Dysmotility-like dyspepsia symptoms are frequent in patients with gluten-sensitive enteropathy (GSE). Current data suggest that patients with mild enteropathy may be present with gluten-sensitive symptoms and complications.
AimTo investigate the prevalence of GSE, including mild enteropathy, in patients with dysmotility-like dyspepsia symptoms.
MethodsWe retrospectively studied 142 patients who presented dysmotility-like dyspepsia symptoms and normal upper gastrointestinal endoscopy. Endoscopic duodenal biopsies were taken and processed using hematoxylin–eosin staining and CD3 immunophenotyping. In patients with enteropathy (number of intraepithelial lymphocytes greater than 25 per 100 enterocytes) we also performed coeliac serology (anti-tissue transglutaminase IgA) and HLA-DQ2/DQ8 genotyping. A gluten-free diet was offered if one of these markers was positive. The final GSE diagnosis was established based on clinical and histopathological response to the gluten-free diet after 18 months of follow-up.
ResultsFifty-one patients (35.9%) had enteropathy; 4 (2.8%) Marsh type 3b, 24 (16.9%) Marsh type 3a, 3 (2.1%) Marsh type 2, and 20 (14.1%) Marsh type 1. A positive serology result was extremely low (6.7%) in mild enteropathy (Marsh type 1–3a) in contrast with Marsh type 3b patients (50%). Most patients with enteropathy had positive HLA DQ2 or -DQ8 genotyping (84.1%). Out of the 37 patients who started a gluten-free diet, 34 (91.9%) improved their symptoms, and 28 of 32 (87.5%) had a histopathological or serological response. A final GSE diagnosis was established in 28 of the 142 patients (19.7%).
ConclusionGluten-sensitive enteropathy can be a frequent and unsuspected cause of dysmotility-like dyspepsia.
La dispepsia de tipo dismotilidad es frecuente en pacientes con enteropatía sensible al gluten (ESG). Los datos actuales sugieren que los pacientes con enteropatía leve pueden presentar síntomas y complicaciones gluten dependientes.
ObjetivoInvestigar la prevalencia de ESG, incluida la enteropatía leve, en pacientes con dispepsia tipo dismotilidad.
MétodosEstudio retrospectivo de 142 pacientes que presentaban dispepsia de tipo dismotilidad y normalidad en la endoscopia digestiva alta. Se realizaron biopsias duodenales y se procesaron mediante tinción de hematoxilina-eosina e inmunofenotipo CD3. En los pacientes con enteropatía (número de linfocitos intraepiteliales superior a 25 por cada 100 enterocitos) también se realizó una serología celíaca (anti-transglutaminasa tisular IgA) y genotipado HLA-DQ2/DQ8. Si uno de estos marcadores resultaba positivo, se ofrecía al paciente iniciar una dieta sin gluten. El diagnóstico final de ESG se estableció en función de la respuesta clínica e histopatológica a la dieta sin gluten después de 18 meses de seguimiento.
ResultadosCincuenta y un pacientes (35,9%) presentaban enteropatía, 4 (2,8%) de Marsh tipo 3b, 24 (16,9%) Marsh tipo 3a, 3 (2,1%) Marsh tipo 2, y 20 (14,1%) Marsh tipo 1. La positividad serológica fue extremadamente baja (6,7%) en la enteropatía leve (Marsh tipo 1-3a), al contrario que en los pacientes con una lesión Marsh tipo 3b (50%). La mayoría de los pacientes con enteropatía presentaban valores positivos para el genotipado HLA DQ2 o -DQ8 (84,1%). De los 37 pacientes que iniciaron una dieta sin gluten, en 34 (91,9%) mejoraron los síntomas, y 28 de 32 (87,5%) presentaron respuesta histopatológica o serológica. Un diagnóstico final de ESG se estableció en 28 de los 142 pacientes (19,7%).
ConclusiónLa enteropatía sensible al gluten puede ser una causa frecuente e insospechada de dispepsia de tipo dismotilidad.
Coeliac disease (CD) is an immune-mediated systemic disorder elicited by gluten and related prolamines in genetically susceptible individuals and characterised by the presence of a variable combination of gluten-dependent clinical manifestations, CD-specific antibodies, HLA-DQ2 or HLA-DQ8 haplotypes, and enteropathy.1,2 Current data suggest that patients with mild enteropathy (not villous atrophy) may present with gluten sensitive symptoms and complications, and that they may show good clinical and histological response to a gluten-free diet (GFD).3–8 The broader term gluten-sensitive enteropathy (GSE) comprises the whole spectrum of gluten-dependent mucosal histopathological lesions, which range from normal villous architecture with intraepithelial lymphocytosis as the only abnormality to total villous atrophy.9 Patients with minimal immunopathological changes in the intestine and gluten-sensitive symptoms have been diagnosed recently as suffering from non-coeliac gluten-sensitivity.10
Dyspepsia is a common symptom of GSE and may be present in up to 60% of these patients, including those with mild enteropathy.11,12 The prevalence of CD, based on the presence of intestinal villous atrophy, in patients with dyspepsia is significantly higher than that in the general population, occurring at a rate of 1.2–5.8%.13–20 This may be because patients with CD frequently experience gastrointestinal motility disturbances.21–23 These motility disorders of the upper gastrointestinal tract may contribute to the development of symptoms such as postprandial fullness, bloating, flatulence, distension, nausea and/or vomiting, as well as regurgitation and heartburn.19,24 To the best of our knowledge, there have been no published reports on the prevalence of the whole spectrum of GSE in patients with dyspepsia.
Therefore, the aim of the present study was to investigate the prevalence of GSE, including mild enteropathy, in patients with dysmotility-like dyspepsia symptoms (DLDS) and normal upper gastrointestinal (GI) endoscopy results.
Materials and methodsPatientsThis observational retrospective study examined a cohort of 142 patients seen at the endoscopy unit of San Jorge Hospital, Huesca, between January 2007 and December 2007 who was presented with DLDS but with no upper GI endoscopic evidence of structural disease that could explain their symptoms. Dysmotility-like dyspepsia definition was consistent with the Rome II criteria including a number of different nonpainful symptoms such as upper abdominal fullness, bloating, nausea, epigastric burning, belching, and vomiting.25 We excluded patients with a previous diagnosis of coeliac disease, gastrectomy and/or severe systemic diseases (e.g., severe cardio-respiratory conditions, kidney or liver failure or neoplasia). Clinical data were obtained using a questionnaire, with special attention being given to symptoms, family history, and conditions associated with GSE.
The study was performed in accordance with the Declaration of Helsinki, and all patients gave their informed consent for upper GI endoscopy and for histological sampling.
Histopathological studiesDuring endoscopy, four endoscopic biopsies from the second and third portion of the duodenum were performed to all subjects. Additionally, gastric biopsies to rule out the presence of Helicobacter pylori by means of urease test were also taken. The duodenum specimens were processed using hematoxylin–eosin and immunohistochemical stains. Immunostaining for intraepithelial lymphocytes (IELs) was performed using monoclonal antibodies against CD3 (DakoCytomation, Glostrup, Denmark) in formalin-fixed, paraffin-embedded sections. The number of IELs was estimated by counting the number of CD3+ cells per 100 epithelial cells.26
Histopathological findings were categorized according to the modified Marsh-Oberhuber criteria9; “infiltrative” lesions with intraepithelial lymphocytosis were defined as Marsh type 1 lesions, “infiltrative/hyperplasic” lesions were defined as Marsh type 2 lesions, and villous atrophy was defined as Marsh type 3 lesions (3a: partial atrophy, 3b: subtotal atrophy, 3c: total atrophy). We assumed a Marsh type 1 lesion or the presence of a lymphocytic enteropathy if the number of IELs was equal to or greater than the proportion of 25/100 epithelial cells.9,27 Patients with lymphocytic enteropathy (Marsh type 1), crypt hyperplasia (Marsh type 2) or partial atrophy (Marsh type 3a) were classified as having mild enteropathy.28
H. pylori studies, serology and HLAIn patients with enteropathy, we also performed H. pylori diagnosis, antibody detection and HLA genotyping. The presence of H. pylori was established by a rapid urease test (CLO-test®, Kimberly-Clark, Roswell, USA) at the time of GI endoscopy or by a 13C-urea breath test (UBTest®, Otsuka Pharmaceutical Europe Ltd., Middlesex, UK) within two months of the endoscopy. The patient was considered positive for H. pylori infection if one of these tests were determined to be positive. Serum IgA-tissue transglutaminase antibodies (t-TGA) were analyzed using a quantitative and automated fluorescence-immunoassay method by means of a commercial available detection kit (EliA™ Celikey™ IgA, Phadia AB, Uppsala, Sweden). These antibodies were considered to be positive if the values exceeded 3U/mL. HLA-DQ2 (DQA1*05 and DQB1*02 alleles) and DQ8 (DQA1*03 and DQB1*0302 alleles) genotyping was performed via PCR amplification. A subject was considered to be genetically at-risk of developing CD when one or both alleles of the HLA-DQ2 heterodimer were positive, and when both alleles of HLA-DQ8 heterodimer were positive.2
Gluten-sensitive enteropathy diagnosis and follow-upA gluten-free diet (GFD) was offered to enteropathy patients who either presented serology markers or were identified as immunogenetic-HLA DQ2- or DQ8-positive. Follow-up with clinical assessment was performed at 3, 6 and 18 months. Finally, a second upper GI endoscopy with duodenal biopsies was performed at 18 months.
A final diagnosis of GSE was considered when the following criteria were fulfilled29: (1) a Marsh types 1–3 lesion was observed in the duodenum biopsies, (2) the presence of either serum coeliac disease or HLA-DQ2 or -DQ8 genotyping, and (3) a demonstration of gluten dependence based on significant and sustained improvement of symptoms and a histopathological or serological response to the GFD. We considered a histopathological response to have occurred when duodenal lesions decreased from Marsh type 3 to Marsh type 1 or Marsh type 0 or, in the case of Marsh type 1 lesions, when the number of IELs was normalized or reduced by 50% compared to the first histopathological study.8
Statistical analysisStatistical analysis was performed using SPSS 14.0 software. Qualitative parameters were expressed as proportions, whereas quantitative variables were expressed as the mean±SD. The χ2-test and Fisher's exact test were used when appropriate for comparison between qualitative variables, while the t-test was used for comparison between quantitative variables. A value of P<0.05 was considered to be significant.
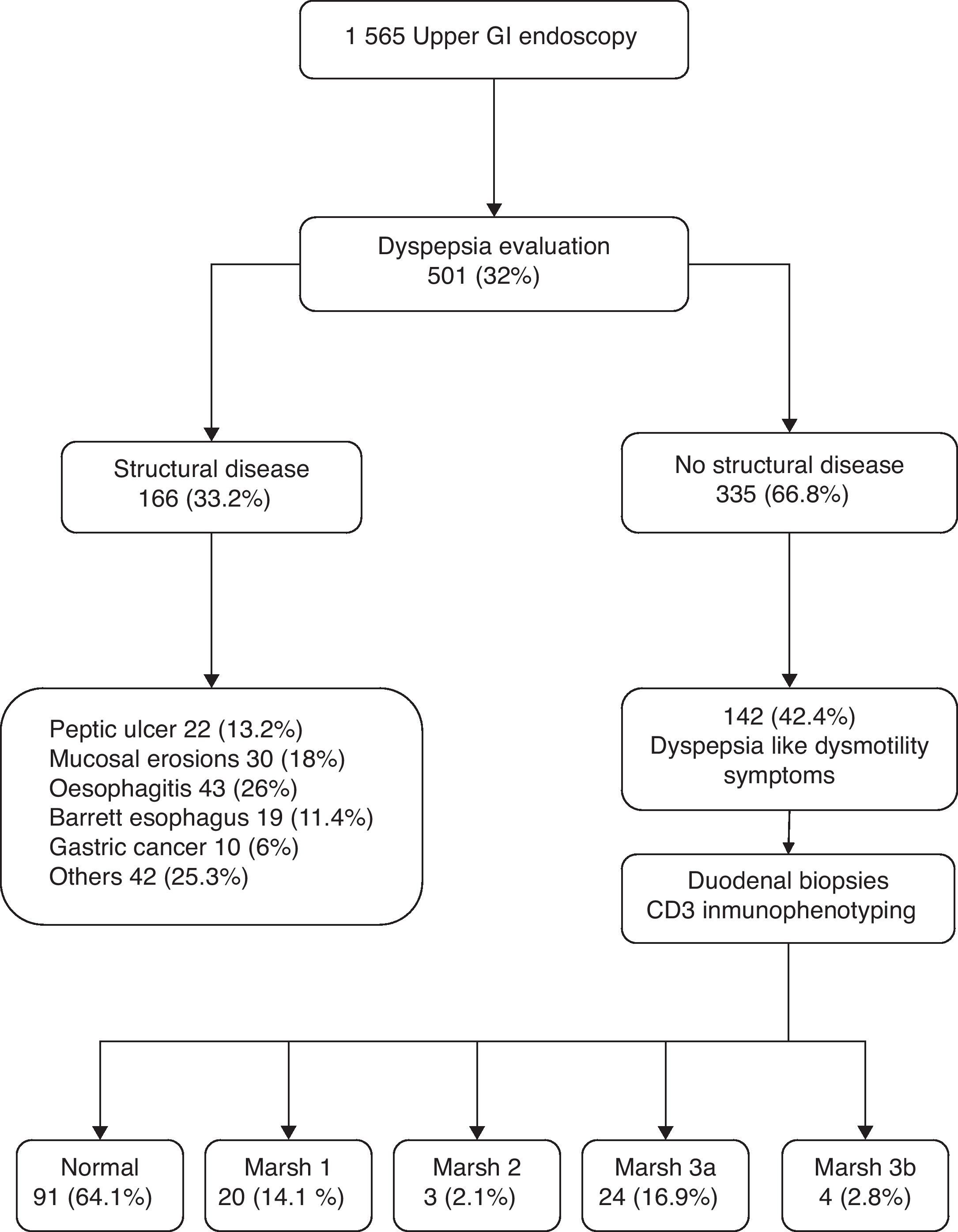
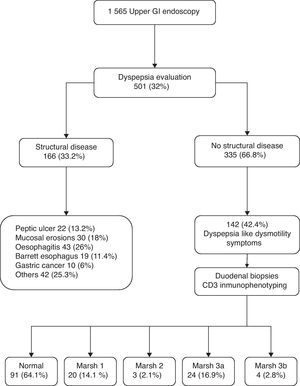
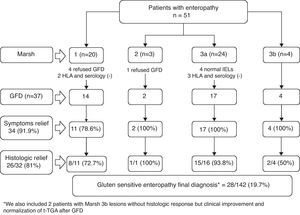
ResultsFrom January to December 2007, we performed a total of 1565 upper GI endoscopies, 501 (32%) of which were performed for dyspepsia evaluation. Of the 335 patients with dyspepsia and normal upper GI endoscopy, 142 with DLDS were included for this study (mean age 45.8±15 years; 73.9% females). Fifty-one of these 142 patients (35.9%) had enteropathy, including 4 (2.8%) with subtotal villous atrophy (Marsh type 3b), 24 (16.9%) with partial villous atrophy (Marsh type 3a), 3 (2.1%) with hyperplasic crypts (Marsh type 2), and 20 (14.1%) with lymphocytic enteropathy (Marsh type 1). None of the patients presented total villous atrophy (Fig. 1).
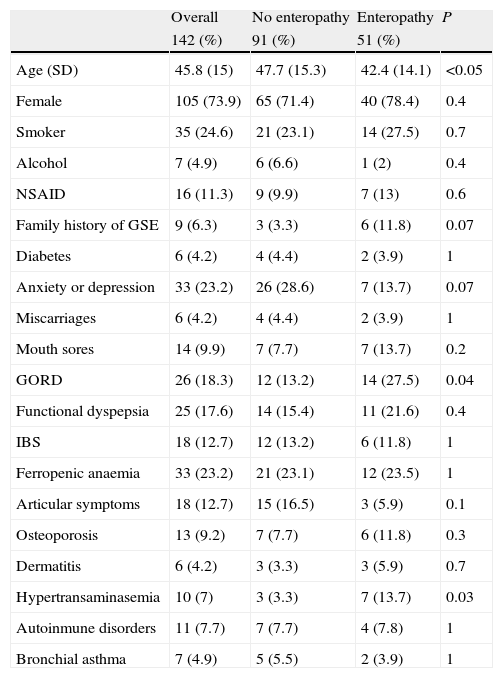
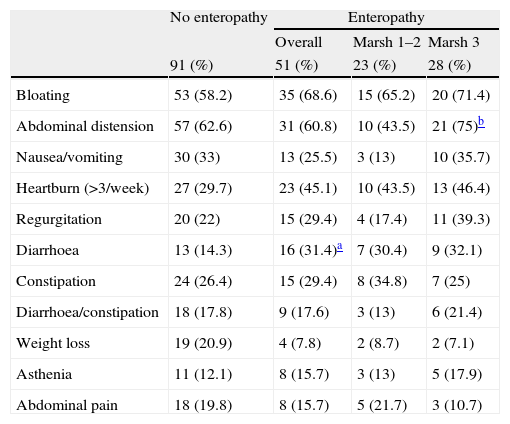
When we compared patients with and without enteropathy, we observed that patients with duodenal histopathological lesions were younger (42.4 vs. 47.7 years, P<0.05), and those patients more frequently had hypertransaminasemia (13.7% vs. 3.3%, P<0.05), and a previous diagnosis of gastro-oesophageal reflux disease (27.5% vs. 13.2%, P<0.05)(Table 1). Regarding the symptoms, only diarrhoea was significantly more common in patients with duodenal histopathological lesions vs. those without (31.4% vs. 14.3%, P<0.05), and only abdominal distension was more frequent in patients with Marsh type 3 lesions than that in patients with Marsh type 1 or type 2 lesions (75% vs. 43.5%, P<0.05) (Table 2).
Associated clinical conditions relating to the presence or absence of enteropathy.
| Overall | No enteropathy | Enteropathy | P | |
| 142 (%) | 91 (%) | 51 (%) | ||
| Age (SD) | 45.8 (15) | 47.7 (15.3) | 42.4 (14.1) | <0.05 |
| Female | 105 (73.9) | 65 (71.4) | 40 (78.4) | 0.4 |
| Smoker | 35 (24.6) | 21 (23.1) | 14 (27.5) | 0.7 |
| Alcohol | 7 (4.9) | 6 (6.6) | 1 (2) | 0.4 |
| NSAID | 16 (11.3) | 9 (9.9) | 7 (13) | 0.6 |
| Family history of GSE | 9 (6.3) | 3 (3.3) | 6 (11.8) | 0.07 |
| Diabetes | 6 (4.2) | 4 (4.4) | 2 (3.9) | 1 |
| Anxiety or depression | 33 (23.2) | 26 (28.6) | 7 (13.7) | 0.07 |
| Miscarriages | 6 (4.2) | 4 (4.4) | 2 (3.9) | 1 |
| Mouth sores | 14 (9.9) | 7 (7.7) | 7 (13.7) | 0.2 |
| GORD | 26 (18.3) | 12 (13.2) | 14 (27.5) | 0.04 |
| Functional dyspepsia | 25 (17.6) | 14 (15.4) | 11 (21.6) | 0.4 |
| IBS | 18 (12.7) | 12 (13.2) | 6 (11.8) | 1 |
| Ferropenic anaemia | 33 (23.2) | 21 (23.1) | 12 (23.5) | 1 |
| Articular symptoms | 18 (12.7) | 15 (16.5) | 3 (5.9) | 0.1 |
| Osteoporosis | 13 (9.2) | 7 (7.7) | 6 (11.8) | 0.3 |
| Dermatitis | 6 (4.2) | 3 (3.3) | 3 (5.9) | 0.7 |
| Hypertransaminasemia | 10 (7) | 3 (3.3) | 7 (13.7) | 0.03 |
| Autoinmune disorders | 11 (7.7) | 7 (7.7) | 4 (7.8) | 1 |
| Bronchial asthma | 7 (4.9) | 5 (5.5) | 2 (3.9) | 1 |
NSAID: non steroidal antiinflammatory drugs; GSE: gluten-sensitive enteropathy; GORD: gastro-oesophageal reflux disease; IBS: irritable bowel syndrome.
Symptoms associated with postprandial fullness in relation to the presence or absence of enteropathy.
| No enteropathy | Enteropathy | |||
| Overall | Marsh 1–2 | Marsh 3 | ||
| 91 (%) | 51 (%) | 23 (%) | 28 (%) | |
| Bloating | 53 (58.2) | 35 (68.6) | 15 (65.2) | 20 (71.4) |
| Abdominal distension | 57 (62.6) | 31 (60.8) | 10 (43.5) | 21 (75)b |
| Nausea/vomiting | 30 (33) | 13 (25.5) | 3 (13) | 10 (35.7) |
| Heartburn (>3/week) | 27 (29.7) | 23 (45.1) | 10 (43.5) | 13 (46.4) |
| Regurgitation | 20 (22) | 15 (29.4) | 4 (17.4) | 11 (39.3) |
| Diarrhoea | 13 (14.3) | 16 (31.4)a | 7 (30.4) | 9 (32.1) |
| Constipation | 24 (26.4) | 15 (29.4) | 8 (34.8) | 7 (25) |
| Diarrhoea/constipation | 18 (17.8) | 9 (17.6) | 3 (13) | 6 (21.4) |
| Weight loss | 19 (20.9) | 4 (7.8) | 2 (8.7) | 2 (7.1) |
| Asthenia | 11 (12.1) | 8 (15.7) | 3 (13) | 5 (17.9) |
| Abdominal pain | 18 (19.8) | 8 (15.7) | 5 (21.7) | 3 (10.7) |
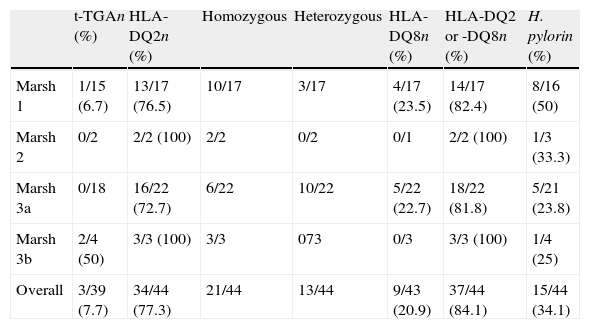
Table 3 lists the frequency of serology, HLA genotyping and H. pylori infection by degree of intestinal lesion. Tissue transglutaminase antibodies were positive in only 3 of 39 (7.7%) patients, including 1 of 15 (6.7%) of those with Marsh type 1 lesions and 2 of 4 (50%) of those with Marsh type 3b lesions. No positive results were recorded for t-TGA in patients with Marsh type 2- or Marsh type 3a lesions. However, most patients with enteropathy had positive HLA DQ2 or DQ8 genotyping (84.1%).
Serology, HLA immunogenetic and H. pylori diagnosis in patients with enteropathy.
| t-TGAn (%) | HLA-DQ2n (%) | Homozygous | Heterozygous | HLA-DQ8n (%) | HLA-DQ2 or -DQ8n (%) | H. pylorin (%) | |
| Marsh 1 | 1/15 (6.7) | 13/17 (76.5) | 10/17 | 3/17 | 4/17 (23.5) | 14/17 (82.4) | 8/16 (50) |
| Marsh 2 | 0/2 | 2/2 (100) | 2/2 | 0/2 | 0/1 | 2/2 (100) | 1/3 (33.3) |
| Marsh 3a | 0/18 | 16/22 (72.7) | 6/22 | 10/22 | 5/22 (22.7) | 18/22 (81.8) | 5/21 (23.8) |
| Marsh 3b | 2/4 (50) | 3/3 (100) | 3/3 | 073 | 0/3 | 3/3 (100) | 1/4 (25) |
| Overall | 3/39 (7.7) | 34/44 (77.3) | 21/44 | 13/44 | 9/43 (20.9) | 37/44 (84.1) | 15/44 (34.1) |
t-TGA: IgA-tissue transglutaminase antibodies; HLA-DQ2: presence of immunogenetic HLA-DQ2 (either one or both alleles); HLA-DQ8: presence of immunogenetic HLA-DQ8 (both alleles).
H. pylori infection was present in 15 of 44 (34.1%) patients with enteropathy and 19 of 40 (47.5%) without enteropathy (P=0.4). A non-statistically significant trend of increased frequency was observed in patients with Marsh types 1–2 lesions (47.4%) compared to patients with Marsh type 3 lesions (24%, P=0.1). Six patients with enteropathy and one patient without enteropathy had a history of successful H. pylori treatment prior to upper GI endoscopy and duodenal biopsies. Three patients with Marsh type 1 lesion (two with immunogenetic-HLA DQ2- and DQ8-negative and one without clinical response to the GFD) underwent H. pylori eradication treatment, but their dyspeptic symptoms did not improve.
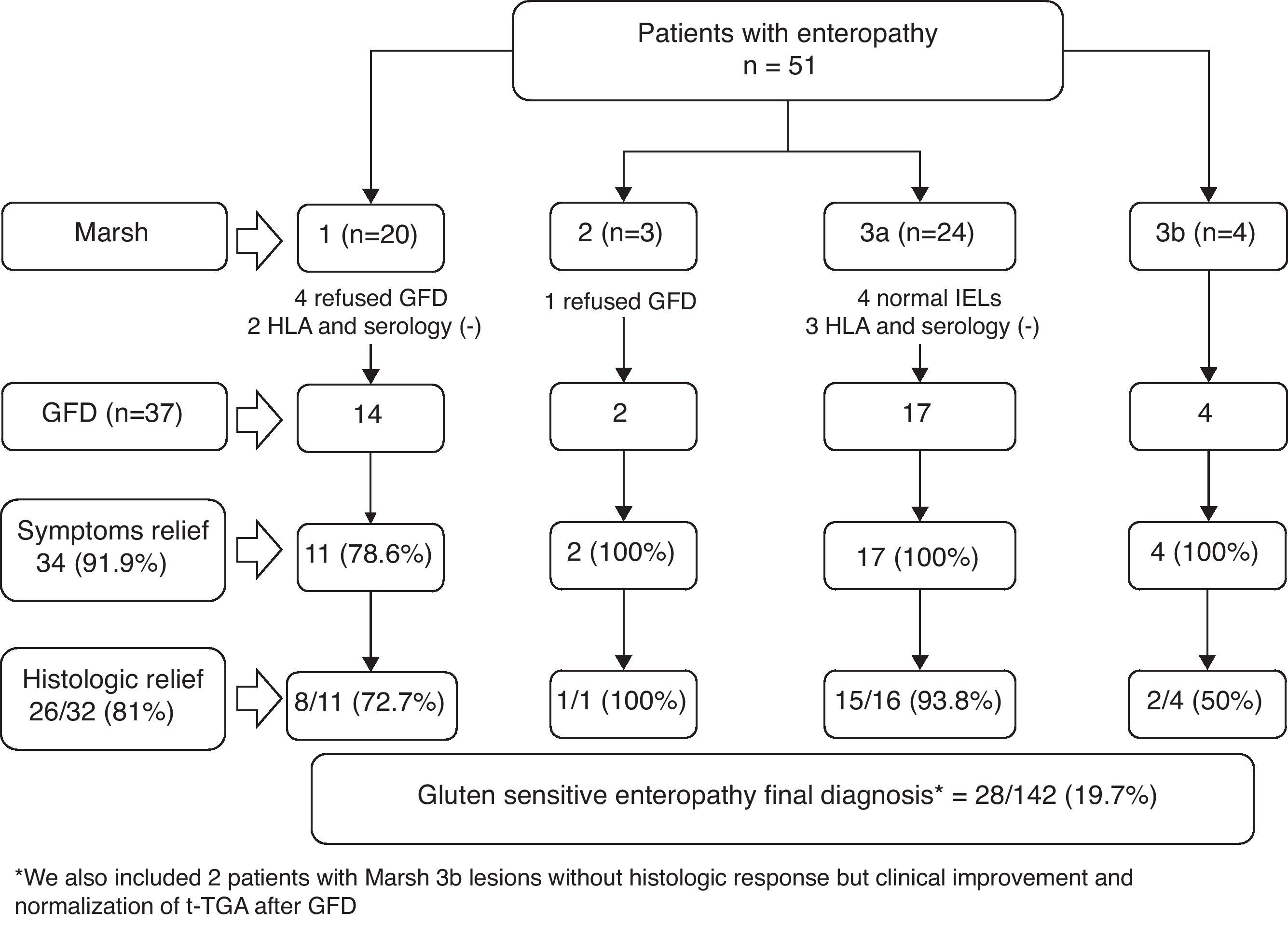
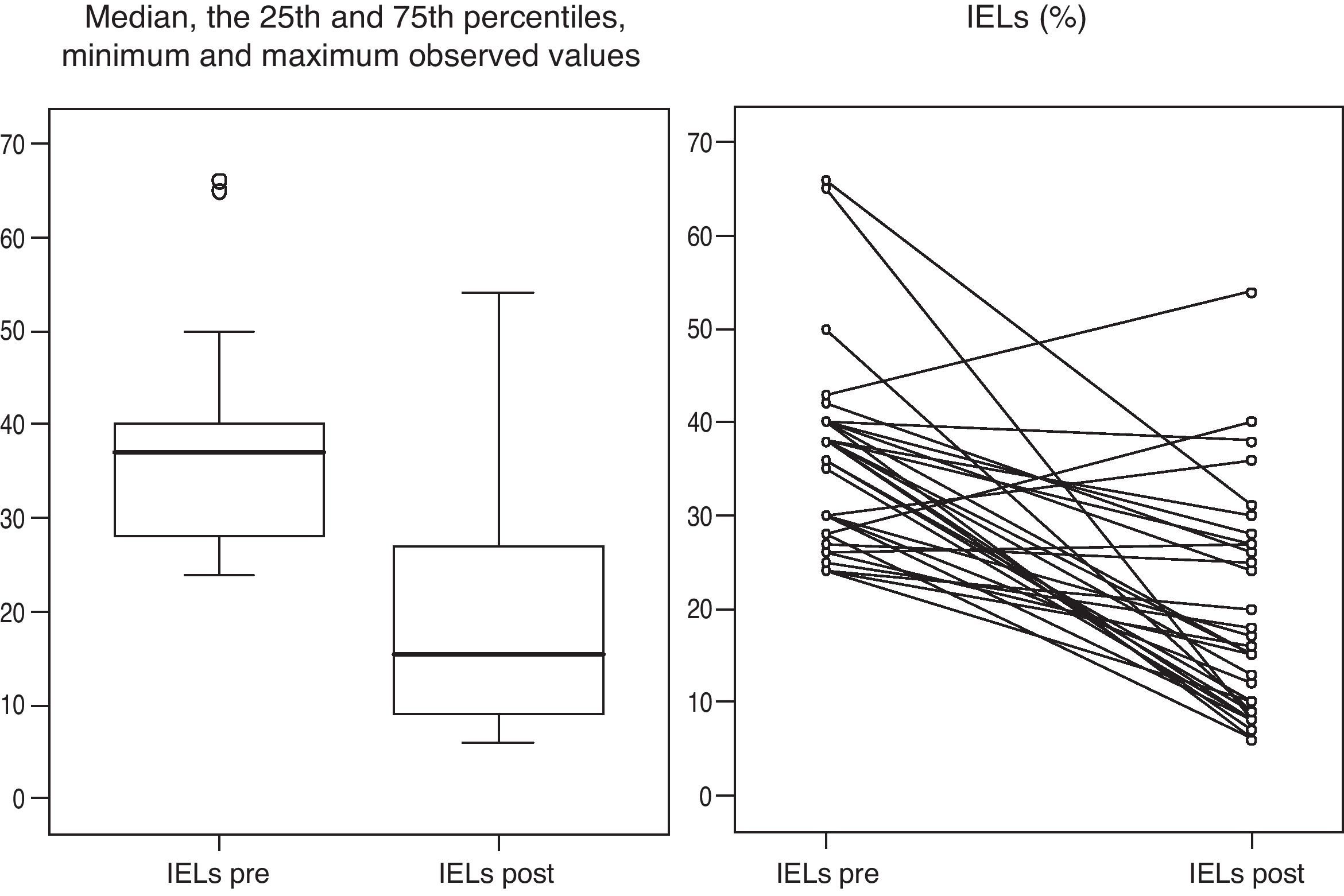
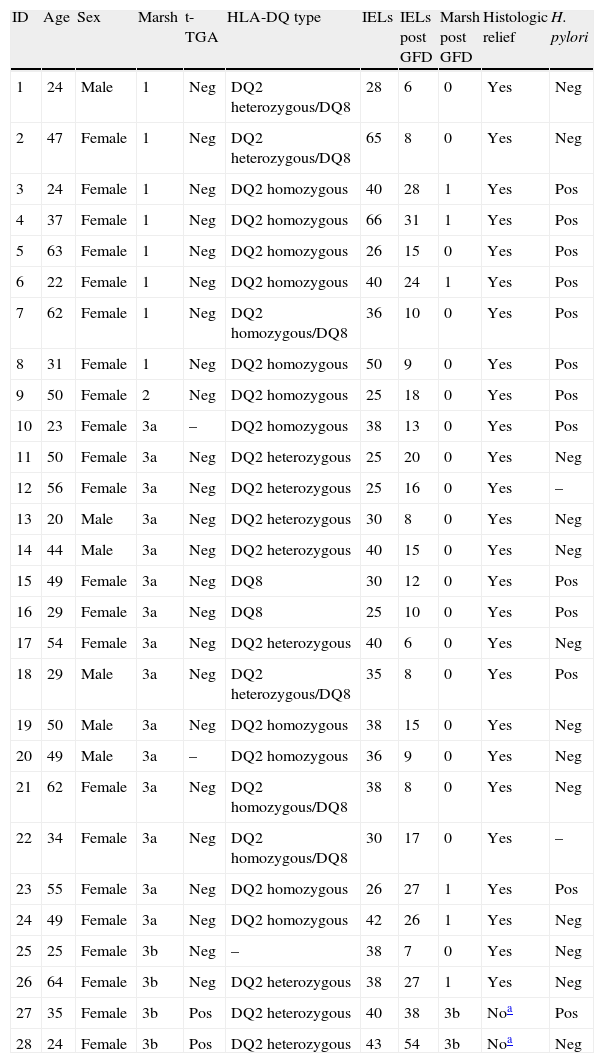
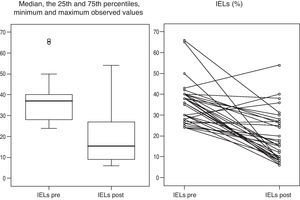
Gluten-free dietFig. 2 presents the clinical evolution and final diagnoses of the 51 patients with enteropathy. Thirty-four of 37 (91.9%) patients who started a gluten-free diet improved their dyspeptic symptoms (78.6% Marsh type 1, 100% Marsh type 2, 100% Marsh type 3a, 100% Marsh type 3b), and 26 of 32 (81%) patients who accepted and received a second upper GI endoscopy presented an improvement of histopathological lesions (72.7% Marsh type 1, 100% Marsh type 2, 93.8% Marsh type 3a, 50% Marsh type 3b). In these patients, duodenal intraepithelial lymphocytes counts also improved following the GFD. The IELs counts and means decreased from 36±10.2 to 19.2±11.9 (P<0.001, Fig. 3). Two patients presenting with Marsh type 3b lesions, the presence of t-TGA and without histopathological improvement, were also diagnosed with GSE based on their clinical and serological responses (i.e., normalization of t-TGA) to the GFD. A final GSE diagnosis was thus established in 28 of 142 patients (19.7%). Table 4 shows serology, HLA-DQ type, histopathology and H. pylori diagnosis in these patients.
Serology, HLA-DQ type, histopathology and H. pylori diagnosis in gluten-sensitive enteropathy patients.
| ID | Age | Sex | Marsh | t-TGA | HLA-DQ type | IELs | IELs post GFD | Marsh post GFD | Histologic relief | H. pylori |
| 1 | 24 | Male | 1 | Neg | DQ2 heterozygous/DQ8 | 28 | 6 | 0 | Yes | Neg |
| 2 | 47 | Female | 1 | Neg | DQ2 heterozygous/DQ8 | 65 | 8 | 0 | Yes | Neg |
| 3 | 24 | Female | 1 | Neg | DQ2 homozygous | 40 | 28 | 1 | Yes | Pos |
| 4 | 37 | Female | 1 | Neg | DQ2 homozygous | 66 | 31 | 1 | Yes | Pos |
| 5 | 63 | Female | 1 | Neg | DQ2 homozygous | 26 | 15 | 0 | Yes | Pos |
| 6 | 22 | Female | 1 | Neg | DQ2 homozygous | 40 | 24 | 1 | Yes | Pos |
| 7 | 62 | Female | 1 | Neg | DQ2 homozygous/DQ8 | 36 | 10 | 0 | Yes | Pos |
| 8 | 31 | Female | 1 | Neg | DQ2 homozygous | 50 | 9 | 0 | Yes | Pos |
| 9 | 50 | Female | 2 | Neg | DQ2 homozygous | 25 | 18 | 0 | Yes | Pos |
| 10 | 23 | Female | 3a | – | DQ2 homozygous | 38 | 13 | 0 | Yes | Pos |
| 11 | 50 | Female | 3a | Neg | DQ2 heterozygous | 25 | 20 | 0 | Yes | Neg |
| 12 | 56 | Female | 3a | Neg | DQ2 heterozygous | 25 | 16 | 0 | Yes | – |
| 13 | 20 | Male | 3a | Neg | DQ2 heterozygous | 30 | 8 | 0 | Yes | Neg |
| 14 | 44 | Male | 3a | Neg | DQ2 heterozygous | 40 | 15 | 0 | Yes | Neg |
| 15 | 49 | Female | 3a | Neg | DQ8 | 30 | 12 | 0 | Yes | Pos |
| 16 | 29 | Female | 3a | Neg | DQ8 | 25 | 10 | 0 | Yes | Pos |
| 17 | 54 | Female | 3a | Neg | DQ2 heterozygous | 40 | 6 | 0 | Yes | Neg |
| 18 | 29 | Male | 3a | Neg | DQ2 heterozygous/DQ8 | 35 | 8 | 0 | Yes | Pos |
| 19 | 50 | Male | 3a | Neg | DQ2 homozygous | 38 | 15 | 0 | Yes | Neg |
| 20 | 49 | Male | 3a | – | DQ2 homozygous | 36 | 9 | 0 | Yes | Neg |
| 21 | 62 | Female | 3a | Neg | DQ2 homozygous/DQ8 | 38 | 8 | 0 | Yes | Neg |
| 22 | 34 | Female | 3a | Neg | DQ2 homozygous/DQ8 | 30 | 17 | 0 | Yes | – |
| 23 | 55 | Female | 3a | Neg | DQ2 homozygous | 26 | 27 | 1 | Yes | Pos |
| 24 | 49 | Female | 3a | Neg | DQ2 homozygous | 42 | 26 | 1 | Yes | Neg |
| 25 | 25 | Female | 3b | Neg | – | 38 | 7 | 0 | Yes | Neg |
| 26 | 64 | Female | 3b | Neg | DQ2 heterozygous | 38 | 27 | 1 | Yes | Neg |
| 27 | 35 | Female | 3b | Pos | DQ2 heterozygous | 40 | 38 | 3b | Noa | Pos |
| 28 | 24 | Female | 3b | Pos | DQ2 heterozygous | 43 | 54 | 3b | Noa | Neg |
t-TGA: IgA-tissue transglutaminase antibodies; IELs: duodenal intraepithelial lymphocytes; GFD: gluten-free diet.
Gluten-sensitive enteropathy has a wide and heterogeneous spectrum of clinical manifestations, ranging from symptomless manifestations to the classical gastrointestinal form with diarrhoea and weight loss with a great variety of atypical clinical manifestations in between, such as anaemia, osteopenia, dermatitis, abdominal pain, amongst others.30 Dyspepsia is also a common symptom that may be present in up to 60% of GSE patients.11,12,24 Moreover, the prevalence of CD, based on the presence of intestinal villous atrophy, in patients with dyspepsia is significantly higher than that in the general population, occurring at a rate that varies from 1.2 to 5.8%.13–20
In the present study, we found a higher prevalence of GSE (19.7%) than that found in previous studies. This unexpected prevalence can be explained by several reasons. Firstly, we included a select subgroup of patients with dyspepsia (namely, those with dysmotility symptoms). All these patients were referred to the endoscopy unit for etiologic diagnosis of dyspepsia, allowing us to include patients in real clinical practice. Secondly, in contrast to previous studies the presence of intestinal villous atrophy and serologic markers were not considered to be necessary criteria for the final diagnosis of GSE. In addition, our pathologists processed the duodenal specimens using CD3 lymphocytic immunophenotyping, a useful diagnostic tool for increasing the likelihood of detecting of lymphocytic enteropathy, especially when atrophy is absent.26
Why have we chosen to include only patients with dyspepsia-like dysmotility symptoms in this study? Patients with GSE frequently have gastrointestinal motor disturbances. There are studies that have shown a delay in oesophageal transit, gastric and gallbladder emptying, in addition to orocecal transit time compared to colonic transit.21 These motility disorders of the upper gastrointestinal tract could contribute to the development of symptoms such as postprandial fullness, bloating, flatulence, distension, nausea and/or vomiting, as well as regurgitation and heartburn.19,24 Based on these reports, we thought that those patients with dyspepsia and dysmotility symptoms may be a risk group for GSE. Motility disorders in patients with GSE have been related to complex interactions among the reduced absorption of certain food constituents (for example, fat or starch), a dysfunction in the autonomic nervous system, and derangements of the secretion of several hormones such as cholecystokinin, neurotensin, plasma peptide YY, ghrelin and somatostatin. Fortunately, most of these changes usually disappear after a GFD is implemented.22,23,31–33
Why have we considered patients with mild enteropathy for the diagnosis of GSE in this study? Current data suggest that patients with Marsh types 1–2 lesions and testing positive for endomysial antibodies may suffer from GI symptoms and complications, such as abnormal bone mineral density, as frequently as patients with overt villous atrophy.34,35 Patients with mild enteropathy and negative coeliac serology may also have gluten-sensitive symptoms and complications, such as abdominal pain, distension, diarrhoea, anaemia, osteopenia or hypertransaminasemia.4 Furthermore, several prospective, although not controlled, studies have suggested that most of these patients may show good clinical and histological responses to a GFD.5–8
However, there are conditions other than GSE in which a duodenal intraepithelial lymphocytosis is possible.36,37 Examples of these include H. pylori infection, non-steroidal anti-inflammatory drugs, peptic duodenitis, small intestinal bacterial overgrowth, food protein intolerance (e.g., cow's milk, peanuts and eggs), tropical sprue, autoimmune disorders, oeosinophilic gastroenteritis, Crohn's disease, lymphocytic colitis and common variable immunodeficiency.9 In the present study, most patients had mild histological lesions and negative coeliac serology, and not all these were finally diagnosed with GSE (Fig. 2). According to the recommendation of Collin et al.,29 we have taken into account further evidence in order to diagnose GSE in these patients with borderline enteropathy. Firstly, we performed tests for immunogenetic markers (HLA DQ2 or DQ8) and for specific antibodies (t-TGA). HLA-DQ2 and DQ8 genotyping has a very high negative predictive value for coeliac disease.38,39 If one of these genetic or serologic markers was positive, we offered the patient the opportunity to start a GFD. Secondly, we proved gluten dependence based on the improvement of symptoms and histologic inflammation at an 18-month follow-up. A final GSE diagnosis was established in those patients who improved their symptoms and histopathological or serology abnormalities after undertaking the GFD. The subset characterization of γδ+ IELs and the immunohistologic detection of IgA anti-transglutaminase subepithelial deposits may offer additional value for the diagnosis of GSE but these techniques are not straightforward for use in clinical practice.40,41
Despite the indication in previous studies of the excellent sensitivity and specificity of serology for GSE diagnosis, we found a surprisingly low positivity when testing for t-TGA (7.7%). This may be explained by the fact that the prevalence of t-TGA may be correlated with the degree of intestinal damage; indeed, it has been reported that these antibodies often remain negative in partial villous atrophy or in the absence of atrophy.42,43 In our study, the great majority of patients had only mild enteropathy. In these patients, positive serum antibodies were extremely low (6.7%) in contrast with patients with subtotal villous atrophy (50%). Consequently, the negativity of the serologic test in the cases of mild enteropathy does not rule out GSE, and these tests performed without histologic evaluation may underestimate the real prevalence of GSE. For this reason, duodenal biopsies are still recommended in patients with strong clinical suspicion of GSE, even if serologic testing is negative.1,44
In view of these results, a change could be suggested in the diagnosis workup for patients with dyspepsia. According to the Roma III criteria, functional dyspepsia is defined as the presence of epigastric pain or burning, postprandial fullness or early satiation in the absence of an underlying organic disease likely to explain the symptoms.25 Upper GI endoscopy is the gold standard for excluding an organic cause of dyspepsia. However, the absence of a structural disease at an upper GI endoscopy is not enough to exclude an organic cause of dyspepsia, such as GSE, the diagnosis of which is based on the presence of histopathological small intestinal mucosal damage. Consequently, duodenal biopsies might improve the etiologic diagnoses in patients presenting dysmotility-like dyspepsia (or postprandial distress syndrome according to Roma III criteria) when an upper GI endoscopy does not provide evidence of a structural disease explaining the symptoms. However, the present study has several shortcomings that may have limited the possible clinical application of these results. Firstly, we did not take biopsies from all dyspepsia patients who underwent endoscopy; thus, we did not have a control group. Secondly, the data from the patients who received a GFD were uncontrolled. Consequently, we cannot definitively rule out a placebo effect for the positive response. Although we provided compelling evidence of clinical and histopathological responses to the GFD, we did not give a subsequent gluten challenge to the patients. Therefore, it will be necessary to confirm these preliminary results in randomized, therapeutic trials to determine whether dyspepsia patients with mild enteropathy and without positive coeliac serology will benefit from a GFD.
In conclusion, the present study shows that GSE can be a frequent and unsuspected cause of postprandial distress or dysmotility-like dyspepsia. If these results are confirmed in randomized, controlled clinical studies, duodenal biopsies with CD3 immunophenotyping should be considered as part of the approach for determining a diagnosis of dyspepsia if there is no evidence for a structural disease that could explain the symptoms at the time of an upper GI endoscopy.
FundingWriting support was provided by American Journal Experts and funded by INDOGASTRO foundation.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank Dr. Maria Esteve and Dr. Fernando Fernandez Bañares from the Department of Gastroenterology at the Hospital Universitari Mútua Terrassa for their valuable suggestions and critical review of the manuscript. We also gratefully thank to Shirley Hooper for the English review in the revised manuscript.
Preliminary results of this study were presented as oral presentation at the 16th UEGW Vienna, 08: Santolaria S, Alcedo J, Cuartero B, Diez I, Lorente S, Abascal M, García Prats M, Marigil M, Vera J, Gimeno J, Montoro M. High prevalence of duodenal histological lesions in patients with dyspepsia and normal upper gastrointestinal endoscopy. Gut 2008;57(Suppl II):A2.