Hepatitis E virus (HEV) infection has been identified as a cause of chronic hepatitis in transplant recipients. Rapid evolution to cirrhosis, clinical decompensation and death has been reported in immunosuppressed patients.1 Ribavirin has antiviral activity against HEV and clinical guidelines recommend at least three months of therapy in chronically infected patients. However, treatment failure may occur and the only therapeutic strategy in such cases is interferon.2 Recent data has shown that sofosbuvir inhibits the replication of HEV genotype-3 in vitro and a few cases of successful antiviral therapy with sofosbuvir have been published.3,4 Here, we present a case of a liver-kidney transplant patient with HEV infection failing to several courses of ribavirin treatment who finally underwent sofosbuvir and ribavirin therapy.
Case reportA 52-year-old man with polycystic kidney disease and decompensated liver cirrhosis secondary to nodular regenerative hyperplasia received simultaneous liver-kidney transplantation in October 2012. In addition, he had been diagnosed with common variable immunodeficiency treated with monthly injections of immunoglobulin. Four months after liver transplantation the patient presented the following liver test abnormalities: alanine aminotransferase 199 (5–40IU/L), aspartate aminotransferase 199 (5–40IU/L), alkaline phosphatase 743 (46–116IU/L), gamma glutamyltransferase 162 (5–40IU/L) and bilirubin 0.6 (<0.6mg/dl). Liver ultrasound findings were normal. Viral serologic tests were negative for cytomegalovirus, human immunodeficiency virus and hepatitis A, B and C viruses. Autoimmune liver disease was also ruled out. Finally, HEV infection was diagnosed based on the presence of HEV-RNA (1.8×108IU/mL by real-time PCR, RealStar® HEV RT-PCR Kit 1.0 Altona; IZASA) and anti-HEV IgM. Phylogenetic analysis showed that the patient was infected with HEV genotype-3. No faecal specimen was available for HEV RNA detection. At the time of infection, immunosuppressant levels were within the normal range, according to our protocol (5–8ng/ml for tacrolimus). He received ribavirin treatment (800mg/day) for 12 weeks, with normalization of liver tests and undetectable HEV-RNA at week 8. However, HEV infection relapsed 6 weeks after the end of therapy.
A new course of ribavirin (800mg/day) was given for 24 weeks; however, he presented with a new virological relapse 24 weeks after the end of treatment. Ribavirin was resumed at a higher dose (1200mg/day) during a 12-month period. During this third course of therapy, a stage IV Burkitt's lymphoma was diagnosed needing R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) chemotherapy plus methotrexate. To further support lymphoma treatment, tacrolimus was reduced to 3mg/dl leading to steady-state through plasma levels of 3–4. Despite achieving complete remission of the lymphoma, HEV-RNA persisted positive with abnormal liver tests. A G1634R mutation in HEV viral polymerase was retrospectively detected in serum samples, which may have increased the replicative capacity of HEV and reduced the efficacy of ribavirin.5
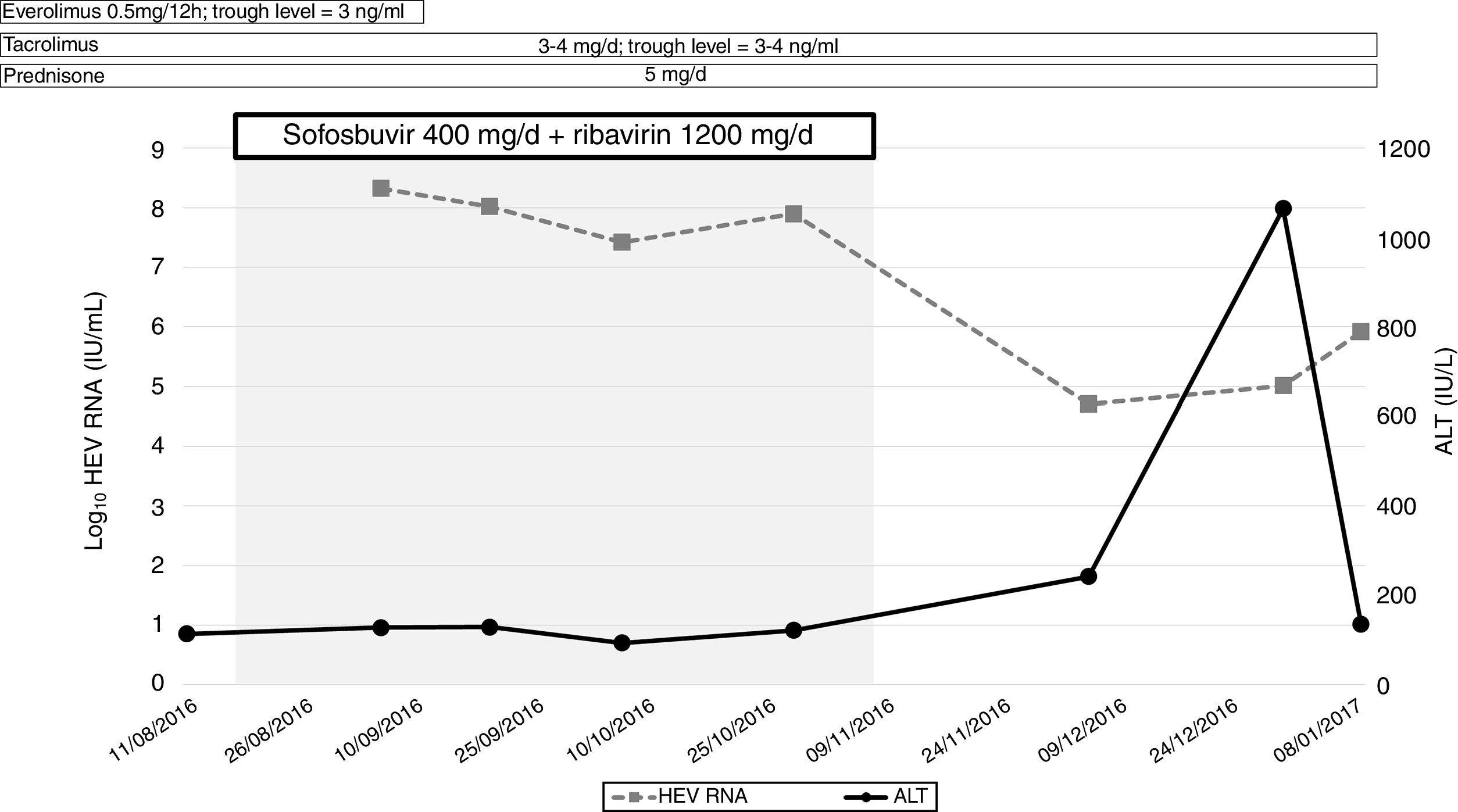
Sofosbuvir 400mg/day in combination with ribavirin (1200mg/day) for 12 weeks was indicated at this point (4 years post-liver transplantation). At that moment, transient elastography (FibroScan®; Echosens, France) showed a value of 4.5kPa and a liver ultrasound excluded signs of cirrhosis. HEV-RNA did not decline, nor did alanine aminotransferase levels normalize (Fig. 1). For this reason, treatment was interrupted at week 10. The patient died 6 months later due to infectious complications.
DiscussionIn summary, we did not observe any significant change in HEV-RNA concentration during therapy. It is unlike that the immune status of the patient would affect sofosbuvir activity, due to its direct mechanism of action. The latter suggests that the concentrations reached may not be enough to inhibit viral replication in some cases. Moreover, the possibility of resistance of sofosbuvir cannot be ruled out, which could explain the differences in efficacy among the reported cases.4 A recent prospective study presented at the Internacional Liver Congress (ILC 2019) assessed the efficacy of sofosbuvir monotherapy in patients with chronic hepatitis E and confirmed the lack of clinical efficacy of the drug, at least in monotherapy (ref 6). The data further support our findings.
Authors’ contributionsAll authors participated in data collection, analysis and drafted the manuscript. All authors read and approved the final manuscript.
Financial supportNo financial support was received in relation to this manuscript.
Conflict of interestXavier Forns has acted as advisor for Gilead and AbbVie and has received unrestricted grant support from AbbVie.