Population-based bowel screening programmes with faecal occult blood (FOB) tests need to achieve high uptake rates and offer quality services. We invited participants in the Barcelona Programme to complete a satisfaction survey, in order to explore factors influencing uptake and respondents’ opinion and satisfaction with each step of the screening process.
Material and methodTelephone survey using an ad hoc questionnaire (see annex) administered to a final sample of 1189 people: 310 non-participants in the programme (NoP), 553 participants with a negative test result (PNeg), and 326 participants with a positive result (PPos).
ResultsHigh scores were obtained for the clarity of the information provided by the programme (mean 8.9 on a scale 0–10), and for the accessibility and attention at the pharmacy as well as its role as the point for collection and return of FOB test cards (mean >9.3). Aspects that were not so highly rated were: preparation for the colonoscopy (41.6% reported quite a lot or a lot of discomfort), and to a lesser extent telephone accessibility (27.1% reported some difficulties). Participants also expressed concern about receiving a positive test result by telephone (78.9% reported some concern).
ConclusionsRespondents’ opinion of the programme was positive overall, and supports the pharmacy as the point for distributing and collecting FOB test cards, as well as the role of the pharmacist in the context of the programme. Some aspects of the screening process will be reviewed in order to improve participant satisfaction and eventually increase uptake.
Los programas poblacionales de cribado del cáncer colorrectal con prueba de detección de sangre oculta en heces deben obtener tasas altas de participación y ofrecer unos servicios de calidad. Para conocer los factores que influyen en la participación, la opinión y la satisfacción relacionadas con cada una de etapas, se lleva a cabo una encuesta de satisfacción del Programa de Barcelona.
Material y métodoEncuesta telefónica mediante cuestionario diseñado ad hoc a una muestra final de 1.189 personas: 310 no participantes en el Programa (NoP), 553 participantes con resultado negativo del test (PNeg) y 326 participantes con resultado positivo (PPos).
ResultadosDestacan las puntuaciones altas obtenidas en claridad de la información en general (8,9 de media, escala 0 a 10) y la atención en la farmacia, su accesibilidad y el papel como centro de recogida y entrega del test (superior a 9,3 de media). Aspectos que no han sido tan bien valorados: la preparación de la colonoscopia (el 41,6% refiere bastantes o muchas molestias, mayor en mujeres y menores de 60 años) y en menor medida la accesibilidad telefónica (27,1% refiere alguna dificultad) y la preocupación al recibir telefónicamente el resultado patológico del test (78,9% refiere alguna preocupación).
ConclusionesLa valoración que la población hace del Programa es positiva y avala la farmacia como punto de distribución y recogida de kits, así como el papel del farmacéutico. Se revisarán algunos aspectos del proceso de cribado con el fin de mejorar la satisfacción del usuario y, eventualmente, incrementar la participación.
Colorectal cancer (CRC) is one of the most common cancers with the highest mortality in Spain.1 Population programmes for early detection or screening with faecal occult blood (FOB) tests have been shown to reduce CRC2–4 incidence and mortality, and this test is recommended by the European quality guidelines for CRC screening.5 Moreover, compared to colonoscopy, they are more widely accepted in our environment.6 In Spain in 2006, the National Health System took on the implementation of population-based CRC screening programmes with FOB tests; in 2013, the Ministry of Health, Social Services and Equality included them in the Common Services Portfolio.
In December 2009, the Early Detection Programme for Colorectal Cancer of Barcelona (PDPCCR-Bcn, www.prevenciocolonbcn.org), whose stand-out feature compared to other programmes is the active participation of pharmacies in the distribution and collection of FOB tests. The organization, operation and main results of the first round of the PDPCCR-Bcn have been previously published in detail.7 In summary, the Programme individually invites men and women aged 50–69 residing in Barcelona by letter to collect the FOB tests from the pharmacies that are collaborating with the Programme. The pharmacist makes sure there are no grounds for exclusion and explains the procedure for collecting the sample before delivering the FOB test. Once the test has been performed, the person in question can return it to any of the collaborating pharmacies and from there it is sent to the Programme's laboratory. People with negative results receive a letter informing them of the result and that they will be invited back in two years. Those with a positive result are contacted by phone to arrange an appointment at the hospital screening visit, where the result of the test is explained and a colonoscopy proposed.
In order to meet the objectives of the screening programmes, it is essential to obtain high participation rates and adherence once participants have taken part, as well as to offer services that ensure quality throughout the process.5 In this regard, it is fundamental to know which factors influence participation, as well as the opinions and satisfaction related to each one of the aspects and stages of screening.
Satisfaction surveys of health service users are an indicator of health care quality that assess the process, structure and outcome of care, as well as the service activities. The concept of satisfaction includes, among other things, aspects related to accessibility, personal treatment and technical assessment, and is a good predictor of user compliance. Analysing user satisfaction through surveys has become a valuable instrument that has been incorporated as a measure of service quality and a means to improve organization.8–10 In breast cancer screening programmes, which have been underway for more than 20 years, the importance of surveys has been demonstrated both in the improvement of services and in increased participation.11,12 However, to date no published work on user satisfaction has been found for CRC population screening programmes with FOB tests, probably due to their more recent implementation. Most of the studies available focus on satisfaction with regard to the colonoscopy and preferences among different screening options.
This study aims to describe the results of the satisfaction survey on the PDPCCR-Bcn, analysing the results by age and sex, and comparing respondents who did not participate in the Programme, participants with negative results and participants with positive results.
Materials and methodBetween December 2013 and February 2014, a cross-sectional study was conducted by telephone survey on men and women between 50 and 69 years of age who had been invited in the two years prior to the PDPCCR-Bcn. In order to minimize memory bias, the last six Basic Health Areas (BHA) in which the Programme was carried out were selected, with a total target population of 35,655 people (comprising a Programme operation period of between February 2012 and September 2013).
The sample size was calculated to be able to estimate the degree of satisfaction independently in each of the following three profiles: 1) non-participants with positive result (NoP); 2) participants with negative result (PNeg) and 3) PPos. Sampling was randomized by quotas according to the target population in each of the six BHAs and their respective rates of participation and positivity. With a total population of 15,533, 13,163 and 703 people, respectively, and accepting an alpha risk of 0.95 for an accuracy of 0.05 in the two-tailed test of an estimated proportion of the acceptable degree of satisfaction (score 6 or more out of 10) of 0.5 and an estimated loss ratio of 20%, a total sample size of 540 for the NoP profile, 564 for PNeg and 343 for PPos was obtained. 258 people (17.7%) were excluded as “ineligible” (death, disability, incorrect telephone number, etc.), of which 230 (89.5%) were in the NoP profile. As such, the final valid sample was 1189 people: 310 from the NoP profile, 553 from the PNeg and 326 from the PPos.
An ad hoc questionnaire was drawn up from the operational circuit of the Programme and the dimensions intended to be explored, comprising 60 structured questions in 9 blocks (Appendix B, see annex with full questionnaire): 1) Perception of colorectal cancer and first contact with the Programme; 2) Informational material and role of the general practitioner; 3) Programme's telephone assistance; 4) Pharmacy care; 5) FOB test collection process; 6) Negative FOB result; 7) Positive FOB result; 8) Reasons for non-participation, and 9) Overall assessment of the Programme.
Data collection was performed by telephone survey and was carried out by an external company (Projecta’m4.3), specialized in the field work of scientific projects in the area of health. The telephone number(s) used were those listed in the Programme's database, which, on the one hand, come from the Central Registry of Insured People—associated with the Spanish health card—and, on the other, are those provided by the individuals at the time of their participation. The company was provided with a secure and confidential file containing the form with the survey, as well as the name, surname(s), telephone number, age and sex of all the individuals in the sample. The phone call protocol included up to 10 attempts on different days and time slots between 9am and 10pm. In order to test the questionnaire and train the people carrying out the field work, a pilot test of the survey was conducted on 50 people representative of the final sample. This test was supervised by one of the investigators and, subsequent to the performance of the same, slight changes were made to the wording of some items in order to improve their understanding.
All the information was collected on an Access form and later processed and analysed with the IBM-SPSS statistical software package, version 22. All three profiles were compared, as well as the NoP vs the participants (both PNeg and PPos). Bivariate analyses were performed, using contingency tables and the chi squared test (p<0.05) for the comparison of categorical variables, and the mean and the Student t test were calculated for the comparison of the quantitative variables. Since the questions are answered by different subgroups of the sample depending on the block in question, the tables show the response percentages as well as the numerators and denominators of each indicator (identified as n1 and n2, respectively).
The entire study was conducted in accordance with current legislation and the current European Union and national conventions and declarations on ethical standards in research projects. All of the individuals contacted by telephone were informed, prior to the survey, of the framework and purpose of the study, the approximate duration of the survey, the voluntary nature of participation in the survey, the confidentiality of their personal data and responses, and the anonymity of all results. Verbal acceptance to answering survey questions was considered as consent to participate. Data processing was performed in accordance with Organic Law 15/1999 on the personal data protection. The protocol of this study was approved by the Parc de Salut Mar Clinical Research Ethics Committee on 14 November 2013.
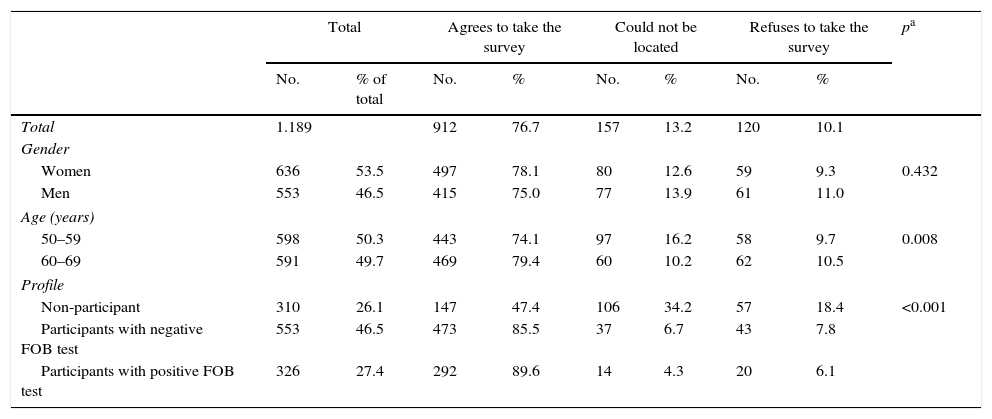
ResultsIn total, 912 people agreed to participate in the survey, representing a response rate of 76.7% (Table 1). People aged 60–69 had a slightly higher response rate than those aged 50–59 (79.4% vs 74.1%, respectively), while those in the 50–59 age bracket could not be located more frequently. The response rate of the individuals in the NoP profile was significantly lower than the rest (NoP 47.4%, PNeg 85.5%, PPos 89.6%, p<0.001), although almost two-thirds of the NoPs who did not respond to the survey could not be located. Of the total number of respondents, 54.5% (497) were women and 48.5% (443) were between 50 and 59 years old.
Characteristics of the sample according to the success of the survey.
| Total | Agrees to take the survey | Could not be located | Refuses to take the survey | pa | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % of total | No. | % | No. | % | No. | % | ||
| Total | 1.189 | 912 | 76.7 | 157 | 13.2 | 120 | 10.1 | ||
| Gender | |||||||||
| Women | 636 | 53.5 | 497 | 78.1 | 80 | 12.6 | 59 | 9.3 | 0.432 |
| Men | 553 | 46.5 | 415 | 75.0 | 77 | 13.9 | 61 | 11.0 | |
| Age (years) | |||||||||
| 50–59 | 598 | 50.3 | 443 | 74.1 | 97 | 16.2 | 58 | 9.7 | 0.008 |
| 60–69 | 591 | 49.7 | 469 | 79.4 | 60 | 10.2 | 62 | 10.5 | |
| Profile | |||||||||
| Non-participant | 310 | 26.1 | 147 | 47.4 | 106 | 34.2 | 57 | 18.4 | <0.001 |
| Participants with negative FOB test | 553 | 46.5 | 473 | 85.5 | 37 | 6.7 | 43 | 7.8 | |
| Participants with positive FOB test | 326 | 27.4 | 292 | 89.6 | 14 | 4.3 | 20 | 6.1 | |
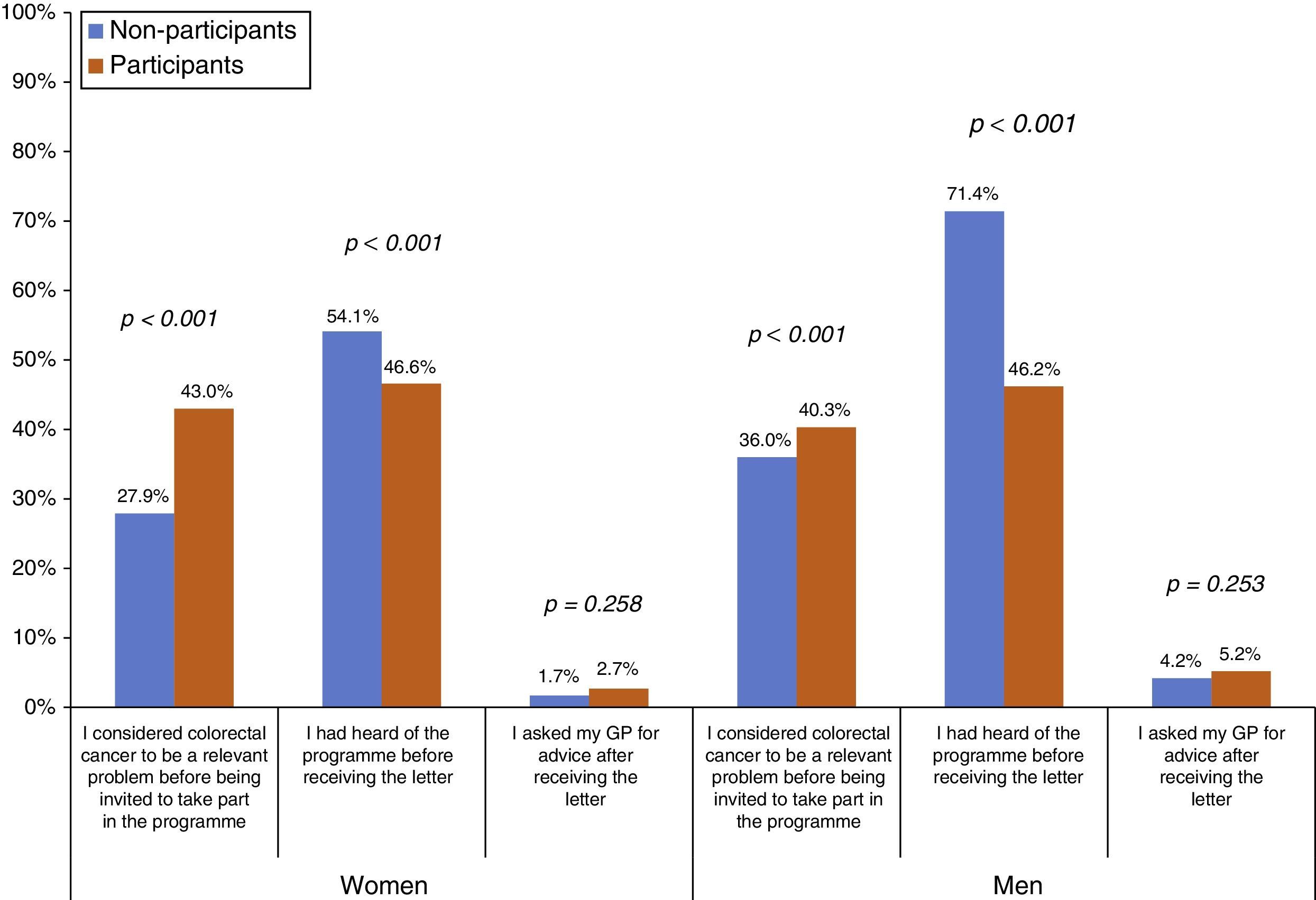
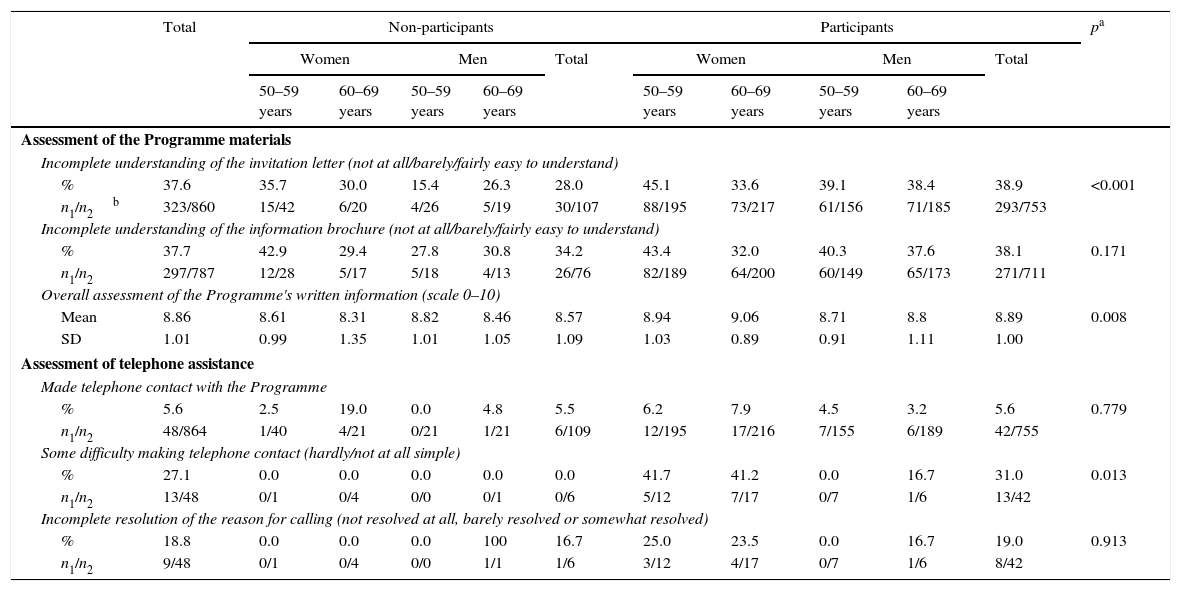
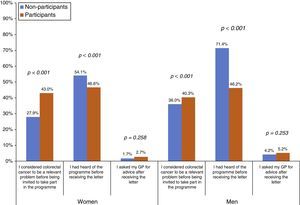
The proportion of people who considered CRC to be a relevant problem before being invited to take part in the Programme was higher among participants than among NoPs, both in women (43.0% vs 27.9%, respectively) and men (40.3% vs 36.0%, respectively) (Fig. 1). However, the NoPs more commonly stated that they had heard of the Programme before receiving the first Programme letter in comparison to the participants, particularly in the case of men (71.4% of NoPs and 46.2% of participants). There was no significant difference between the percentage of NoPs and participants reporting that they had sought advice from their GP after receiving information about the Programme. Regarding the assessment of the Programme material (Table 2), 37.6% stated that their understanding of the invitation letter was incomplete (grouping of “not at all”, “barely” or “fairly” easy to understand), although less than 1% put “not at all” or “barely”. Participants reported an incomplete understanding more frequently than NoPs (38.9% vs 28.0%, respectively). The percentage of incomplete understanding was similar (37.7%) for the brochure, with no differences between the profiles. The average overall assessment of the Programme's written information was 8.86 on a scale of 0–10, being slightly higher among the participants.
Assessment of Programme materials and telephone assistance.
| Total | Non-participants | Participants | pa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women | Men | Total | Women | Men | Total | |||||||
| 50–59 years | 60–69 years | 50–59 years | 60–69 years | 50–59 years | 60–69 years | 50–59 years | 60–69 years | |||||
| Assessment of the Programme materials | ||||||||||||
| Incomplete understanding of the invitation letter (not at all/barely/fairly easy to understand) | ||||||||||||
| % | 37.6 | 35.7 | 30.0 | 15.4 | 26.3 | 28.0 | 45.1 | 33.6 | 39.1 | 38.4 | 38.9 | <0.001 |
| n1/n2b | 323/860 | 15/42 | 6/20 | 4/26 | 5/19 | 30/107 | 88/195 | 73/217 | 61/156 | 71/185 | 293/753 | |
| Incomplete understanding of the information brochure (not at all/barely/fairly easy to understand) | ||||||||||||
| % | 37.7 | 42.9 | 29.4 | 27.8 | 30.8 | 34.2 | 43.4 | 32.0 | 40.3 | 37.6 | 38.1 | 0.171 |
| n1/n2 | 297/787 | 12/28 | 5/17 | 5/18 | 4/13 | 26/76 | 82/189 | 64/200 | 60/149 | 65/173 | 271/711 | |
| Overall assessment of the Programme's written information (scale 0–10) | ||||||||||||
| Mean | 8.86 | 8.61 | 8.31 | 8.82 | 8.46 | 8.57 | 8.94 | 9.06 | 8.71 | 8.8 | 8.89 | 0.008 |
| SD | 1.01 | 0.99 | 1.35 | 1.01 | 1.05 | 1.09 | 1.03 | 0.89 | 0.91 | 1.11 | 1.00 | |
| Assessment of telephone assistance | ||||||||||||
| Made telephone contact with the Programme | ||||||||||||
| % | 5.6 | 2.5 | 19.0 | 0.0 | 4.8 | 5.5 | 6.2 | 7.9 | 4.5 | 3.2 | 5.6 | 0.779 |
| n1/n2 | 48/864 | 1/40 | 4/21 | 0/21 | 1/21 | 6/109 | 12/195 | 17/216 | 7/155 | 6/189 | 42/755 | |
| Some difficulty making telephone contact (hardly/not at all simple) | ||||||||||||
| % | 27.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 41.7 | 41.2 | 0.0 | 16.7 | 31.0 | 0.013 |
| n1/n2 | 13/48 | 0/1 | 0/4 | 0/0 | 0/1 | 0/6 | 5/12 | 7/17 | 0/7 | 1/6 | 13/42 | |
| Incomplete resolution of the reason for calling (not resolved at all, barely resolved or somewhat resolved) | ||||||||||||
| % | 18.8 | 0.0 | 0.0 | 0.0 | 100 | 16.7 | 25.0 | 23.5 | 0.0 | 16.7 | 19.0 | 0.913 |
| n1/n2 | 9/48 | 0/1 | 0/4 | 0/0 | 1/1 | 1/6 | 3/12 | 4/17 | 0/7 | 1/6 | 8/42 | |
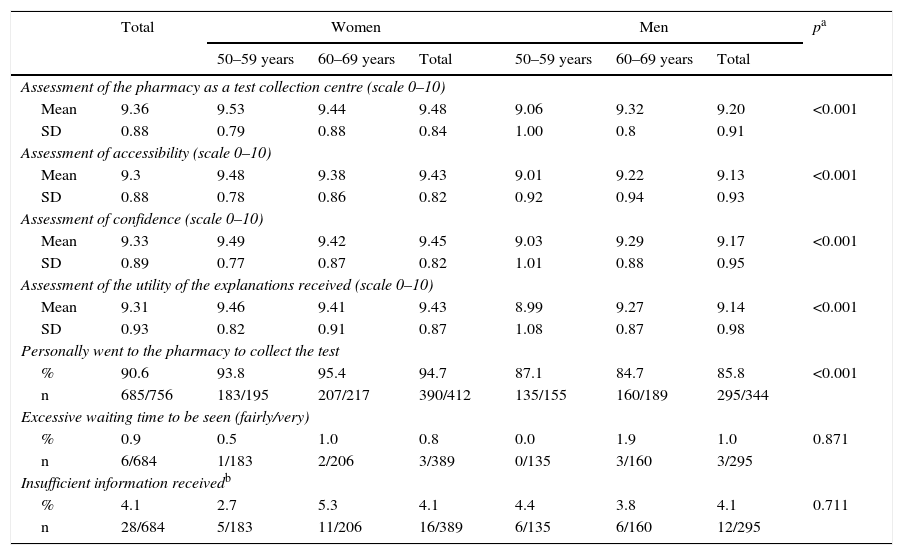
Regarding telephone service (Table 2), 5.6% of the respondents reported having made telephone contact with the Programme, with no differences between the profiles, and of these, 27.1% reported having had difficulty making contact, the majority being participants. 18.8% felt that their reason for calling was not resolved at all, barely resolved or somewhat resolved (“incomplete resolution”). The pharmacy received average values above 9 (scale from 0 to 10) for all aspects assessed: as a test collection centre, in terms accessibility and confidence, and regarding the utility of the explanations received there; women gave better scores more frequently than men (Table 3). 90.6% of the respondents who participated reported that they went to the pharmacy themselves to collect the test, and this figure was higher in women. 0.9% reported that the waiting time to be seen at the pharmacy was excessive (fairly or very) and 4.1% lacked information that would have been useful to them.
Assessment of pharmacy care.
| Total | Women | Men | pa | |||||
|---|---|---|---|---|---|---|---|---|
| 50–59 years | 60–69 years | Total | 50–59 years | 60–69 years | Total | |||
| Assessment of the pharmacy as a test collection centre (scale 0–10) | ||||||||
| Mean | 9.36 | 9.53 | 9.44 | 9.48 | 9.06 | 9.32 | 9.20 | <0.001 |
| SD | 0.88 | 0.79 | 0.88 | 0.84 | 1.00 | 0.8 | 0.91 | |
| Assessment of accessibility (scale 0–10) | ||||||||
| Mean | 9.3 | 9.48 | 9.38 | 9.43 | 9.01 | 9.22 | 9.13 | <0.001 |
| SD | 0.88 | 0.78 | 0.86 | 0.82 | 0.92 | 0.94 | 0.93 | |
| Assessment of confidence (scale 0–10) | ||||||||
| Mean | 9.33 | 9.49 | 9.42 | 9.45 | 9.03 | 9.29 | 9.17 | <0.001 |
| SD | 0.89 | 0.77 | 0.87 | 0.82 | 1.01 | 0.88 | 0.95 | |
| Assessment of the utility of the explanations received (scale 0–10) | ||||||||
| Mean | 9.31 | 9.46 | 9.41 | 9.43 | 8.99 | 9.27 | 9.14 | <0.001 |
| SD | 0.93 | 0.82 | 0.91 | 0.87 | 1.08 | 0.87 | 0.98 | |
| Personally went to the pharmacy to collect the test | ||||||||
| % | 90.6 | 93.8 | 95.4 | 94.7 | 87.1 | 84.7 | 85.8 | <0.001 |
| n | 685/756 | 183/195 | 207/217 | 390/412 | 135/155 | 160/189 | 295/344 | |
| Excessive waiting time to be seen (fairly/very) | ||||||||
| % | 0.9 | 0.5 | 1.0 | 0.8 | 0.0 | 1.9 | 1.0 | 0.871 |
| n | 6/684 | 1/183 | 2/206 | 3/389 | 0/135 | 3/160 | 3/295 | |
| Insufficient information receivedb | ||||||||
| % | 4.1 | 2.7 | 5.3 | 4.1 | 4.4 | 3.8 | 4.1 | 0.711 |
| n | 28/684 | 5/183 | 11/206 | 16/389 | 6/135 | 6/160 | 12/295 | |
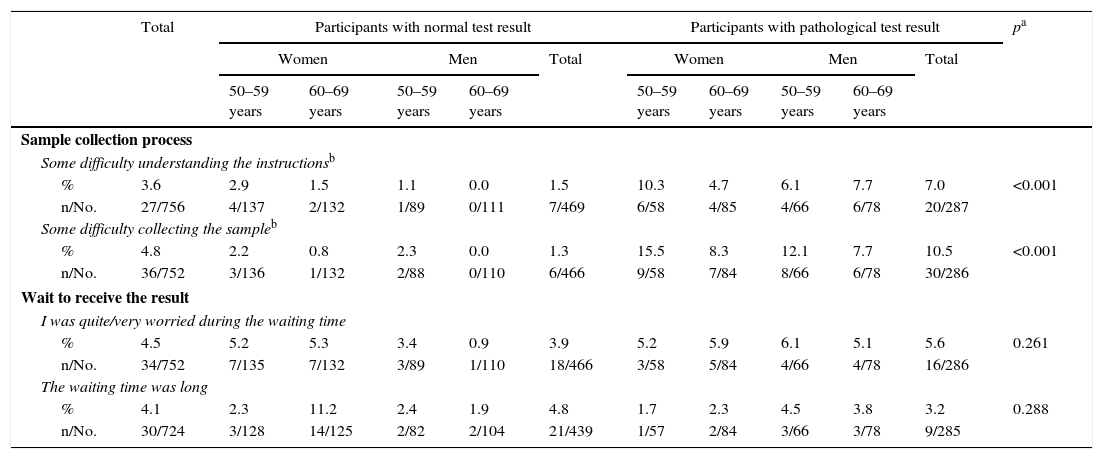
In relation to the sample collection process, the participants with a pathological test result reported greater difficulties than the participants with a normal result (Table 4): 1.5% of PNeg and 7.0% of PPos reported some difficulty in understanding the instructions, and 1.3 and 10.5%, respectively, some difficulty in collecting the sample. 4.1% of the respondents felt that the waiting time to receive the result was excessively long and 4.5% said that they were worried during that time.
Assessment of the process of collecting the sample and waiting to receive the result.
| Total | Participants with normal test result | Participants with pathological test result | pa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women | Men | Total | Women | Men | Total | |||||||
| 50–59 years | 60–69 years | 50–59 years | 60–69 years | 50–59 years | 60–69 years | 50–59 years | 60–69 years | |||||
| Sample collection process | ||||||||||||
| Some difficulty understanding the instructionsb | ||||||||||||
| % | 3.6 | 2.9 | 1.5 | 1.1 | 0.0 | 1.5 | 10.3 | 4.7 | 6.1 | 7.7 | 7.0 | <0.001 |
| n/No. | 27/756 | 4/137 | 2/132 | 1/89 | 0/111 | 7/469 | 6/58 | 4/85 | 4/66 | 6/78 | 20/287 | |
| Some difficulty collecting the sampleb | ||||||||||||
| % | 4.8 | 2.2 | 0.8 | 2.3 | 0.0 | 1.3 | 15.5 | 8.3 | 12.1 | 7.7 | 10.5 | <0.001 |
| n/No. | 36/752 | 3/136 | 1/132 | 2/88 | 0/110 | 6/466 | 9/58 | 7/84 | 8/66 | 6/78 | 30/286 | |
| Wait to receive the result | ||||||||||||
| I was quite/very worried during the waiting time | ||||||||||||
| % | 4.5 | 5.2 | 5.3 | 3.4 | 0.9 | 3.9 | 5.2 | 5.9 | 6.1 | 5.1 | 5.6 | 0.261 |
| n/No. | 34/752 | 7/135 | 7/132 | 3/89 | 1/110 | 18/466 | 3/58 | 5/84 | 4/66 | 4/78 | 16/286 | |
| The waiting time was long | ||||||||||||
| % | 4.1 | 2.3 | 11.2 | 2.4 | 1.9 | 4.8 | 1.7 | 2.3 | 4.5 | 3.8 | 3.2 | 0.288 |
| n/No. | 30/724 | 3/128 | 14/125 | 2/82 | 2/104 | 21/439 | 1/57 | 2/84 | 3/66 | 3/78 | 9/285 | |
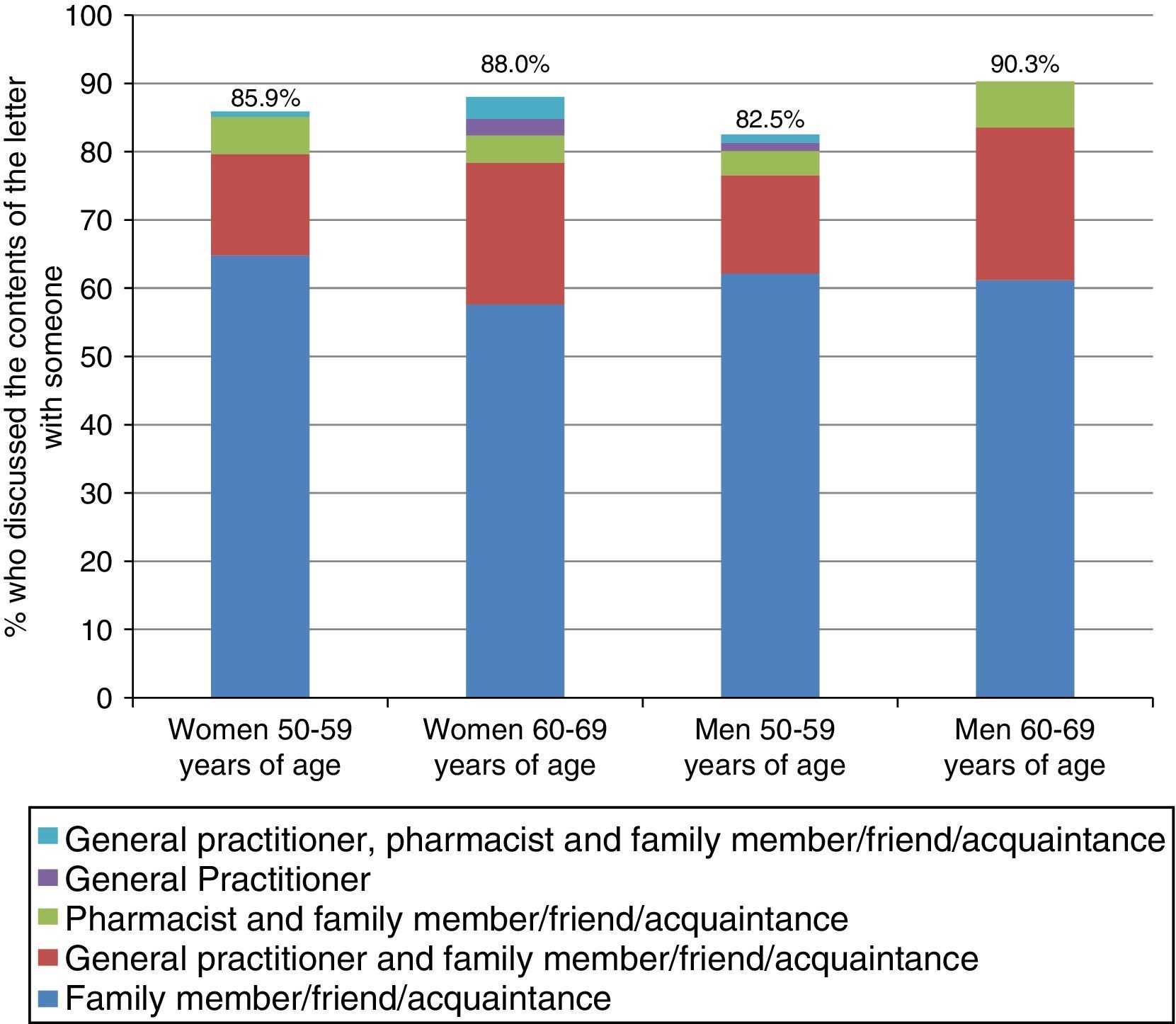
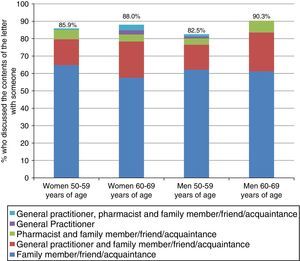
Fig. 2 shows the proportion of PNeg respondents who discussed their negative outcome letter with someone (87.4%) and the distribution of the person(s) with whom they discussed it. People between 60 and 69 years of age engaged in a discussion most often, with no differences between sexes. Most respondents who discussed it with someone did so with a relative, friend or acquaintance in all age and sex groups (more than 65%). 17.3% of those aged 50–59 and 24.1% of those aged 60–69 also discussed it with their GP, and about 5% also discussed it with their pharmacist.
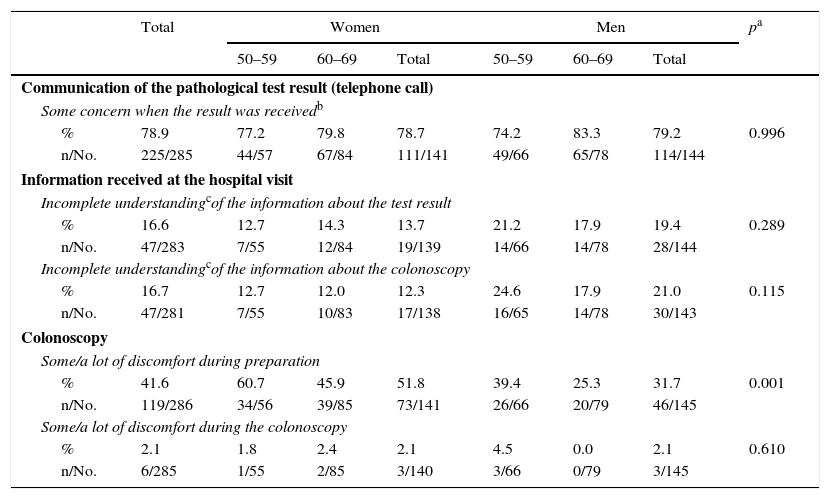
78.9% of respondents with a pathological result reported experiencing some concern when receiving the call (sum of “a lot”, “some” or “little” concern), although only 6.5% (19 people) stated “a lot of concern” (Table 5). Regarding the screening visit, 16.6% and 16.7% reported an incomplete understanding of the information (sum of “some”, “little” or “no understanding”) about the test result and colonoscopy, respectively. 41.6% of those who underwent a colonoscopy as part of the Programme reported some or a lot of discomfort during preparation, with differences seen in gender (51.8% of women and 31.7% of men) and age (greater among people aged 50–59). The colonoscopy involved “some” or “a lot of” discomfort in 2.1% of the respondents, with no differences between gender or age.
Assessment of the communication of the pathological test, the information received at the hospital visit and the colonoscopy.
| Total | Women | Men | pa | |||||
|---|---|---|---|---|---|---|---|---|
| 50–59 | 60–69 | Total | 50–59 | 60–69 | Total | |||
| Communication of the pathological test result (telephone call) | ||||||||
| Some concern when the result was receivedb | ||||||||
| % | 78.9 | 77.2 | 79.8 | 78.7 | 74.2 | 83.3 | 79.2 | 0.996 |
| n/No. | 225/285 | 44/57 | 67/84 | 111/141 | 49/66 | 65/78 | 114/144 | |
| Information received at the hospital visit | ||||||||
| Incomplete understandingcof the information about the test result | ||||||||
| % | 16.6 | 12.7 | 14.3 | 13.7 | 21.2 | 17.9 | 19.4 | 0.289 |
| n/No. | 47/283 | 7/55 | 12/84 | 19/139 | 14/66 | 14/78 | 28/144 | |
| Incomplete understandingcof the information about the colonoscopy | ||||||||
| % | 16.7 | 12.7 | 12.0 | 12.3 | 24.6 | 17.9 | 21.0 | 0.115 |
| n/No. | 47/281 | 7/55 | 10/83 | 17/138 | 16/65 | 14/78 | 30/143 | |
| Colonoscopy | ||||||||
| Some/a lot of discomfort during preparation | ||||||||
| % | 41.6 | 60.7 | 45.9 | 51.8 | 39.4 | 25.3 | 31.7 | 0.001 |
| n/No. | 119/286 | 34/56 | 39/85 | 73/141 | 26/66 | 20/79 | 46/145 | |
| Some/a lot of discomfort during the colonoscopy | ||||||||
| % | 2.1 | 1.8 | 2.4 | 2.1 | 4.5 | 0.0 | 2.1 | 0.610 |
| n/No. | 6/285 | 1/55 | 2/85 | 3/140 | 3/66 | 0/79 | 3/145 | |
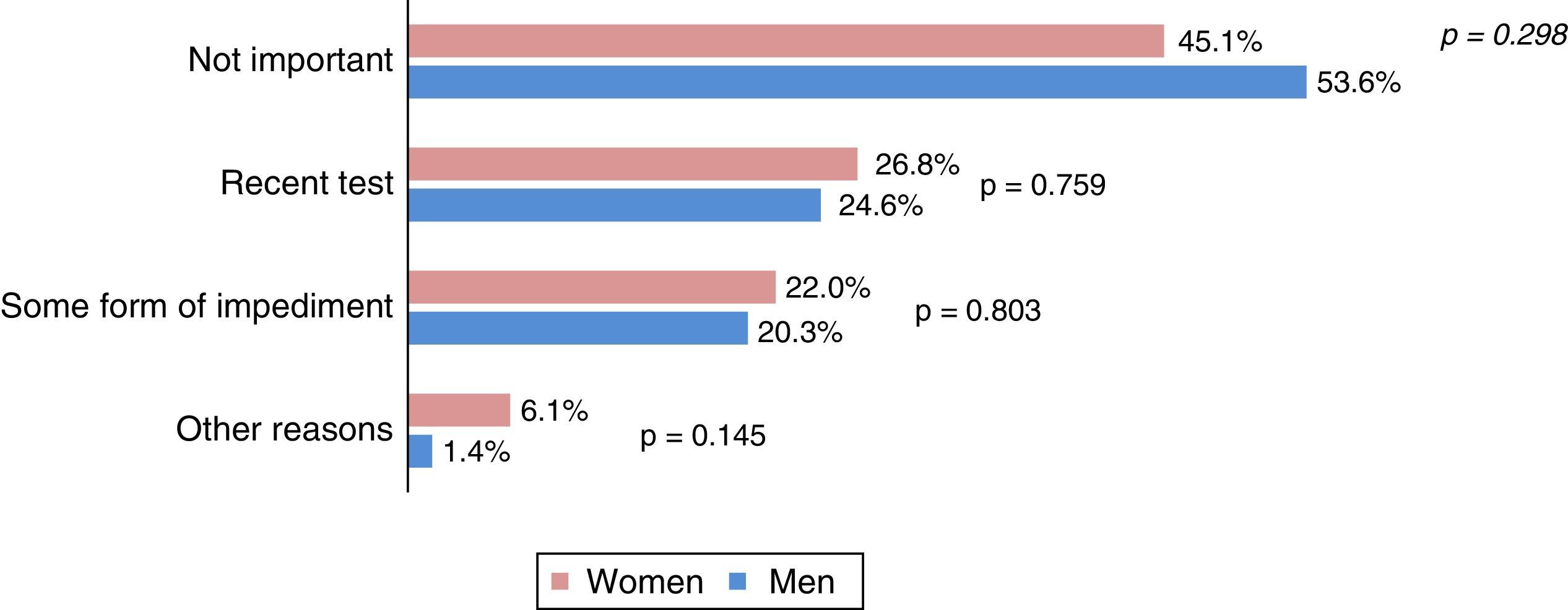
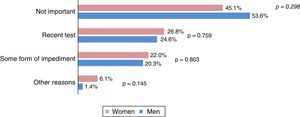
Fig. 3 shows the reasons for non-participation arranged in groups. The most frequent reason was “not important” (45.1% of women and 53.6% of men), followed by the existence of a recent colonic pathology test (25.8%) and some form of personal impediment (21.1%). The average overall score awarded to the Programme by the participants was 9.5 on a scale of 0–10 (SD 0.7), with 67.4% (508) giving a score of 10.
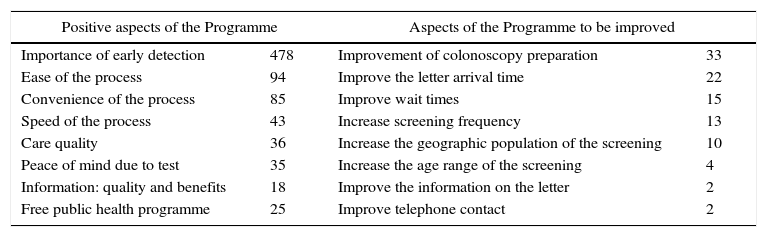
Finally, Table 6 shows the Programme's positive aspects and aspects to be improved, which are most frequently mentioned in the open-ended questions at the end of the survey. The relevance of early cancer detection is by far the most cited positive aspect, followed by the ease, convenience and speed of the screening process. The respondents highlighted the colonoscopy preparation and the waiting times for receiving results letters as the main aspects for improvement. Although only 27 people list it, it is worth mentioning the request to increase the Programme's expansion in terms of frequency, geographic population and age range.
Positive aspects and aspects to be improved spontaneously by the respondents (arranged in groups and number of times mentioned).
| Positive aspects of the Programme | Aspects of the Programme to be improved | ||
|---|---|---|---|
| Importance of early detection | 478 | Improvement of colonoscopy preparation | 33 |
| Ease of the process | 94 | Improve the letter arrival time | 22 |
| Convenience of the process | 85 | Improve wait times | 15 |
| Speed of the process | 43 | Increase screening frequency | 13 |
| Care quality | 36 | Increase the geographic population of the screening | 10 |
| Peace of mind due to test | 35 | Increase the age range of the screening | 4 |
| Information: quality and benefits | 18 | Improve the information on the letter | 2 |
| Free public health programme | 25 | Improve telephone contact | 2 |
This study shows the first results published on satisfaction with the screening process of a population invited to participate in an early, population-based CRC detection programme using FOB tests. Overall, the respondents gave a positive assessment of PDPCCR-Bcn and the “pharmacy strategy”. The high scores obtained for the clarity of information (8.9 on average) and pharmacy care, its accessibility and the role as a test collection and drop-off centre (above 9.3 on average) are particularly noteworthy. Aspects that were not so highly assessed include the colonoscopy preparation (41.6% state some or a lot of discomfort) and, to a lesser extent, telephone accessibility and concern about receiving the pathological FOB test result.
The authors of this study have not found any publication regarding a satisfaction survey on a population-based screening programme for early colorectal cancer detection. However, there are some studies that mention some of the aspects considered in the survey, such as reasons for participation and non-participation,13–20 knowledge and beliefs about CRC,14–17,21 preferences about different screening strategies17,22–24 and satisfaction with the colonoscopy.25,26
Before being invited to take part, Programme participants considered CRC to be a problem that was relevant to their health to a greater extent than the NoPs, which coincides with the notion that the CRC awareness level is positively associated with participation.14,21 Men also listed a lack of concern as a reason for non-participation more frequently than women, which is also in agreement with other authors,14 despite the fact that the percentage of men and women who considered CRC to be a relevant problem before being invited to take part in the Programme is similar. The lower tendency of men to perform preventive practices has been related to psychosocial aspects, less knowledge and interest in cancer and prevention, and less contact with primary care.15 Thus, because in our Programme, as in other programmes using FOB tests, men participate less than women,7 it seems reasonable to adapt the Programme's communication strategies according to gender. On the other hand, a quarter of the NoPs cite “prior tests”, which would have implied their exclusion from the Programme had they actively communicated this.
Less than 3% of the NoPs and 4% of the participants stated that they had gone over the invitation letter with their GP. Although there are no data with which to compare these figures, different studies emphasize that having talked to a doctor about screening or having received screening recommendations on a doctor's behalf are independent and important predictors of subsequent participation.16,19,24,27 Promoting consultations with GPs could lead to an increase in participation.
The Programme material is assessed positively overall (8.86 on a scale of 0–10). However, about a third report that both the letter and the brochure were “not at all”, “barely” or “fairly” easy to understand, answers that the authors deemed to mean an “incomplete understanding”. While it is true that the vast majority of them responded “fairly easy to understand”, it is worth considering why comprehension was not complete and to think about how it could be improved. In this sense, it would be interesting to assess the relationship between this understanding and the socio-economic profile of the people surveyed, a variable that has been related to “health literacy” and to the understanding of the information and process of colorectal cancer screening,28,29 but which was not available for this study. The study design took into account that the six BHAs selected had sufficient variability in the socio-economic indicators of the area, so that, although the answers cannot be compared according to this variable, it can be assumed that, overall, the surveyed sample is representative of the socio-economic level of the Programme's target population. Regarding telephone assistance, difficulties making contact with the Programme (27.1%) and incomplete call resolutions (18.8%) were noteworthy. These aspects will be studied to analyse opportunities for improvement. The involvement of the pharmacy was positively assessed for all aspects studied, which demonstrates the population's satisfaction with the “pharmacy strategy” of the PDPCCR-Bcn.
Both the understanding of the instructions and the sample collection process proved to be more difficult for PPos (7.0%) than for PNegs (1.5%). This difference may be explained by memory bias: the memory of an event is modified according to the diagnosis of a disease related to that event; in this case, the memory of the FOB test experience may have been modified by discovering a positive test result and what it implies. 87.4% discussed the negative outcome letter with someone, most with a relative, friend or acquaintance. The fact that about a fifth also discussed it with their GP could reflect their desire to involve their doctor and make them aware of the result, but might also indicate a lack of understanding of the letter.
The majority (78.9%) of the people with a positive test reported some concern about receiving the result by telephone, a concern that can be explained by the need for additional tests, the scheduling of a hospital visit and uncertainty regarding the possible cause of bleeding. This finding is similar to that reported for breast cancer screening and one of the adverse effects of the most relevant screening programmes.30 It will subject to a more in-depth analysis to investigate whether it is possible to reduce such concern.
Respondents reported no significant discomfort regarding the performance of colonoscopy, but more than 40% felt that they experienced “some” or “a lot of” discomfort during the preparation, with this percentage being higher in women. The laxative preparation prior to colonoscopy is considered the most uncomfortable aspect and the greatest barrier to the performance of colonoscopy in several studies.19,25 Its higher frequency in women has been published previously31 but could be partially explained by a memory bias related to the diagnosis obtained, similar to the one explained above: since the positive predictive value of FOB is lower in women, they will observe its benefit less frequently than men and this can modify the perception of the preparation. In any case, and although this is an aspect in which the authors consider there to be little room for improvement, less uncomfortable alternatives to laxative preparations will be assessed in the Programme.
This study suffers some limitations. First, although the overall response rate of the survey was 76.7%, the NoP profile did not reach 50%. This is related, on the one hand, to the fact that only when the person participates in the Programme is the personal contact information checked and completed and, on the other, to a greater rejection to performing the survey, probably linked to non-participation in the Programme. While it is feasible to think that the people who refused to perform the survey had a lower overall assessment of the Programme than those who accepted from the same profile, it is difficult to predict the bias in people who have not been located. On the other hand, the results are inevitably subject to memory bias, a bias that we attempted to minimize in the study design (by selecting the most recent areas) and that could affect, as already mentioned, the interpretation of certain results. Finally, given that there were no other satisfaction questionnaires in our context, the questionnaire was designed ad hoc from the Programme's operating circuit and agreed upon by the authors of the study and other people in the Programme. The questionnaire was satisfactorily piloted with a sample of 50 people but the validation of the same was considered to fall outside of the scope of this investigation. However, and depending on the need for it, its validation will be considered in the future.
In summary, the population's assessment of the PDPCCR-Bcn is positive and endorses the pharmacy as a kit distribution and collection point, as well as the role of the pharmacist in the Programme. The results of this study, however, will allow us to review some aspects of the screening process, such as telephone accessibility, the invitation letter and the communication process of the positive test result, with a view to improve user satisfaction and, potentially, increase participation.
FundingThe field work of this survey was funded mainly with the prize obtained in the category of Best Institutional Initiative in the Pharmaceutical Area at the 4th Edition of the Esteve Awards: United for Patient Care, and completed by means of a minor financial contribution from Palex, the provider of the Programme's faecal occult blood test.
Conflicts of interestThe authors declare that they have no conflicts of interest. The financial contributions described above did not influence either the methodology or the interpretation of the study results in any way.
To Cristina Hernández, for her role in the preparation of the questionnaire in Access format, and Oleguer Parés-Badell, for his help updating the bibliographic review.
Please cite this article as: Burón A, Posso M, Sivilla J, Grau J, Guayta R, Castells X, et al. Análisis de la satisfacción de los participantes en el Programa de detección precoz de cáncer colorrectal de Barcelona: valoración positiva de la farmacia comunitaria. Gastroenterol Hepatol. 2017;40:265–275.