Autoimmune hepatitis is a chronic liver disease that impacts on morbidity and mortality of patients. Few epidemiological data exist of this in Latin America and Colombia.
ObjectivesThe aim of this study is to describe the demographic, clinical and laboratory characteristics of the patients; the treatment and the response to it, the evolution and course of the disease, requirement of liver transplantation and mortality.
MethodsHistorical cohort study that include patients attended at an University Hospital in Medellin, Colombia between January 2010 and December 2016 with ≥16 years age at the time of diagnosis of autoimmune hepatitis. Data collection was done from the review of medical records. Statistical analysis was performed using SPSS version 20.
ResultsThe study included 278 patients, 90% of the patients were women, the median age at diagnosis was 50 years. 37.8% were cirrhotic at the time of diagnosis. The biochemical remission was 85%. In patients who developed cirrhosis it was found a higher proportion of men (21.2 vs. 7.8%, p=0.027), a greater frequency of overlap autoimmune-primary sclerosant cholangitis (6.0 vs. 0% p=0.006) and a greater frequency of non-response to treatment (12.1 vs. 1.6%, p=0.004).
ConclusionAutoimmune hepatitis is not a rare disease in Colombian population; it predominates in women but has a less favourable course in men. An important number of patients are cirrhotic at the time of diagnosis, the response to treatment and complications in our population are similar to those described worldwide.
La hepatitis autoinmune es una hepatopatía con impacto en la morbimortalidad de los pacientes. Pocos datos epidemiológicos existen sobre esta en Latinoamérica y Colombia.
ObjetivosDescribir las características demográficas, clínicas y de laboratorio de los pacientes con hepatitis autoinmune; el tratamiento y la respuesta al mismo, la evolución y curso de la enfermedad, requerimiento de trasplante hepático y mortalidad.
MétodosEstudio retrospectivo que incluyó una cohorte histórica de pacientes atendidos en un hospital universitario de Medellín, Colombia entre enero de 2010 y diciembre de 2016 con ≥16 años de edad al momento del diagnóstico de hepatitis autoinmune. Se recolectó la información a partir del registro de historias clínicas. Análisis estadístico utilizando SPSS versión 20.
ResultadosSe incluyeron 278 pacientes, el 90% eran mujeres, la mediana de edad al diagnóstico fue de 50 años. Un 37,8% estaban cirróticos al momento del diagnóstico. La remisión bioquímica fue del 85%. En los pacientes que progresaron a cirrosis se encontró una mayor proporción de hombres (21,2 vs. 7,8%, p=0,027), una mayor frecuencia de síndrome de solapamiento HAI-CEP (6,0 vs. 0%, p=0,006), una menor remisión bioquímica (57,6 vs. 89%, p=<0,001) y una mayor frecuencia de no respuesta al tratamiento (12,1 vs. 1,6%, p=0,004).
ConclusiónLa hepatitis autoinmune no es infrecuente en la población colombiana, predomina en mujeres pero tiene un comportamiento desfavorable en hombres. Un importante número de pacientes están cirróticos al momento del diagnóstico, la respuesta al tratamiento y complicaciones en nuestra población son similares a las descritas mundialmente.
Autoimmune hepatitis (AIH) is a chronic liver disease of unknown aetiology that was first described by Dr Waldenström in 1950.1 It has an effect on morbidity and mortality in those affected, and places an economic burden on health systems.2 It is a potential cause of acute liver failure and progression to cirrhosis of the liver.3,4
Its pathophysiology is known to be due to autoimmunity, as in other autoimmune diseases, with self-antigens and a loss of immune tolerance generating an immune response against the tissues, with the liver being the main organ affected.4 It is more prevalent in women, with a female-male ratio of 4:1, which can increase to 10:1 in type 2 AIH.5 It particularly affects young people, although it has been reported in all age groups.6,7
Some studies have found a genetic predisposition to the development of AIH,8 which could lead to variability in data on the incidence and prevalence of the disease according to the geographical area studied. The Caucasian population is one of the most widely described,9,10 with an annual incidence of 1.07 cases per 100,000 inhabitants,11 which is very similar in all European countries.
In Latin America, there are no clear epidemiological data. Some studies show genetic polymorphisms in the major histocompatibility complex in the Latin American population that are different from those found in populations on other continents.12,13 Available information in Colombia is from descriptive studies with a very small patient population.14
The aim of this study is to describe the demographic, clinical, laboratory and treatment characteristics of a cohort of patients with AIH at a University Hospital in Medellín, Colombia, and to describe the incidence of cirrhosis, relapse, required liver transplantation, post-transplantation recurrence and mortality.
Materials and methodsThis retrospective cohort study included patients treated by the emergency department, hospital wards and outpatient clinic of Hospital Pablo Tobón Uribe in Medellín, Colombia, between 1 January 2010 and 31 December 2016.
A search was performed in the electronic medical record system for patients diagnosed with AIH (code K754) according to the International Classification of Diseases (ICD-10). In order to identify patients with AIH-primary biliary cholangitis (PBC) (AIH-PBC) and AIH-primary sclerosing cholangitis (PSC) (AIH-PSC) overlap syndromes, those patients diagnosed with PBC (code K743) were also evaluated.
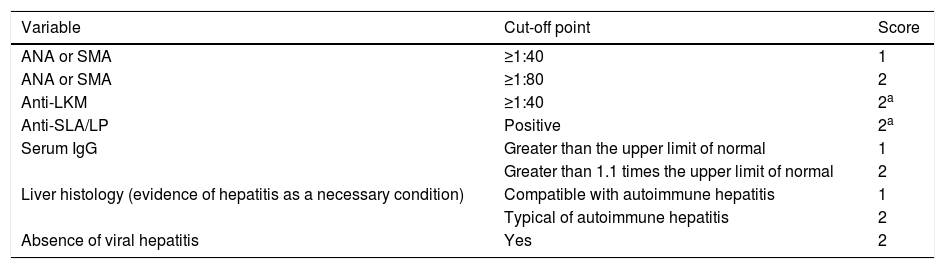
Patients diagnosed with AIH after the age of 16 according to the simplified criteria for the diagnosis of AIH published in 2008 by the International Autoimmune Hepatitis Group (IAIHG)15 (Table 1) and those with overlap syndrome that met the biochemical features, autoantibodies profile, liver histology features and cholangiographic features16 were included.
Simplified criteria for the diagnosis of autoimmune hepatitis according to the International Autoimmune Hepatitis Group (IAIHG), 2008.
| Variable | Cut-off point | Score |
|---|---|---|
| ANA or SMA | ≥1:40 | 1 |
| ANA or SMA | ≥1:80 | 2 |
| Anti-LKM | ≥1:40 | 2a |
| Anti-SLA/LP | Positive | 2a |
| Serum IgG | Greater than the upper limit of normal | 1 |
| Greater than 1.1 times the upper limit of normal | 2 | |
| Liver histology (evidence of hepatitis as a necessary condition) | Compatible with autoimmune hepatitis | 1 |
| Typical of autoimmune hepatitis | 2 | |
| Absence of viral hepatitis | Yes | 2 |
≥6 points: probable autoimmune hepatitis.
≥7 points: definite autoimmune hepatitis.
The maximum number of points for all autoantibodies is 2.
Typical histological features of autoimmune hepatitis: interface hepatitis, lymphocytic/lymphoplasmacytic infiltrates in portal tracts and extending into the lobule, emperipolesis, rosette formation.
Histology compatible with autoimmune hepatitis: chronic hepatitis with lymphocytic infiltration without all the features considered typical of autoimmune hepatitis.
ANA: antinuclear antibodies; LKM: liver–kidney microsomal antibodies; SLA/LP: soluble liver antigen/liver–pancreas antibodies; SMA: smooth muscle antibodies.
Patients with no clinical, biochemical and histological data in their medical records were excluded.
Demographic, clinical, serological, radiological, histological and treatment variables were collected.
According to the clinical presentation at the time of diagnosis, patients were classified as: asymptomatic with abnormal biochemical tests of liver function, and patients with no symptoms but with the presence of abnormal liver function tests. Non-specific symptoms: asthenia, anorexia, pruritus, weight loss and abdominal pain with evidence of abnormal biochemical tests of liver function. Acute hepatitis: right upper quadrant pain, nausea and jaundice, with a pattern of hepatocellular injury shown in the laboratory tests. Cirrhosis of the liver: presence of clinical (gynaecomastia, telangiectasia, palmar erythema, collateral circulation, ascites and encephalopathy), biochemical (hypoalbuminaemia, thrombocytopaenia, prolongation of prothrombin time) and imaging signs of cirrhosis. Acute liver failure: acute symptoms of hepatitis with coagulopathy and development of encephalopathy within 26 weeks of the onset of jaundice according to the definition given by O’Grady et al.17 De novo autoimmune hepatitis: patients with a history of liver transplant (without a pre-transplant diagnosis of AIH) and who developed AIH during the post-transplant period.
The degree of biopsy-proven hepatic fibrosis was evaluated using the METAVIR scoring system, with stages from F0 to F4, where F0 is no fibrosis and F4 is advanced fibrosis with cirrhosis; likewise, histological features were classified as: not compatible, compatible or typical of AIH according to the IAIHG.15
In the response to treatment evaluation, response was classified as: biochemical remission for the normalisation of transaminases and IgG; partial remission for clinical improvement and reduction in transaminases without reaching normalisation; and no response for those who did not manage at least a 25% decrease in transaminase levels, taking the level at which treatment started as the reference point.
Relapse was defined as ALT levels more than threefold the upper limit of normal and elevated IgG levels or worsening of histological features after achieving remission with drug treatment.
Data were collected by reviewing medical records in the hospital's electronic registry using a previously designed data collection instrument. To ensure the quality of the data and reduce the risk of information bias, 10% of the medical records were double-checked by the investigators.
Follow-up of the patients was conducted until 31 December 2016 or until the last date documented in the medical record, after which it was considered censored data if none of the measured outcomes had occurred.
The Universidad de Antioquia's registered SPSS statistical software, version 20, was used to perform the statistical analysis. Categorical variables are presented as absolute and relative frequencies, and continuous variables are presented as mean and standard deviation, if the dataset follows a normal or median distribution, or median and interquartile range (IQR) if it does not follow a normal distribution according to the Kolmogorov–Smirnov test.
The incidence density of complications, such as cirrhosis, required liver transplantation and death, was estimated.
Sample size was not estimated a priori since all patients diagnosed with AIH and treated during the study period were included.
The study followed the guidelines of the Declaration of Helsinki, version 2013, on research involving human beings and Resolution 008430 of 1993 on clinical research in Colombia, and was approved by the hospital's ethics committee.
The final manuscript followed STROBE recommendations for the reporting of observational studies.18
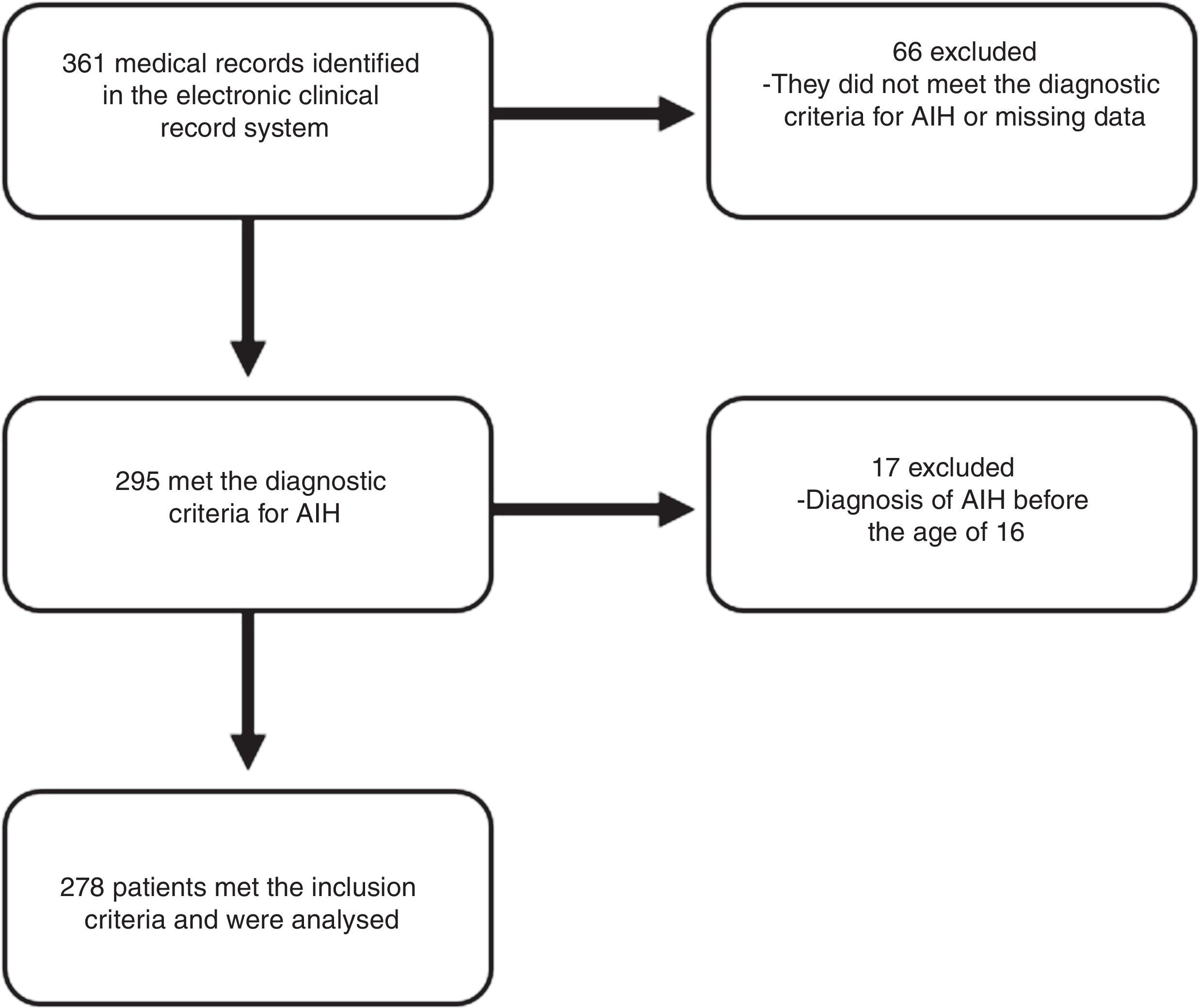
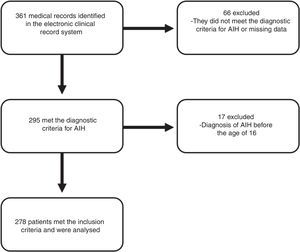
ResultsA total of 278 patients met the inclusion criteria (Fig. 1). The median follow-up time was 41 months (IQR 16–74 months) with a range from 0 to 241 months.
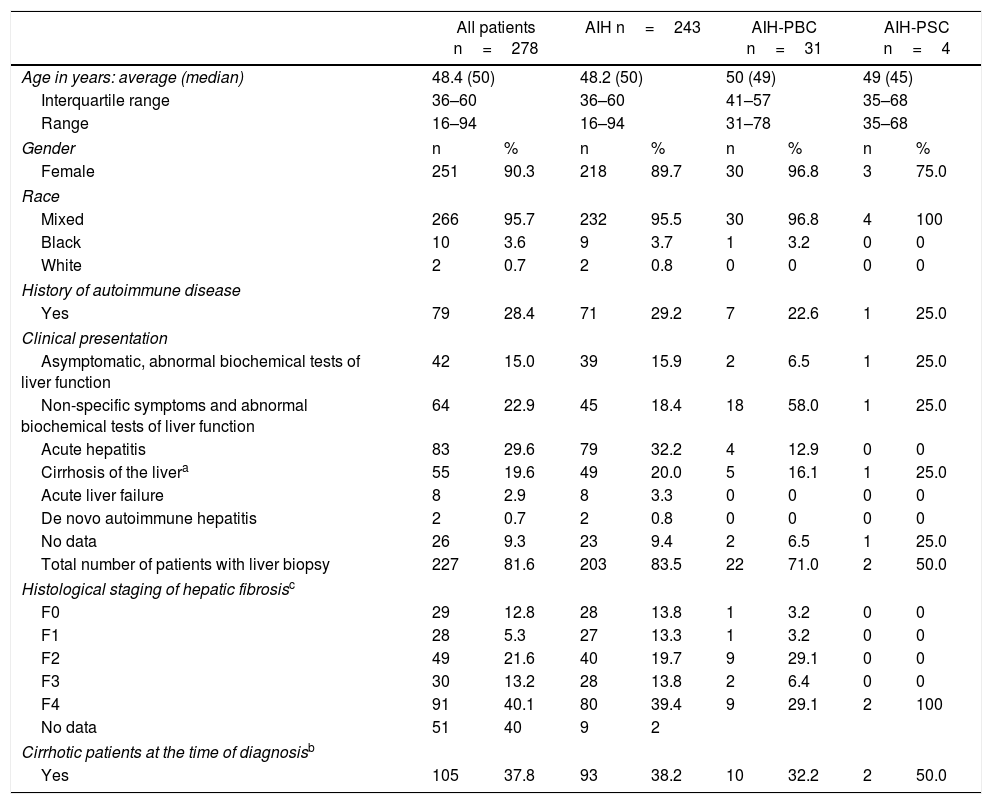
90% of the patients were women, and the majority were of mixed race. The median age at diagnosis was 50 years with a range from 16 to 94 years. The average age at diagnosis in patients with acute liver failure was younger (35 years). A total of 35 (12.6%) patients had AIH-PBC and AIH-PSC overlap syndrome (Table 2).
Clinical features of patients.
| All patients n=278 | AIH n=243 | AIH-PBC n=31 | AIH-PSC n=4 | |||||
|---|---|---|---|---|---|---|---|---|
| Age in years: average (median) | 48.4 (50) | 48.2 (50) | 50 (49) | 49 (45) | ||||
| Interquartile range | 36–60 | 36–60 | 41–57 | 35–68 | ||||
| Range | 16–94 | 16–94 | 31–78 | 35–68 | ||||
| Gender | n | % | n | % | n | % | n | % |
| Female | 251 | 90.3 | 218 | 89.7 | 30 | 96.8 | 3 | 75.0 |
| Race | ||||||||
| Mixed | 266 | 95.7 | 232 | 95.5 | 30 | 96.8 | 4 | 100 |
| Black | 10 | 3.6 | 9 | 3.7 | 1 | 3.2 | 0 | 0 |
| White | 2 | 0.7 | 2 | 0.8 | 0 | 0 | 0 | 0 |
| History of autoimmune disease | ||||||||
| Yes | 79 | 28.4 | 71 | 29.2 | 7 | 22.6 | 1 | 25.0 |
| Clinical presentation | ||||||||
| Asymptomatic, abnormal biochemical tests of liver function | 42 | 15.0 | 39 | 15.9 | 2 | 6.5 | 1 | 25.0 |
| Non-specific symptoms and abnormal biochemical tests of liver function | 64 | 22.9 | 45 | 18.4 | 18 | 58.0 | 1 | 25.0 |
| Acute hepatitis | 83 | 29.6 | 79 | 32.2 | 4 | 12.9 | 0 | 0 |
| Cirrhosis of the livera | 55 | 19.6 | 49 | 20.0 | 5 | 16.1 | 1 | 25.0 |
| Acute liver failure | 8 | 2.9 | 8 | 3.3 | 0 | 0 | 0 | 0 |
| De novo autoimmune hepatitis | 2 | 0.7 | 2 | 0.8 | 0 | 0 | 0 | 0 |
| No data | 26 | 9.3 | 23 | 9.4 | 2 | 6.5 | 1 | 25.0 |
| Total number of patients with liver biopsy | 227 | 81.6 | 203 | 83.5 | 22 | 71.0 | 2 | 50.0 |
| Histological staging of hepatic fibrosisc | ||||||||
| F0 | 29 | 12.8 | 28 | 13.8 | 1 | 3.2 | 0 | 0 |
| F1 | 28 | 5.3 | 27 | 13.3 | 1 | 3.2 | 0 | 0 |
| F2 | 49 | 21.6 | 40 | 19.7 | 9 | 29.1 | 0 | 0 |
| F3 | 30 | 13.2 | 28 | 13.8 | 2 | 6.4 | 0 | 0 |
| F4 | 91 | 40.1 | 80 | 39.4 | 9 | 29.1 | 2 | 100 |
| No data | 51 | 40 | 9 | 2 | ||||
| Cirrhotic patients at the time of diagnosisb | ||||||||
| Yes | 105 | 37.8 | 93 | 38.2 | 10 | 32.2 | 2 | 50.0 |
The most common comorbidity was hypothyroidism (34.2%). Of the total number of patients, 79 (28.4%) had another autoimmune disease (other than hypothyroidism); the most common were Sjögren's syndrome (6.8%), systemic lupus erythematosus (6.4%) and rheumatoid arthritis (2.5%).
Three (1%) patients were infected with hepatitis B virus, one (0.3%) with human immunodeficiency virus and none with hepatitis C virus.
The main clinical presentation in patients with AIH was acute hepatitis (29.6%), while in patients with AIH-PBC it was non-specific symptoms with abnormal biochemical tests of liver function (58%). Eight (2.8%) patients had acute liver failure and two (0.7%) had de novo AIH (all in the AIH group).
In 26 (9.3%) patients, no information was obtained on clinical presentation at the time of diagnosis. All patients in this group met the criteria for diagnosis of AIH and/or overlap syndromes.
AIH was considered to be drug-induced in 13 (4.6%) patients; nitrofurantoin was the most commonly associated drug (9 of the 13 cases) followed by non-steroidal anti-inflammatory drugs (2 cases), propylthiouracil (one case) and adalimumab (one case). None of these cases had acute liver failure, and one developed cirrhosis during follow-up.
At the time of AIH diagnosis, 105 (37.8%) patients were clinically, radiologically, biochemically or histologically cirrhotic.
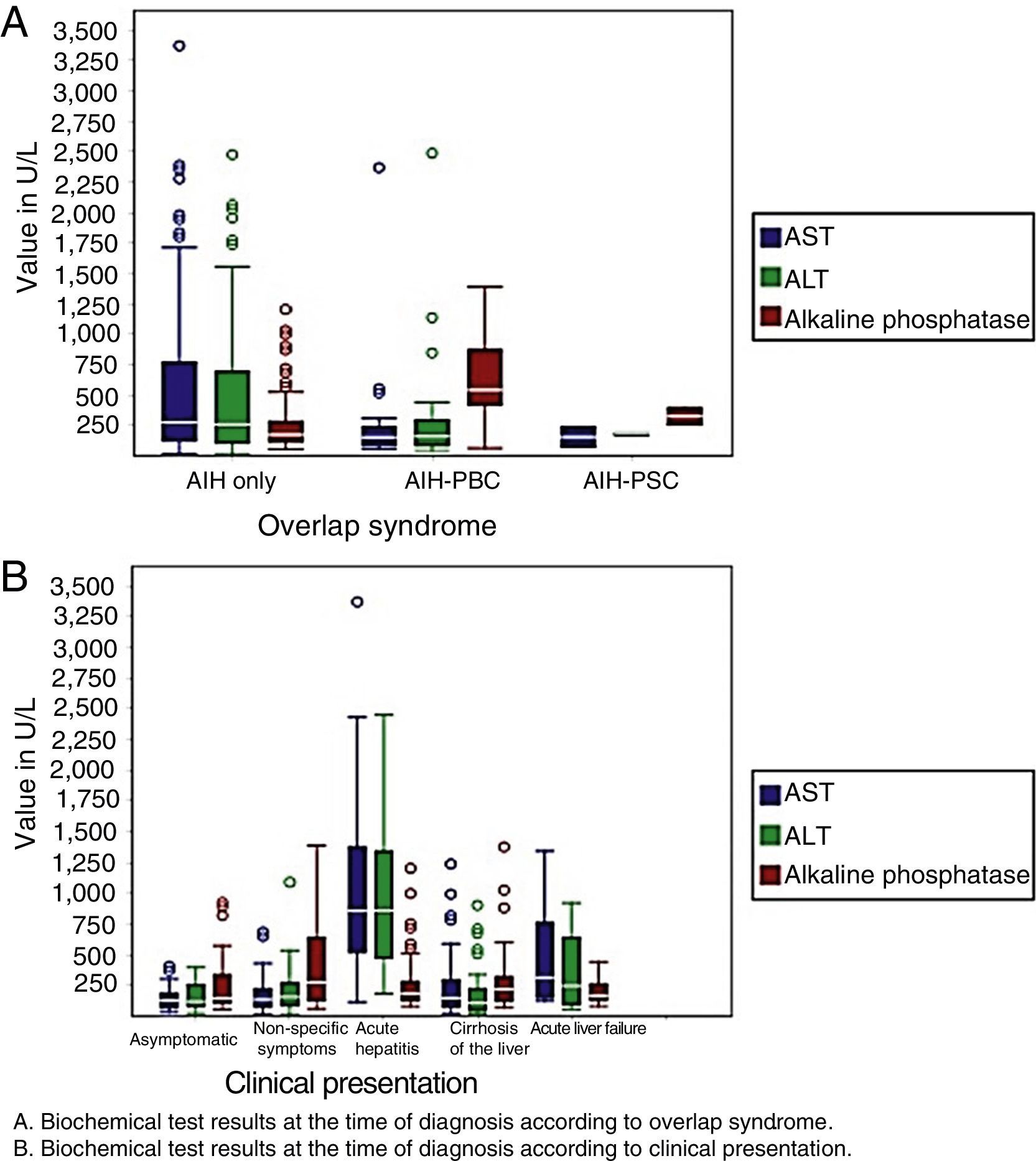
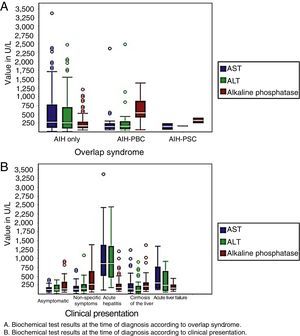
With regard to laboratory variables, patients with acute hepatitis and acute liver failure had higher levels of transaminases at the time of diagnosis, as did patients with AIH. Patients with AIH-PBC and AIH-PSC had higher levels of alkaline phosphatase when compared with patients with AIH (Fig. 2).
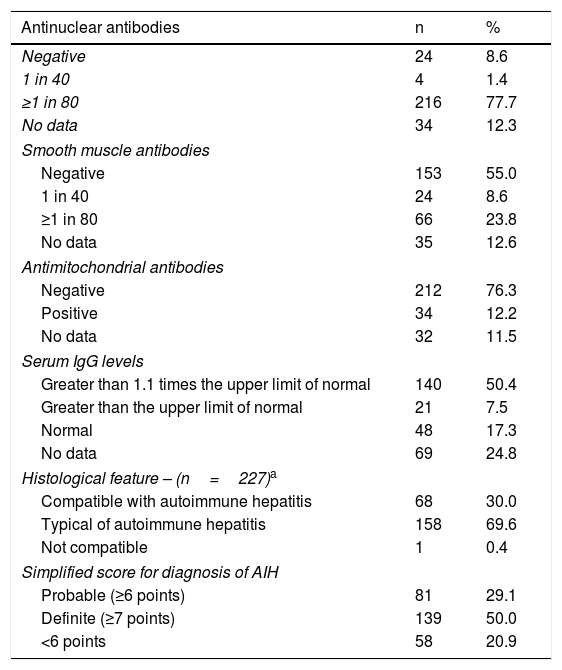
In total, 233 (83.8%) patients had at least one positive autoantibody (antinuclear or smooth muscle antibodies) and 25 (81%) of the 31 patients with AIH-PBC had positive antimitochondrial antibodies (AMA). Anti-SLA/LP or LKM antibodies were not evaluated in 276 (99.2%) patients.
Two hundred and twenty-seven (81.7%) of all the patients underwent a liver biopsy and 226 (99.6%) of these showed features compatible with (chronic hepatitis with lymphocytic infiltration) or typical of AIH (interface hepatitis, lymphocytic/lymphoplasmacytic infiltrates in portal tracts and extending into the lobule, emperipolesis and rosette formation). Thirty-one (88.5%) of the patients with overlap syndrome underwent a liver biopsy and 21 (67.7%) of these had typical features of AIH; all met the diagnostic, serological, imaging and histological criteria for overlap syndrome.16
As shown in Table 3, the simplified score for diagnosis in 220 (79.1%) of the 278 patients was ≥6 points (probable or definite AIH); of the 58 patients who scored <6 points, 43 (74%) received induction therapy and 85% of these achieved biochemical remission. Likewise, of the 51 patients who did not have a liver biopsy, 18 (35%) had a clinical diagnosis of cirrhosis and 30 (58.8%) received treatment, with 23 (76.6%) of these achieving biochemical remission (Table 4).
Serological and histological features of patients.
| Antinuclear antibodies | n | % |
|---|---|---|
| Negative | 24 | 8.6 |
| 1 in 40 | 4 | 1.4 |
| ≥1 in 80 | 216 | 77.7 |
| No data | 34 | 12.3 |
| Smooth muscle antibodies | ||
| Negative | 153 | 55.0 |
| 1 in 40 | 24 | 8.6 |
| ≥1 in 80 | 66 | 23.8 |
| No data | 35 | 12.6 |
| Antimitochondrial antibodies | ||
| Negative | 212 | 76.3 |
| Positive | 34 | 12.2 |
| No data | 32 | 11.5 |
| Serum IgG levels | ||
| Greater than 1.1 times the upper limit of normal | 140 | 50.4 |
| Greater than the upper limit of normal | 21 | 7.5 |
| Normal | 48 | 17.3 |
| No data | 69 | 24.8 |
| Histological feature – (n=227)a | ||
| Compatible with autoimmune hepatitis | 68 | 30.0 |
| Typical of autoimmune hepatitis | 158 | 69.6 |
| Not compatible | 1 | 0.4 |
| Simplified score for diagnosis of AIH | ||
| Probable (≥6 points) | 81 | 29.1 |
| Definite (≥7 points) | 139 | 50.0 |
| <6 points | 58 | 20.9 |
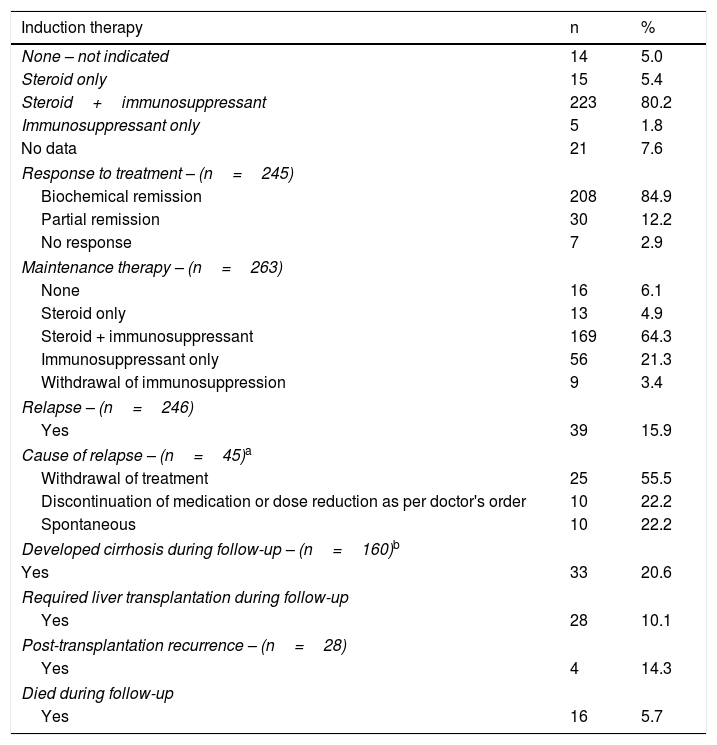
Treatment, response to treatment and evolution over time.
| Induction therapy | n | % |
|---|---|---|
| None – not indicated | 14 | 5.0 |
| Steroid only | 15 | 5.4 |
| Steroid+immunosuppressant | 223 | 80.2 |
| Immunosuppressant only | 5 | 1.8 |
| No data | 21 | 7.6 |
| Response to treatment – (n=245) | ||
| Biochemical remission | 208 | 84.9 |
| Partial remission | 30 | 12.2 |
| No response | 7 | 2.9 |
| Maintenance therapy – (n=263) | ||
| None | 16 | 6.1 |
| Steroid only | 13 | 4.9 |
| Steroid + immunosuppressant | 169 | 64.3 |
| Immunosuppressant only | 56 | 21.3 |
| Withdrawal of immunosuppression | 9 | 3.4 |
| Relapse – (n=246) | ||
| Yes | 39 | 15.9 |
| Cause of relapse – (n=45)a | ||
| Withdrawal of treatment | 25 | 55.5 |
| Discontinuation of medication or dose reduction as per doctor's order | 10 | 22.2 |
| Spontaneous | 10 | 22.2 |
| Developed cirrhosis during follow-up – (n=160)b | ||
| Yes | 33 | 20.6 |
| Required liver transplantation during follow-up | ||
| Yes | 28 | 10.1 |
| Post-transplantation recurrence – (n=28) | ||
| Yes | 4 | 14.3 |
| Died during follow-up | ||
| Yes | 16 | 5.7 |
Prednisolone and azathioprine were the drugs most commonly used for induction and maintenance therapy. For induction therapy, patients were administered prednisolone 0.5–1mg/kg/day and azathioprine 1mg/kg/day, with a gradual tapering of the prednisolone dose over the following three months and subsequent increase of the azathioprine dose up to 2mg/kg/day, according to tolerance and response to treatment.
Patients with AIH-PBC also received ursodeoxycholic acid at a dose of 15mg/kg/day.
The median time to remission was 13.4 weeks (IQR 7.2–34.4 weeks).
Steroids were discontinued in 56 (21.3%) of the 263 patients receiving maintenance therapy, and steroids plus immunosuppressant therapy were discontinued in 9 (3.4%) of the patients. Drug treatment was discontinued completely in 11 (84.6%) of the 13 cases of drug-induced AIH.
Rescue therapy was required in 20 (7.2%) patients, firstly for not achieving biochemical remission and, secondly, for the development of adverse effects, especially to azathioprine. The drugs used were mycophenolate mofetil, ciclosporin and tacrolimus in 80%, 15% and 5%, respectively. Of these patients, 11 (55%) were cirrhotic at the time of diagnosis, 60% had biochemical remission, 25% had partial remission and 15% did not respond to treatment.
A total of 78 (28%) patients had treatment-associated adverse effects, with the most common adverse effects being osteoporosis, infectious complications and Cushing's syndrome. Of those patients who received azathioprine, 2.4% had abnormal haematological parameters, 1.2% gastric intolerance and 0.4% pneumonitis.
On evaluating the group of patients who developed cirrhosis during follow-up and comparing them with those who did not develop cirrhosis, we found a higher proportion of men (21.2 vs. 7.8%, p=0.027), a higher frequency of AIH-PSC overlap syndrome (6.0 vs. 0%, p=0.006), lower biochemical remission rate (57.6 vs. 89%, p=<0.001) and a higher frequency of no response to treatment (12.1 vs. 1.6%, p=0.004).
Twenty-eight (10.1%) patients received transplants; the main indication was cirrhosis of the liver and complications due to portal hypertension and development of hepatocellular carcinoma. The eight patients with acute liver failure met criteria for poor prognosis. Five (62.5%) received a liver transplant, and the remaining three did not receive a transplant due to having contraindications for transplant (two patients had severe intracranial hypertension and one had haemorrhagic pancreatitis). In post-transplantation patients, the rate of AIH recurrence was 14.3%.
During follow-up, 16 (5.7%) patients died (4.8% of the women and 14.8% of the men). Of these, four (25%) had acute liver failure (the three with contraindications for transplant and one who received a transplant but died one year later due to septic shock).
The main cause of death was multiple organ failure secondary to liver failure and related infectious complications.
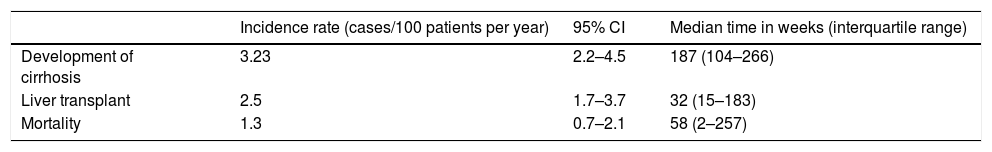
The incidence rates for liver cirrhosis, required liver transplantation and mortality are shown in Table 5.
Incidence of cirrhosis, liver transplantation and mortality in patients with AIH.
| Incidence rate (cases/100 patients per year) | 95% CI | Median time in weeks (interquartile range) | |
|---|---|---|---|
| Development of cirrhosis | 3.23 | 2.2–4.5 | 187 (104–266) |
| Liver transplant | 2.5 | 1.7–3.7 | 32 (15–183) |
| Mortality | 1.3 | 0.7–2.1 | 58 (2–257) |
This study includes the largest cohort of patients with AIH published in Colombia and Latin America. According to worldwide publications,9,19–22 its prevalence in women over the age of 40 is corroborated in the Colombian population. Also, although more cases are reported among white and Caucasian populations,9,23 the findings of this study show that AIH also affects patients of other races, especially mixed race, which is predominant in Latin America. The variability in incidence of AIH among the different races is possibly explained by the different polymorphisms in the human leucocyte antigen (HLA) system, as demonstrated by Duarte-Rey et al.,13 where HLA DQ2 and DR52 are risk factors for AIH among the Latin American population and HLA DR3 and DQ2 are risk factors on other continents, especially Europe and North America. Likewise, HLA could also explain the different clinical presentation and clinical course of AIH according to gender. Al-Chalabi et al.19 describe different HLA haplotypes expressed more frequently in men who developed AIH at an earlier age with more relapses. This study found a more aggressive clinical course of AIH in Colombian men, with a higher frequency of cirrhosis, lower biochemical remission rates, higher frequency of required liver transplantation and death.
The presentation of AIH is variable, with acute hepatitis being the most common,19,22,24,25 unlike overlap syndromes such as AIH-PBC where non-specific symptoms, such as pruritus, are more prevalent (58% in this study). In 15% of the patients, the only manifestation was abnormal liver function tests (elevated transaminases in AIH and elevated alkaline phosphatase in overlap syndromes); therefore, AIH should always be considered as a differential diagnosis in patients with abnormal liver function tests. These findings are also described by other authors.19,21,22 37.8% of the patients were cirrhotic in the baseline biopsy, but only half of these had clinical signs of liver disease, which confirms the poor diagnostic accuracy of physical examination for the diagnosis of cirrhosis26 and the risk of complications that these patients face when cirrhosis is not clinically detected.
Liver biopsy is a valuable tool for the diagnosis of AIH27; however, the patients’ clinical condition is sometimes not compatible with liver biopsy and, therefore, the percentage of patients undergoing a liver biopsy in the published AIH studies varies (usually less than 85%).19,22,23,28 Therefore, this study is representative for the description of AIH in Latin America, since histological confirmation was obtained in 81% of patients, of which 99% had features typical of or compatible with AIH.
The simplified score for the diagnosis of AIH has >80% sensitivity and >90% specificity with a cut-off of ≥6 points15; therefore, diagnosing patients with a score of <6 points is a challenge. In 21% of the patients, the simplified score was <6 points, which was mainly explained by: no available serum IgG levels, no liver biopsy in 28 (48%) of these patients and hepatitis B virus infection in one patient. Although the lack of a liver biopsy could be limiting to rule out other aetiologies (e.g. non-alcoholic steatohepatitis), biochemical response to treatment helped corroborate the diagnosis in these patients, as described in the treatment guidelines.27
There was a higher frequency of negative AMA (19%) in patients with AIH-PBC compared with that described in other studies; however, all studies met the Paris criteria (alkaline phosphatase ≥5 times the upper limit of normal and histological features of ductal lesions or destructive cholangitis),16 which have shown good accuracy for AIH-PBC overlap syndrome diagnosis.29
The combination of prednisolone and azathioprine was the most common induction therapy regimen and is the treatment of choice according to different guidelines.27,30,31 Deferred initiation of azathioprine (2 weeks after starting prednisolone) is recommended to avoid adverse effects related to azathioprine.27 Simultaneous initiation of these 2 drugs during induction therapy was not associated with a higher frequency of adverse effects, and the remission rate achieved was comparable with that described worldwide using this same regimen (84.9%).28,32
Interestingly, 13 patients had drug-induced AIH and the main drug involved was nitrofurantoin. This association was clearly established by Björnsson et al. in a Mayo Clinic study28 in which such patients had a less aggressive clinical course with higher biochemical remission rates, allowing withdrawal of immunosuppression in a greater percentage of patients. In this study, immunosuppression could be withdrawn in 85% of drug-induced cases, unlike the non-drug-induced group in which it was possible to withdraw glucocorticoids in only 21.3% of cases, with total withdrawal of treatment in only 3%.
15% of patients had a relapse, and the main cause was non-adherence to treatment (55%). A multidisciplinary approach in Latin American countries could reduce this rate.
Recurrence of post-transplantation AIH has been reported in 12–46% of patients33; in this study, post-transplantation recurrence is reported in four (14.3%) patients. In three of the four patients, graft function improved with modified immunosuppression and one patient required liver re-transplantation due to graft cirrhosis. The same patient experienced recurrence again 6 years after re-transplantation with progression to graft cirrhosis.
As mentioned by Czaja,34 it is not clear why AIH relapses, although several possible reasons have been suggested, such as: early withdrawal of glucocorticoids, donor–recipient HLA incompatibility, different immunosuppression regimens35 and, more recently, disease activity shown histologically and in liver function tests before transplantation.36
Prognosis based on mortality rates, development of cirrhosis, acute liver failure and transplant-free survival is variable, and is influenced by the severity of the initial clinical presentation, early recognition of the disease, coexistence of other conditions affecting the liver, establishment of timely and appropriate treatment, adherence to treatment by patients and the degree of response to treatment.27
Progression-to-cirrhosis, liver transplantation and mortality rates were similar to those reported worldwide.24
The study had several limitations. The first was a risk of information bias due to being a retrospective study with data collected from medical records. However, the quality of the data collected was guaranteed as 10% of the medical records were double-checked, and hepatologists from the hospital worked together with highly homogeneous work ethics. Second, it was a single-centre study; however, the centre involved is a widely known liver disease and liver transplantation centre throughout Colombia.
One of its strengths is the number of patients involved, since it is the AIH study with the most patients reported in Colombia and in Latin America. Also, more than 80% of the patients had a liver biopsy, allowing highly reliable diagnosis of AIH. Finally, another strength was the follow-up time (median of 41 months and range up to 241 months) since it revealed the behaviour and progression of AIH among the Colombian population.
ConclusionAIH is not a rare disease in our geographical region in Colombia. It is more prevalent in women, but has an unfavourable impact in men. It has a wide variability in clinical presentation, and a large number of patients are cirrhotic at the time of diagnosis. Timely diagnosis is required to start adequate treatment, and prevent disease progression and complications. Response to treatment and complications developed among the Colombian population are similar to those described by other centres around the world.
Conflict of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Díaz-Ramírez GS, Marín-Zuluaga JI, Donado-Gómez JH, Muñoz-Maya O, Santos-Sánchez Ó, Restrepo-Gutiérrez JC. Caracterización de los pacientes con hepatitis autoinmune de un hospital universitario, Medellín-Colombia: estudio de cohorte. Gastroenterol Hepatol. 2018;41:87–96.