Vedolizumab (VDZ) is a monoclonal antibody that selectively targets α4β7 integrin, approved to treat moderate-to-severe inflammatory bowel disease (IBD). It has a safety profile related to its mechanism of action, characterized by a gut-selectivity.1,2 Pre-marketing data, in an analysis that included over 2800 VDZ-exposed patients only showed a 0.82% of hepatobiliary events.2 No cases of cholestasis were reported.
In this report, we describe the case of an 82-year-old man, without toxic habits and with hypertension, past hepatitis B, prostatectomy for adenocarcinoma and Parkinson's disease. He was diagnosed with severe ulcerative colitis (UC) and was treated with different therapies such as mesalazine, steroids and infliximab (IFX). The latest one was discontinued because of secondary loss of response. Two months later, VDZ was started, three doses of 300mg intravenous infusions at weeks 0, 2 and 6.
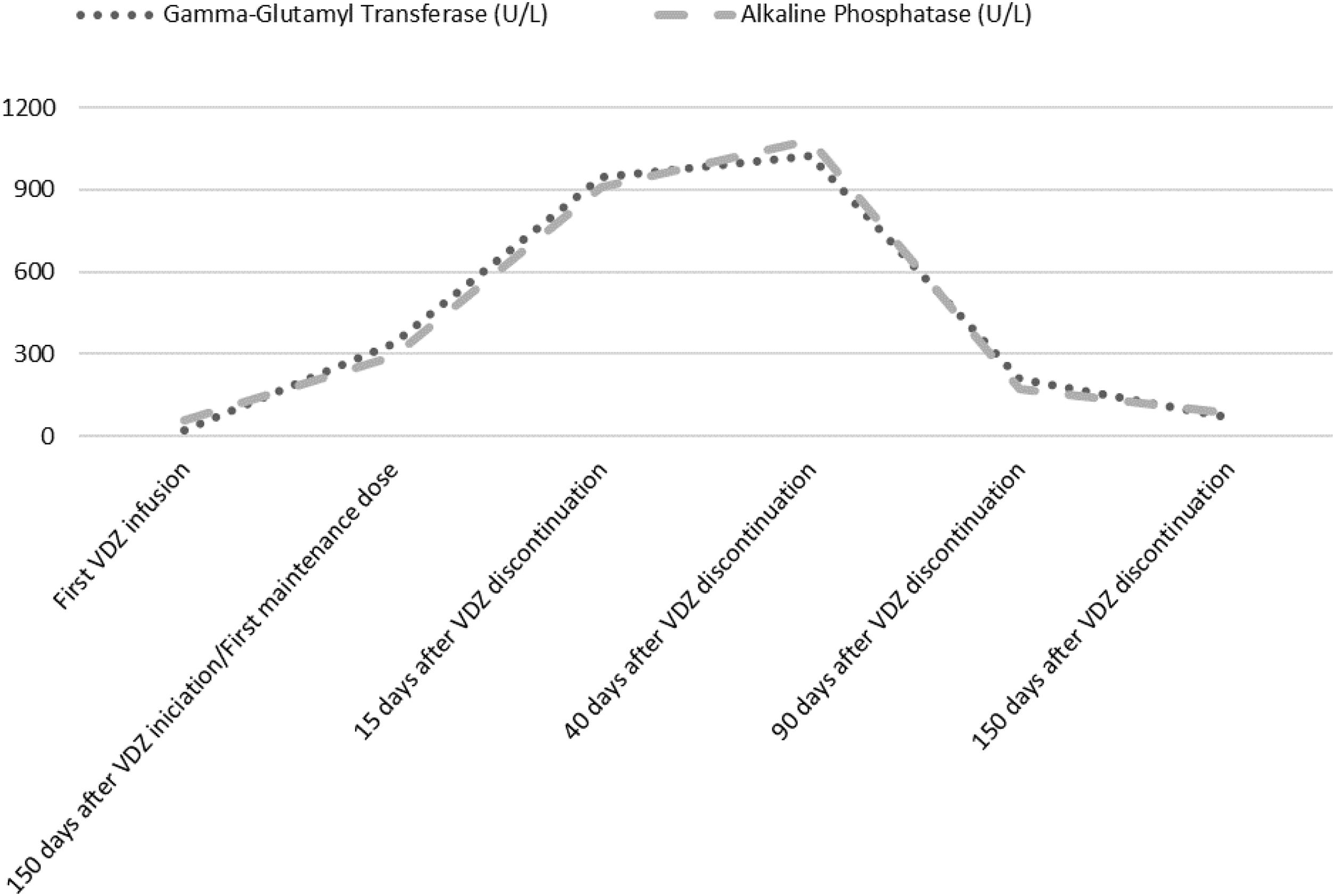
Laboratory analysis at the beginning of the treatment showed a normal hepatic profile. Before the first maintenance session, laboratory tests showed an anicteric cholestasis, with high levels of Gamma-Glutamyl Transferase (GGT) and Alkaline Phosphatase (ALP) (GGT 948U/L, ALP 909U/L). Aspartate aminotransferase (AST) was 132U/L and alanine aminotransferase (ALT) 169U/L. The patient remained asymptomatic. He denied any change in his usual medication. Serological markers of autoimmunity were positive (presence of antinuclear (ANA) and smooth muscle antibodies (ASMA) in a titre=1/320). Immunoglobulin levels, other laboratory tests, abdominal ultrasound and a nuclear colangioresonance were normal. Cholestatic liver injury attributable to VDZ was assumed. The Council for International Organizations of medical Sciences (CIOMS) scale3 classified the event as probable (score of 8), according to its likelihood to be drug-induced liver injury (DILI). Liver biopsy was not considered because of the frailty of the patient. The drug was withdrawn and ursodeoxycholic acid at a dosage of 15mg/kg for two weeks was recommended.
The patient received oral prednisone, 50mg/day for two weeks, that was tapered and maintained at 20mg/day for 12 weeks, because of UC relapse. Cholestasis lasted for 8 months (Fig. 1). Serum enzymes remained normal, ASMA became negative and ANA titre was 1/160 after corticosteroid cessation. No liver biochemical abnormalities have been observed after a follow-up of 2 years.
DILI classified by the criteria of the International Consensus Conference, corresponded to a cholestatic pattern. Previous studies described few cases of hepatocellular type of DILI that obliged to interrupt VDZ. Our patient had a cholestatic liver injury, which could be explained because of his advanced age, as older patients are more prone to a cholestatic pattern of DILI.4 After a review of the published scientific literature, we have found just a single case of cholestatic liver injury attributable to VDZ.5 It should be noted that in both cases, DILI occurred after the induction therapy of VDZ. Unlike our case, it occurred in a young patient and it progressed to chronic liver injury despite prompt drug withdrawal and steroid treatment.
The main limitation of our case is the lack of a liver biopsy which was not considered because of the favorable outcome and the advanced age of the patient. Moreover, in suspected DILI cases it does not provide definitive diagnostic information in most instances.4 Our diagnostic was supported by the CIOMS scale3 and the clinical evolution. Although ANA and ASMA were found positive, serologic markers of autoimmunity have also been described in DILI from other biological treatment, as IFX. Autoimmune hepatitis (AIH) and AIH-DILI,4 a particular phenotype of hepatotoxicity, were not considered as alternative causes because rises in ALT and AST were mild. Although potential autoimmune etiology can not be ruled out, the history of exposure to the medication and the resolution of biochemical abnormalities without relapse, support the diagnosis of DILI.4
In conclusion, although VDZ is a safe treatment, monitoring hepatic enzymes during VDZ therapy should be recommended.
Authors’ contributionsRojas-Feria M. treated the patient. Rodríguez-Fernández M. collected the patient's clinical data. Rodríguez-Fernández M. and Rojas-Feria M. designed the report. Rodríguez-Fernández M., Rojas-Feria M., Castro-Fernández M. and Suárez-García E. wrote the paper.
Informed consent statementConsent was obtained from relatives of the patient for publication of this report.
Financial supportNone.
Conflicts of interestThe authors declare that they have no conflicts of interest.