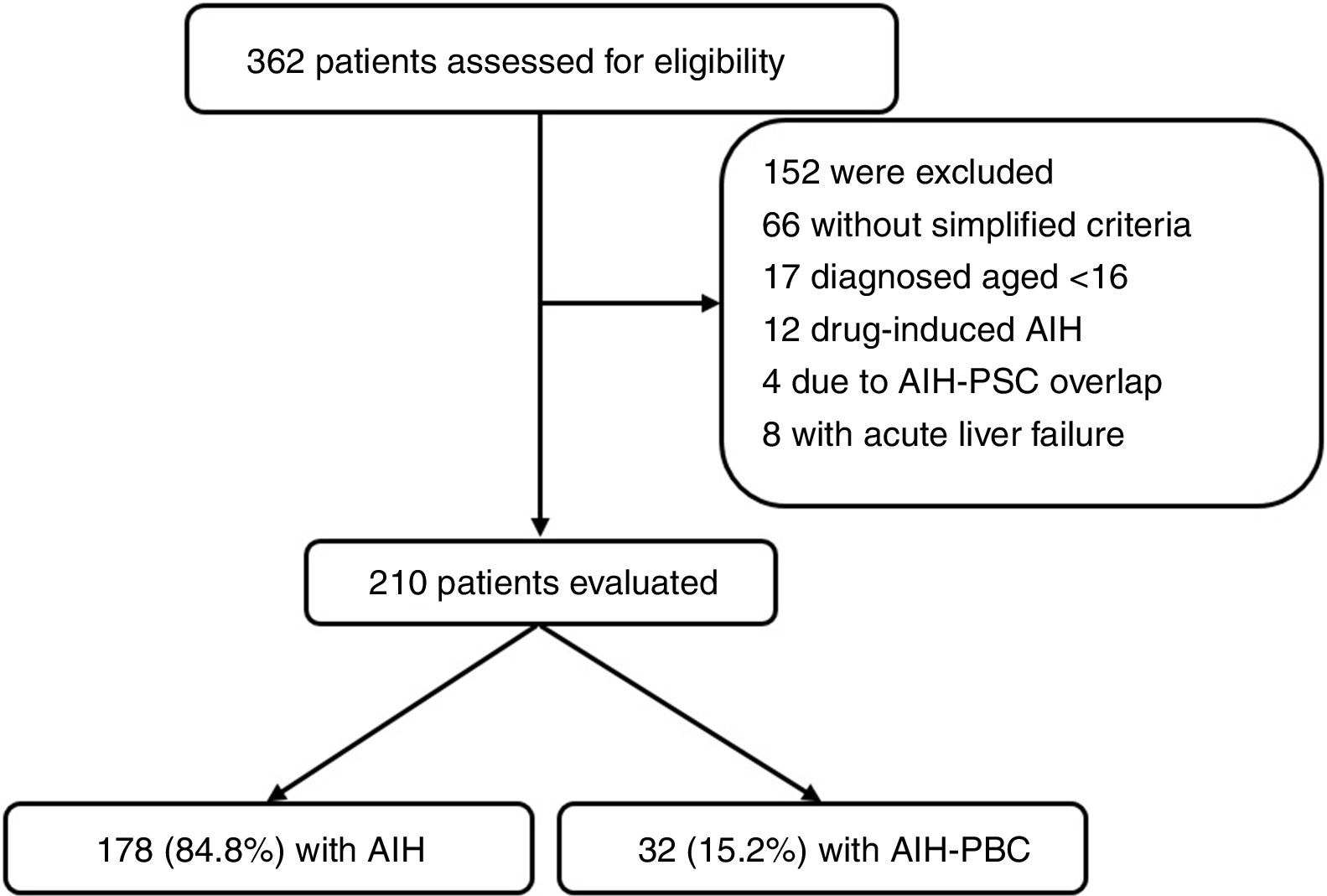
We included patients with a complete medical history and initial diagnosis of AIH and AIH-PBC overlap confirmed by biopsy and simplified autoimmune hepatitis scoring3 and Paris criteria4 respectively, from the moment diagnosis was suspected, through follow-up by the treating hepatologist, and up to the last outpatient or hospital medical record. The reasons for exclusion were: patient aged under 16; drug-induced AIH; acute liver failure; score below 6 according to the simplified AIH criteria; or less than two criteria for each disorder (AIH and PBC) according to Paris criteria for diagnosis of AIH and overlap respectively. Patients with no available liver biopsy were also excluded.
All the included patients had test results ruling out different aetiologies, such as viral hepatitis (anti HCV, anti-HBc, HBsAg and anti-HBs), toxic or metabolic hepatitis (α-1 antitrypsin, transferrin saturation, ferritin, ceruloplasmin) and other autoimmune liver diseases (PSC).
The computerised database was created by the gastrohepatology group, with variables according to standard definitions. Sociodemographic variables (age, gender, race) and clinical variables were included, such as the form of presentation: asymptomatic alteration of liver biochemistry; nonspecific symptoms (asthenia, adynamia, hyporexia and pyrexia); acute hepatitis (jaundice, abdominal pain, pyrexia and elevation of transaminases at least three times the upper limit of normal); or liver cirrhosis diagnosed by clinical and any imaging or biopsy technique. Also included were the levels of anti-nuclear antibodies (ANA), anti-smooth muscle antibodies (ASMA), anti-mitochondrial antibodies (AMA), levels of serum IgG, AST, ALT, alkaline phosphatase, total bilirubin, serum albumin, prothrombin time, and INR at diagnosis and changes in these parameters with the treatment.
The histological findings were evaluated at diagnosis by two pathologists highly experienced in liver biopsy. They were not informed of the patients’ clinical or follow-up data. The findings were classified as: typical histology of autoimmune hepatitis if they had interface hepatitis, lymphoplasmacytic infiltrate in portal tracts extending into the lobule, emperipolesis and rosette formation; compatible histology if they had chronic hepatitis with lymphocytic infiltration; and atypical histology if they had histological signs other than AIH, such as steatohepatitis.3 The histological feature needed for PBC in overlap syndrome was florid lesion of interlobular and septal bile ducts.
Liver fibrosis stage was assessed with the METAVIR scoring system (F0–F4): stage 0 (F0) no fibrosis; stage 1 (F1) mild fibrosis; stage 2 (F2) moderate fibrosis; stage 3 (F3) severe fibrosis; stage 4 (F4) cirrhosis.13
A simplified diagnostic criteria score from the International Autoimmune Hepatitis Group (IAIHG) was included (<6 points: not compatible; probable if >6 points, or definite if ≥7 points),3 or calculated if it had not been recorded. For the Paris criteria, at least two out of three criteria for PBC and AIH need to be present for the diagnosis of overlap syndrome. AIH: I) elevation of ALT≥5 times the upper limit of normal (ULN); II) serum IgG levels≥twice the ULN and/or positive ASMA; and III) liver biopsy with moderate or severe periportal or periseptal lobular inflammation. PBC: (I) ALP≥twice ULN or GGT≥5 times ULN; II) AMA≥1:40; III) biopsy with florid bile duct lesion.4
Biochemical remission, incomplete response, treatment failure, relapse, progression to cirrhosis, indication of liver transplantation, cirrhosis-related death and all-cause mortality were all assessed for both the induction and maintenance treatment.
Biochemical remission was defined as the return to normal of transaminase and IgG levels; incomplete response as improvement without reaching remission levels; relapse as a new elevation of ALT>3 times the ULN according to the IAIHG criteria or increased levels of IgG; and treatment failure as clinical, biochemical and histological worsening despite the treatment.1 The cholestatic component of PBC in overlap syndrome was assessed as follows: ALP≥1.5 times ULN or bilirubin>1mg/dl after a year to determine treatment failure14; this was evaluated in conjunction with the hepatocellular component to define whether or not any response was achieved.
Statistical analysisCategorical variables are presented as absolute frequencies or percentages, and continuous variables as mean and standard deviation if normal or median distribution, and interquartile range (IQR) where distribution was not normal according to the Kolmogorov–Smirnov test. Differences between groups were established with the Chi-square test for categorical variables and the Mann–Whitney U test for median difference. The actuarial probabilities were calculated using the Kaplan–Meier method; the data were censored at the date of the event, orthotopic liver transplantation (OLT), date of last visit or at the end of the follow-up period; the patients with OLT were censored as still alive. All the p-values were calculated for two tails, with statistical significance considered when p<0.05. All the analyses were performed with the statistical software program SPSS version 20.1 (SPSS Inc.).
The preparation and final presentation of the article took into account the STROBE recommendations for the reporting of observational studies.15
The study was conducted at all times in accordance with international parameters for studies with humans, such as the Declaration of Helsinki and Colombian regulations supported by resolution 8430 of 1993, and considered as risk-free research. The protocol was evaluated and approved by the HPTU ethics committee and human research committee. Given the retrospective nature of the study, informed consent was not required.
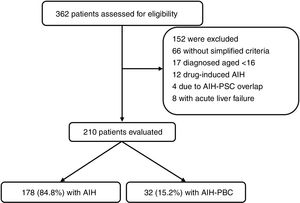
ResultsFrom January 2010 to December 2016 a total of 362 patients were registered by ICD-10 diagnostic code as potentially having AIH; of these, 152 were excluded (Fig. 1). The main reasons for exclusion were drug-induced AIH, overlap syndrome (AIH-PBC) and acute liver failure (due to negative immune profile, negative IgG or limitations in carrying out liver biopsy). In total, 210 were included for the analysis, 178 (84.8%) of whom had AIH and 32 (15.2%) AIH-PBC overlap; median follow-up was for 47.8 months (IQR 14–69) and 52 months (IQR 23.7–68.5) respectively, with no significant differences (p=0.4).
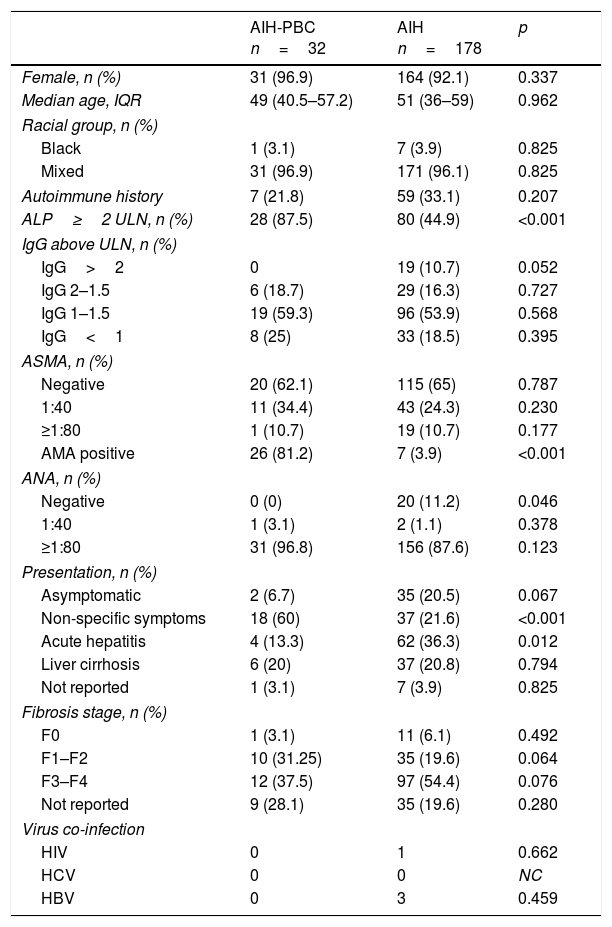
The age range was 16–82 in the AIH group and 31–78 in the AIH-PBC group, 92.1% and 96.9% respectively were female, and 96.1% and 96.9% respectively were of mixed race (Table 1).
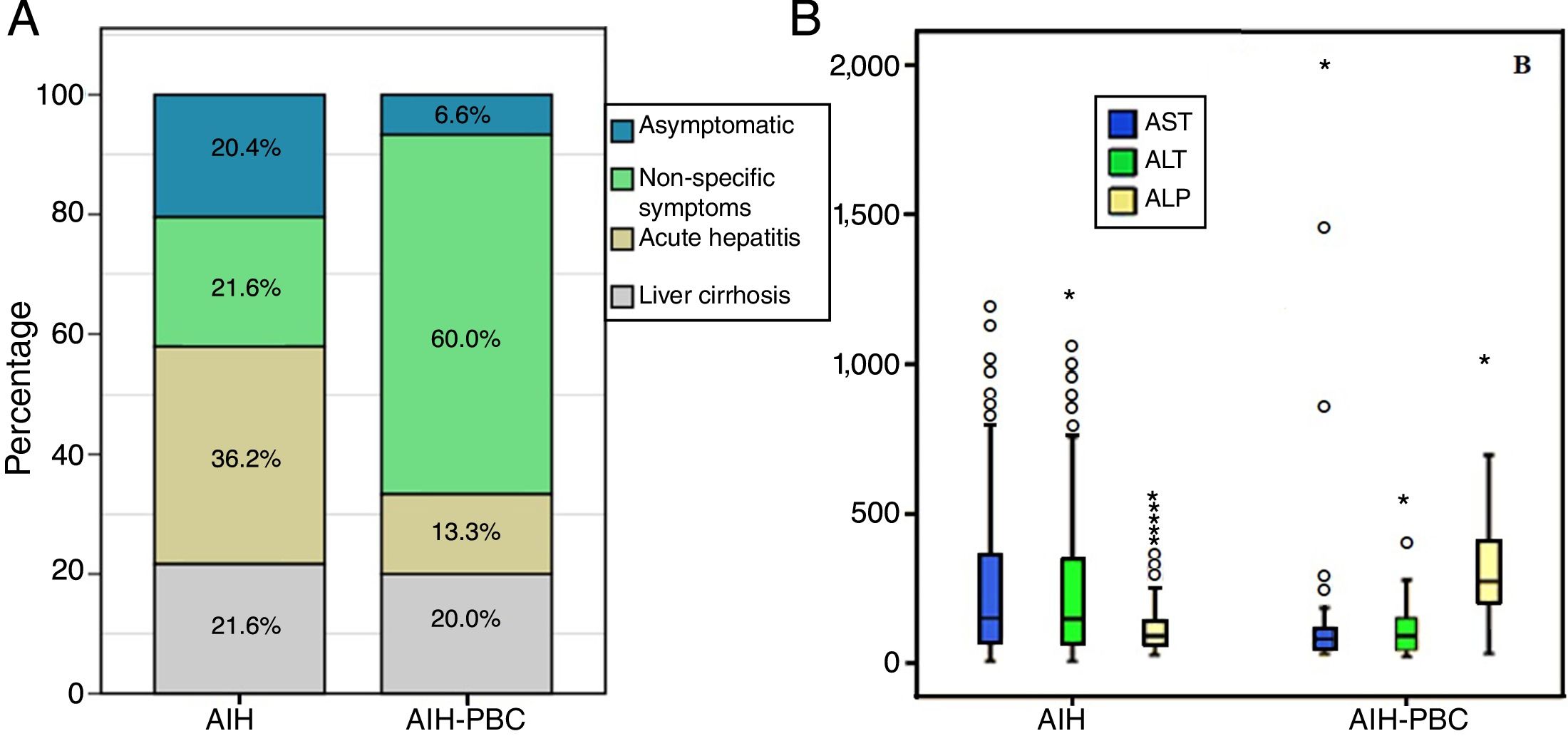
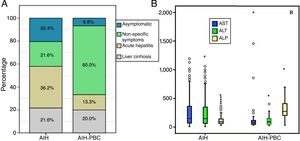
At the time of diagnosis no statistically significant differences were found in history of autoimmunity. However, statistically significant differences were found in the form of clinical presentation, with acute hepatitis being more common in AIH (36.3%, n=62% vs 13.3%, n=2; p=0.012) than in those with overlap syndrome, in whom non-specific symptoms was more common (21.6%, n=37% vs 60%, n=18; p<0.001) (Fig. 2A). There were no differences in the degree of liver fibrosis at the time of diagnosis; however, this information was not reported in 35 patients with AIH and in 9 patients with AIH-PBC, respectively.
According to the differential liver analysis at diagnosis, patients with AIH-PBC had significantly higher levels of alkaline phosphatase (539 vs 229U/l; p<0.001), and a higher proportion were AMA positive (81.2% vs 3.9%; p<0.001) (Tables 1 and 2). In contrast, the medians of AST and ALT were significantly higher in patients with AIH (p=0.011 and p=0.033, respectively) than in those with overlap syndrome (Fig. 2B).
Baseline characteristics. Comparison of demographic characteristics, seropositivity, AIH score, clinical presentation and histology at diagnosis.
| AIH-PBC n=32 | AIH n=178 | p | |
|---|---|---|---|
| Female, n (%) | 31 (96.9) | 164 (92.1) | 0.337 |
| Median age, IQR | 49 (40.5–57.2) | 51 (36–59) | 0.962 |
| Racial group, n (%) | |||
| Black | 1 (3.1) | 7 (3.9) | 0.825 |
| Mixed | 31 (96.9) | 171 (96.1) | 0.825 |
| Autoimmune history | 7 (21.8) | 59 (33.1) | 0.207 |
| ALP≥2 ULN, n (%) | 28 (87.5) | 80 (44.9) | <0.001 |
| IgG above ULN, n (%) | |||
| IgG>2 | 0 | 19 (10.7) | 0.052 |
| IgG 2–1.5 | 6 (18.7) | 29 (16.3) | 0.727 |
| IgG 1–1.5 | 19 (59.3) | 96 (53.9) | 0.568 |
| IgG<1 | 8 (25) | 33 (18.5) | 0.395 |
| ASMA, n (%) | |||
| Negative | 20 (62.1) | 115 (65) | 0.787 |
| 1:40 | 11 (34.4) | 43 (24.3) | 0.230 |
| ≥1:80 | 1 (10.7) | 19 (10.7) | 0.177 |
| AMA positive | 26 (81.2) | 7 (3.9) | <0.001 |
| ANA, n (%) | |||
| Negative | 0 (0) | 20 (11.2) | 0.046 |
| 1:40 | 1 (3.1) | 2 (1.1) | 0.378 |
| ≥1:80 | 31 (96.8) | 156 (87.6) | 0.123 |
| Presentation, n (%) | |||
| Asymptomatic | 2 (6.7) | 35 (20.5) | 0.067 |
| Non-specific symptoms | 18 (60) | 37 (21.6) | <0.001 |
| Acute hepatitis | 4 (13.3) | 62 (36.3) | 0.012 |
| Liver cirrhosis | 6 (20) | 37 (20.8) | 0.794 |
| Not reported | 1 (3.1) | 7 (3.9) | 0.825 |
| Fibrosis stage, n (%) | |||
| F0 | 1 (3.1) | 11 (6.1) | 0.492 |
| F1–F2 | 10 (31.25) | 35 (19.6) | 0.064 |
| F3–F4 | 12 (37.5) | 97 (54.4) | 0.076 |
| Not reported | 9 (28.1) | 35 (19.6) | 0.280 |
| Virus co-infection | |||
| HIV | 0 | 1 | 0.662 |
| HCV | 0 | 0 | NC |
| HBV | 0 | 3 | 0.459 |
AIH, autoimmune hepatitis; ALP, alkaline phosphatase; AMA, antimitochondrial antibodies; ANA, antinuclear antibodies; ASMA, anti-smooth muscle antibodies; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IgG, immunoglobulin G; IQR, interquartile range; PBC, primary biliary cholangitis; ULN, upper limit of normal.
Differences of proportions calculated by chi-square method.
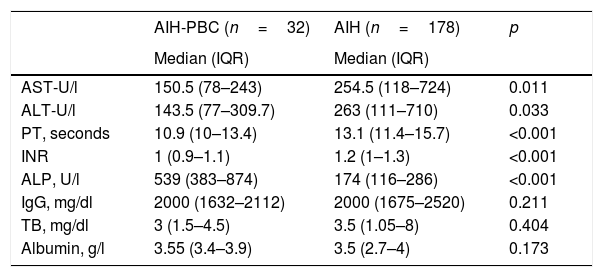
Comparison of liver biochemical and function tests with the diagnosis of overlap syndrome and AIH.
| AIH-PBC (n=32) | AIH (n=178) | p | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| AST-U/l | 150.5 (78–243) | 254.5 (118–724) | 0.011 |
| ALT-U/l | 143.5 (77–309.7) | 263 (111–710) | 0.033 |
| PT, seconds | 10.9 (10–13.4) | 13.1 (11.4–15.7) | <0.001 |
| INR | 1 (0.9–1.1) | 1.2 (1–1.3) | <0.001 |
| ALP, U/l | 539 (383–874) | 174 (116–286) | <0.001 |
| IgG, mg/dl | 2000 (1632–2112) | 2000 (1675–2520) | 0.211 |
| TB, mg/dl | 3 (1.5–4.5) | 3.5 (1.05–8) | 0.404 |
| Albumin, g/l | 3.55 (3.4–3.9) | 3.5 (2.7–4) | 0.173 |
AIH, autoimmune hepatitis; ALT, alanine aminotransferase; AST, aspartate aminotransferase; IgG, immunoglobulin G; INR, international normalised ratio; IQR: interquartile range; PBC, primary biliary cholangitis; PT, prothrombin time; TB, total bilirubin.
Median difference analysis was performed by non-normal distribution.
The difference in medians in liver function tests such as PT and INR was significantly higher in the group with overlap syndrome than in the AIH group (p<0.001) and, although albumin levels were also higher (3.55 vs 3.5g/l; p=0.173), and median total bilirubin lower (3 vs 3.5, p=0.082), these last two differences were not statistically significant (Table 2).
The difference in medians or discrete intervals of IgG was not significant (Table 1) and nor was the percentage of patients with positive ANA. However, there was a significant difference in the percentage of patients with negative ANA, with this finding being more common in AIH than in patients with overlap syndrome (11.2%, n=20% vs 0%, n=0; p<0.046).
Treatment and patient progressThere were no significant differences between the groups in either induction or maintenance treatment (Table 3), except for UDCA, which was indicated more often in AIH-PBC than in AIH: 31 (96.8%) and 39 (21.9%), respectively (p<0.001); the prescribed dose in all cases was 13–15mg/kg body weight/day once diagnosis had been confirmed.
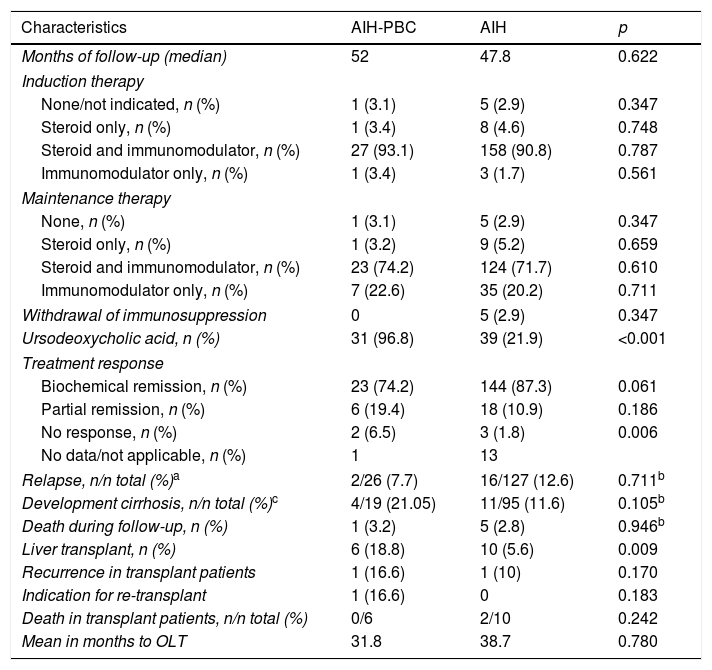
Follow-up and results in patients with AIH and overlap.
| Characteristics | AIH-PBC | AIH | p |
|---|---|---|---|
| Months of follow-up (median) | 52 | 47.8 | 0.622 |
| Induction therapy | |||
| None/not indicated, n (%) | 1 (3.1) | 5 (2.9) | 0.347 |
| Steroid only, n (%) | 1 (3.4) | 8 (4.6) | 0.748 |
| Steroid and immunomodulator, n (%) | 27 (93.1) | 158 (90.8) | 0.787 |
| Immunomodulator only, n (%) | 1 (3.4) | 3 (1.7) | 0.561 |
| Maintenance therapy | |||
| None, n (%) | 1 (3.1) | 5 (2.9) | 0.347 |
| Steroid only, n (%) | 1 (3.2) | 9 (5.2) | 0.659 |
| Steroid and immunomodulator, n (%) | 23 (74.2) | 124 (71.7) | 0.610 |
| Immunomodulator only, n (%) | 7 (22.6) | 35 (20.2) | 0.711 |
| Withdrawal of immunosuppression | 0 | 5 (2.9) | 0.347 |
| Ursodeoxycholic acid, n (%) | 31 (96.8) | 39 (21.9) | <0.001 |
| Treatment response | |||
| Biochemical remission, n (%) | 23 (74.2) | 144 (87.3) | 0.061 |
| Partial remission, n (%) | 6 (19.4) | 18 (10.9) | 0.186 |
| No response, n (%) | 2 (6.5) | 3 (1.8) | 0.006 |
| No data/not applicable, n (%) | 1 | 13 | |
| Relapse, n/n total (%)a | 2/26 (7.7) | 16/127 (12.6) | 0.711b |
| Development cirrhosis, n/n total (%)c | 4/19 (21.05) | 11/95 (11.6) | 0.105b |
| Death during follow-up, n (%) | 1 (3.2) | 5 (2.8) | 0.946b |
| Liver transplant, n (%) | 6 (18.8) | 10 (5.6) | 0.009 |
| Recurrence in transplant patients | 1 (16.6) | 1 (10) | 0.170 |
| Indication for re-transplant | 1 (16.6) | 0 | 0.183 |
| Death in transplant patients, n/n total (%) | 0/6 | 2/10 | 0.242 |
| Mean in months to OLT | 31.8 | 38.7 | 0.780 |
AIH, autoimmune hepatitis; OLT, orthotopic liver transplant; PBC, primary biliary cholangitis.
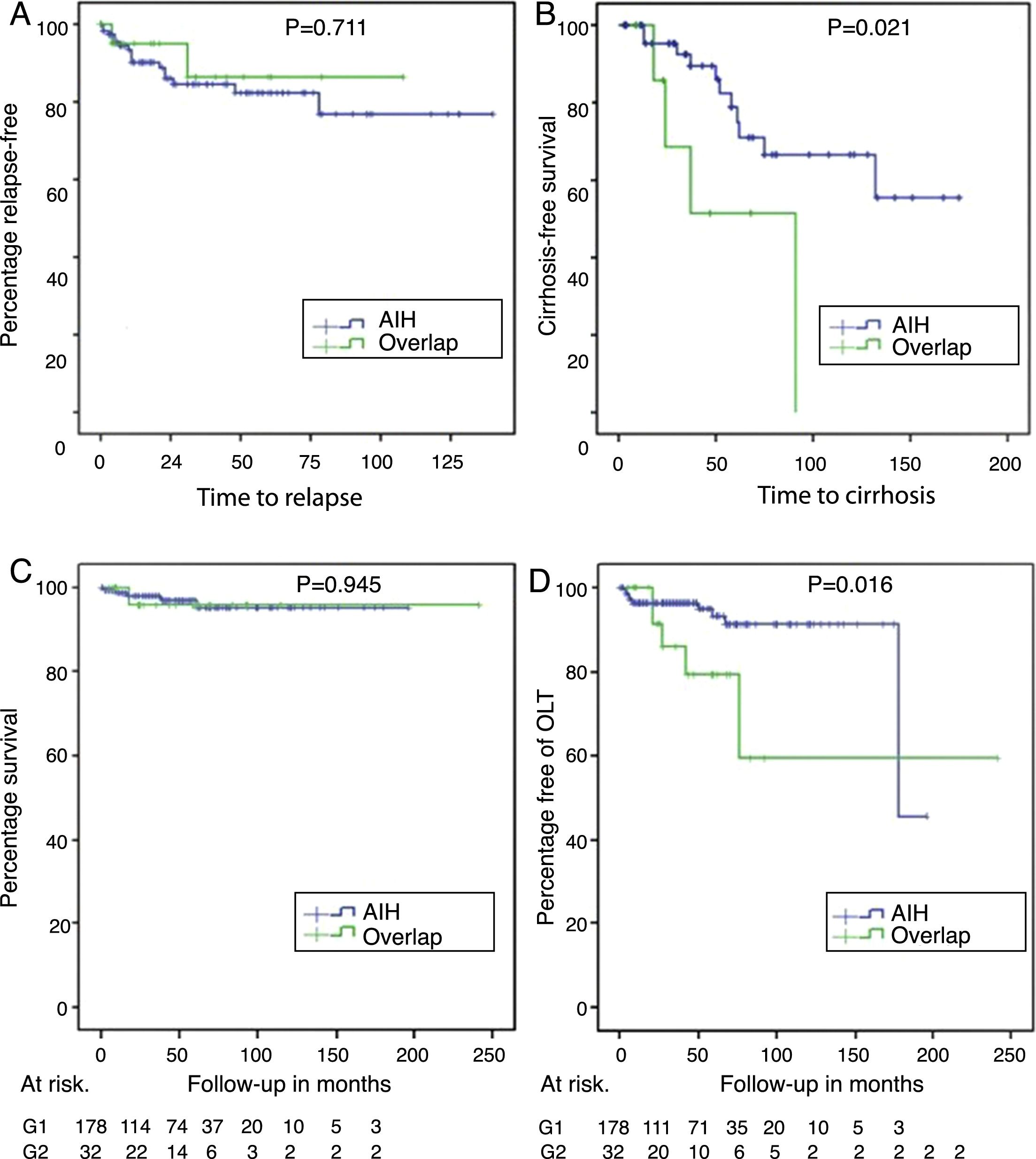
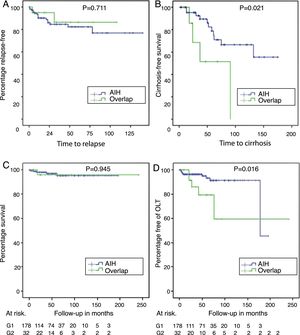
With a non-significant trend, there was a higher percentage of patients with a biochemical response in AIH (144 [87.3%]) than in AIH-PBC (23 [74.2%]) (p=0.061); at the same time, the proportion of relapses in patients for whom complete or partial remission was recorded during follow-up was 12.6% in AIH vs 7.7% in AIH-PBC (p=0.711) (Fig. 3A).
Relapse-free survival in those who achieved biochemical or partial remission (panel A), cirrhosis-free survival in those with F3 or unreported fibrosis (panel B), differential survival in patients with AIH and overlap syndrome (panel C) and transplant-free survival in AIH and overlap (panel D).
Of the patients who did not have cirrhosis or who were F4 at the time of diagnosis, 21% of those with AIH-PBC and 11.6% with AIH progressed to cirrhosis during the follow-up period; the differences were not statistically significant (p=0.105). No cases of progression to cirrhosis were documented in patients with stage 2 fibrosis or lower. However, cases were documented among both those with stage 3 fibrosis and those whose stage was not reported in the biopsy report (4 out of 8 [50%] with overlap syndrome and 11 out of 49 [22.4%] with AIH, this difference being significant [p=0.024]) (Fig. 3B), including in patients with documented biochemical response without relapse during the follow-up period (50%, n=3 out of 6 vs 11.7%, n=4 out of 34; p=0.009).
OLT was significantly more common in overlap syndrome (18%, n=6 vs 5.6%, n=10; p=0.009), as were the rates for re-transplant (3.1%, n=1 vs 0; p=0.183) and liver-related mortality (Fig. 3C, D), although these last two differences were not significant statistically. Although follow-up of transplant patients was shorter in AIH (28.2 months) than in overlap syndrome (56.8 months), again the difference was not significant.
DiscussionThis study presents the results of a comparative retrospective cohort of patients with AIH and AIH-PBC overlap syndrome in a reference centre for patients with liver diseases. In both groups, the majority of the population was female, similar to that reported in previous studies,6,9,11 with the mean age and age range similar to those published in other series for patients with overlap syndrome16 and no marked difference in relation to those with AIH. According to the two largest published series, using the up-to-date criteria most widely accepted in the different studies, the registered prevalence with respect to those with PBC is from 4.8% to 9.2%.4,16 However, they did not determine the prevalence with respect to patients with AIH; this was 15.2% in our series, the first time such an estimate has been made, and higher than the overall prevalence reported for autoimmune liver diseases.17
Just as reported in 2007 by Heurgué et al.17 in a multicentre comparative series in France of 15 patients with overlap syndrome, 48 patients with AIH and 52 with PBC, no differences were found for associated autoimmune comorbidities, in the mean levels of total bilirubin or serum albumin or in fibrosis stage at diagnosis, although there were differences in the mean alkaline phosphatase levels and prothrombin time. However, in this cohort, differences were found in the median ALT values.
The clinical and biochemical response in patients with overlap syndrome has been the subject of study,4,18 with clear recommendations for PBC and AIH, but a continuing lack of consensus in AIH-PBC. Some studies suggest that patients with AIH-PBC and alkaline phosphatase levels less than twice the ULN respond similarly to those with AIH without UDCA, as was found by Czaja,18 where up to 75% achieved biochemical and/or histological remission and 81% biochemical and histological normalisation or near normalisation with immunosuppressants. In our series, only four patients had levels less than twice the ULN. Two of these patients had F4 fibrosis, one of whom was not on UDCA as part of their management and required OLT, but had no disease recurrence. The other patient with F4 fibrosis and the remaining two were all taking UDCA and azathioprine and an adequate biochemical response was documented.
In patients with alkaline phosphatase levels >twice the ULN, the use of UDCA in addition to immunomodulators has been proposed as a management strategy. As demonstrated by Chazouillères et al.4 in 1998, in 12 patients with overlap syndrome, this strategy leads to significant improvement in alkaline phosphatase (ALP), GGT and ALT levels. Moreover, as demonstrated eight years later by the same author,19 this time with 17 patients, the strategy also helps prevent progression to cirrhosis.
All of the 32 patients in our series, except the one mentioned with overlap syndrome, received UDCA as part of their management. None of the 11 (34%) with fibrosis stage lower than F3 progressed to cirrhosis. One of the two patients detected with stage F3 fibrosis progressed to cirrhosis without indication for OLT until the last follow-up. One of the nine patients without data on fibrosis stage in the biopsy report was lost to follow-up, and three (37.5%) of the remaining patients progressed to cirrhosis despite combined management with UDCA and immunosuppression, with one of these having indication for OLT at the end of the study period. These outcomes are worse than those reported by Chazouillères et al.,19 who did not report any cases of progression to fibrosis in those initially treated with combined management (UDCA and immunosuppression). This would suggest that UDCA is effective from the initial stages of the disease.
Just as reported by Silveira et al.5 in their comparative study of 26 patients with AIH-PBC and 109 with PBC followed up for over six years at the Mayo Clinic in Rochester, when comparing patients with AIH-PBC and those with PBC, patients with AIH-PBC have worse outcomes, such as bleeding, oesophageal varices, death and indication for OLT. These findings differ from those previously reported by Lohse et al.,9 who detected a similar biochemical response (defined by reduction greater than twice the ULN) in those with PBC, leading them to propose abandoning the name “overlap syndrome”; and from those of Joshi et al.,17 in a Canadian multicentre study with the Mayo Clinic of 16 patients with overlap syndrome, which showed no difference in survival or need for liver transplantation at seven years compared to those with PBC with or without positive ANA. These patients also had a significantly higher rate of progression to cirrhosis, a higher percentage of non-response to management and a higher rate of indication for transplantation, as we demonstrated in our series.
We are aware of a number of limitations to our study, including the fact that it was a single-centre study with convenience sampling in a reference hospital where the disease spectrum may include those with greater severity and so induce reference bias. There is also the retrospective nature of the study with a limited number of patients in the AIH-PBC overlap syndrome group, the length of follow-up and losses to follow-up in some patients limiting the detection of possible significant differences in mortality and re-transplantation rates, and the lack of complete data in some patients.
Additionally, during the follow-up, serial determinations of clinical parameters were made at the discretion of the treating physician, such as transaminase, bilirubin and albumin levels, but no differential profile of human leucocyte antigen (HLA) or its prognostic implications; in many cases, no histological control was performed to determine histological response due to an apparent adequate biochemical response, and some of these cases were later documented as having progression to cirrhosis.
Multinational, multicentre randomised clinical trials could overcome these limitations and thus detect possible differences in response to the management and relapses not detected in our series. Such studies would also help to standardise the best methods for follow-up and management in these patients.
The strengths of our study include the fact that, according to a systematic search of the different databases and grey literature, this cohort represents the study with the largest number of patients included in each group for comparison and the first attempt to estimate the prevalence of this form of overlap with respect to patients with AIH. Our sample was well characterised, with relatively long periodic clinical follow-up available, with serial biochemical measurements to determine response, and it was representative of the population, as it included patients from different regions of Colombia.
In conclusion, AIH-PBC overlap syndrome represents a not-insignificant portion of patients with respect to those with AIH and therefore should be investigated when suspected. It is important for AIH-PBC overlap syndrome to be recognised, as the disease course can be more severe in these patients and, in view of the higher risk of progression to cirrhosis in advanced fibrosis, even with documented biochemical remission, their prognosis is poor. Added to that is their greater need for liver transplant and possibly even re-transplant, although these figures were not statistically significant in our series.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Martínez Casas OY, Díaz Ramírez GS, Marín Zuluaga JI, Santos Ó, Muñoz Maya O, Donado Gómez JH, et al. Síndrome de superposición: hepatitis autoinmune y colangitis biliar primaria. Resultados a largo plazo de una cohorte retrospectiva en un hospital universitario. Gastroenterol Hepatol. 2018;41:544–552.