Constipation is a very common disorder that adversely affects well-being and quality of life. Evidence-based clinical practice guidelines are an essential element for proper patient management and safe, effective treatment.
The aim of these guidelines is to provide health care professionals who care for patients with chronic constipation with a tool that allows them to make the best decisions about the prevention, diagnosis and treatment of constipation.
The methodology used to draw up these guidelines is described in the Part 1. In this article we will discuss the recommendations for the diagnostic and therapeutic management of constipation.
El estreñimiento es un trastorno muy frecuente que afecta negativamente el bienestar y la calidad de vida de las personas. Para el correcto manejo y tratamiento eficiente y seguro de los pacientes, las guías de práctica clínica basadas en la evidencia son un elemento esencial.
El objetivo de esta guía es proporcionar a los profesionales sanitarios encargados de la asistencia a pacientes con estreñimiento crónico una herramienta que les permita tomar las mejores decisiones sobre la prevención, el diagnóstico y el tratamiento del estreñimiento.
La metodología utilizada en la elaboración de esta guía de práctica clínica se describe en la Parte 1. En este artículo expondremos las recomendaciones en el manejo, tanto diagnóstico como terapéutico del estreñimiento.
High-fibre diet
Water intake
Physical exercise
Pharmacological measuresBulk-forming laxatives
Osmotic laxatives
- -
Polyethylene glycol is recommended as a treatment option in people with chronic constipation (moderate evidence, strong recommendation in favour).
- -
Lactulose is recommended as a treatment option in people with chronic constipation (low evidence, strong recommendation in favour).
- -
The use of polyethylene glycol is preferred over lactulose (moderate evidence, weak recommendation in favour).
Emollient and lubricant laxatives
- -
The use of stimulant laxatives is recommended as a rescue treatment option (moderate evidence, strong recommendation in favour).
- -
The use of stimulant laxatives is suggested as a treatment option in people with chronic constipation who have not responded to bulk-forming and/or osmotic laxatives (moderate evidence, weak recommendation in favour).
Prokinetic laxatives
Secretory laxatives
Other treatmentsBiofeedback (pelvic floor rehabilitation)
Sacral neuromodulation
Surgery (colectomy)
- -
Colectomy should only be considered as a treatment option in people with severe refractory chronic constipation that does not respond to other treatments and in whom extensive involvement of intestinal motility has been ruled out using special tests, including gastrointestinal manometry (low evidence, weak recommendation in favour).
Constipation is a very common disorder that adversely affects people's well-being and quality of life. Evidence-based clinical practice guidelines are an essential element for proper patient management and safe, effective treatment.
The aim of these guidelines is to provide health care professionals who care for patients with chronic constipation with a tool that allows them to make the best decisions about the prevention, diagnosis and treatment of constipation.
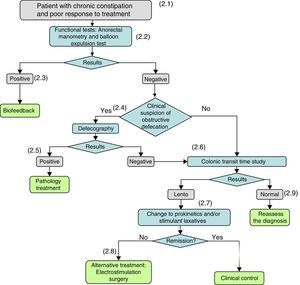
The methodology used to draw up these guidelines is described in Part 1. In this article, we will discuss the recommendations for the diagnostic and therapeutic management of constipation (Figs. 1 and 2, Table 1).
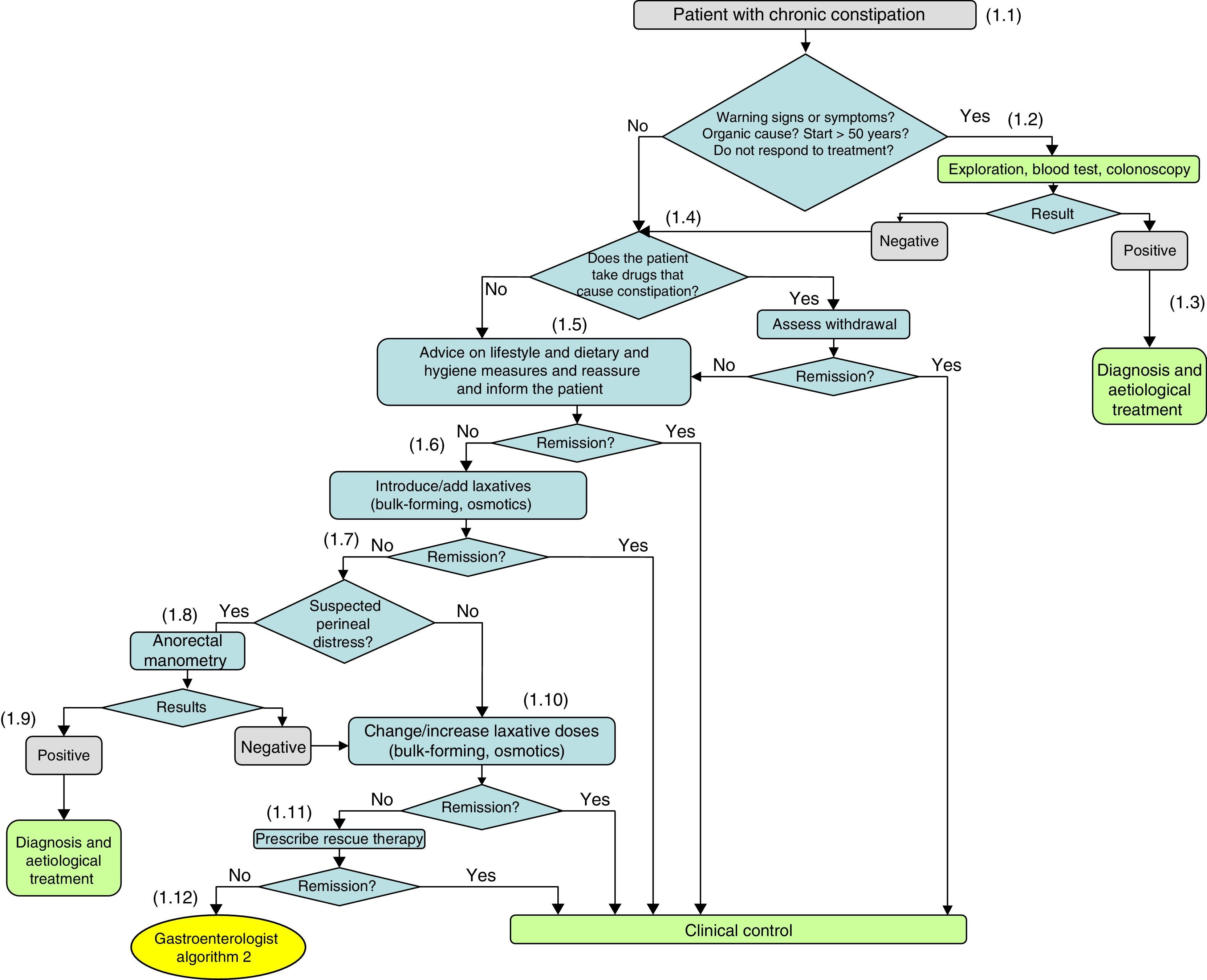
Diagnosis and initial treatment of chronic constipation.
(1.1) In most cases there is no underlying organic cause, with it being classified as chronic idiopathic constipation after a full medical history and negative physical examination. Patients who meet the Rome III criteria for functional constipation do not generally require diagnostic testing.
(1.2) When there are warning signs in the medical history, a possible organic cause is suspected or constipation begins in a person over 50 years of age, diagnostic tests should be performed: blood test and colonoscopy. These diagnostic studies are also indicated in patients with constipation who do not respond to treatment.
(1.3) Secondary chronic constipation may be associated with a wide range of diseases. According to the results, the physician will proceed with the diagnosis and aetiological treatment of constipation. The possible organic causes are diverse and can be consulted in Table 4 of the full guidelines.
(1.4) If the result is negative and no aetiological diagnosis has been identified, it is necessary to consider whether the patient takes drugs that cause constipation and if they can be withdrawn. The list of drugs that can cause constipation is extensive and can be found in Table 5 of the full guidelines.
(1.5) If possible organic causes have been ruled out and drugs that cause constipation and cannot be withdrawn (or their doses reduced), the initial recommendations are non-pharmacological measures. Assess the advice on lifestyle and dietary and hygiene measures: fibre-rich diet, physical exercise and good hydration.
(1.6) Medical treatment with laxatives usually begins after the failure of diet and lifestyle modifications. Currently, there is a wide variety of therapeutic options. First-line treatment involves introducing bulk-forming and/or osmotic laxatives. If the patient is already taking either of these laxatives, changing or adding laxatives from these groups should be considered.
(1.7) If there is no response to bulk-forming and/or osmotic laxatives, emphasis should be placed on whether the constipation is causing perianal distress that favours the onset or chronification of anorectal disease. The onset of haemorrhoidal inflammation or bleeding during defecation effort, the recurrence of anal fissure during defecation effort and involuntary urine output may be warning signs of perineal distress.
(1.8) If perineal distress is suspected, the possibility of anorectal manometry should be considered to assess the presence of a defecation disorder.
(1.9) If the result of the manometry is positive, the physician will proceed with the diagnosis and aetiological treatment.
(1.10) If the condition itself was not suggestive of perineal distress or when a negative anorectal manometry has been performed, treatments with bulk-forming and/or osmotic laxatives should be reviewed and the procedure should be changed or the dose increased.
(1.11) If there is no response to this modification of bulk-forming and/or osmotic laxatives, the possibility of adding rescue therapy with stimulant laxatives, cleansing enemas or suppositories (second-line treatment) should be considered.
(1.12) If they do not respond to this modification, or there is a need for progressive increases in the laxative dose, continuous liquid stools to be able to evacuate, abdominal pain, or worsening of the pelvic floor condition, the patient will be referred to the gastroenterologist.
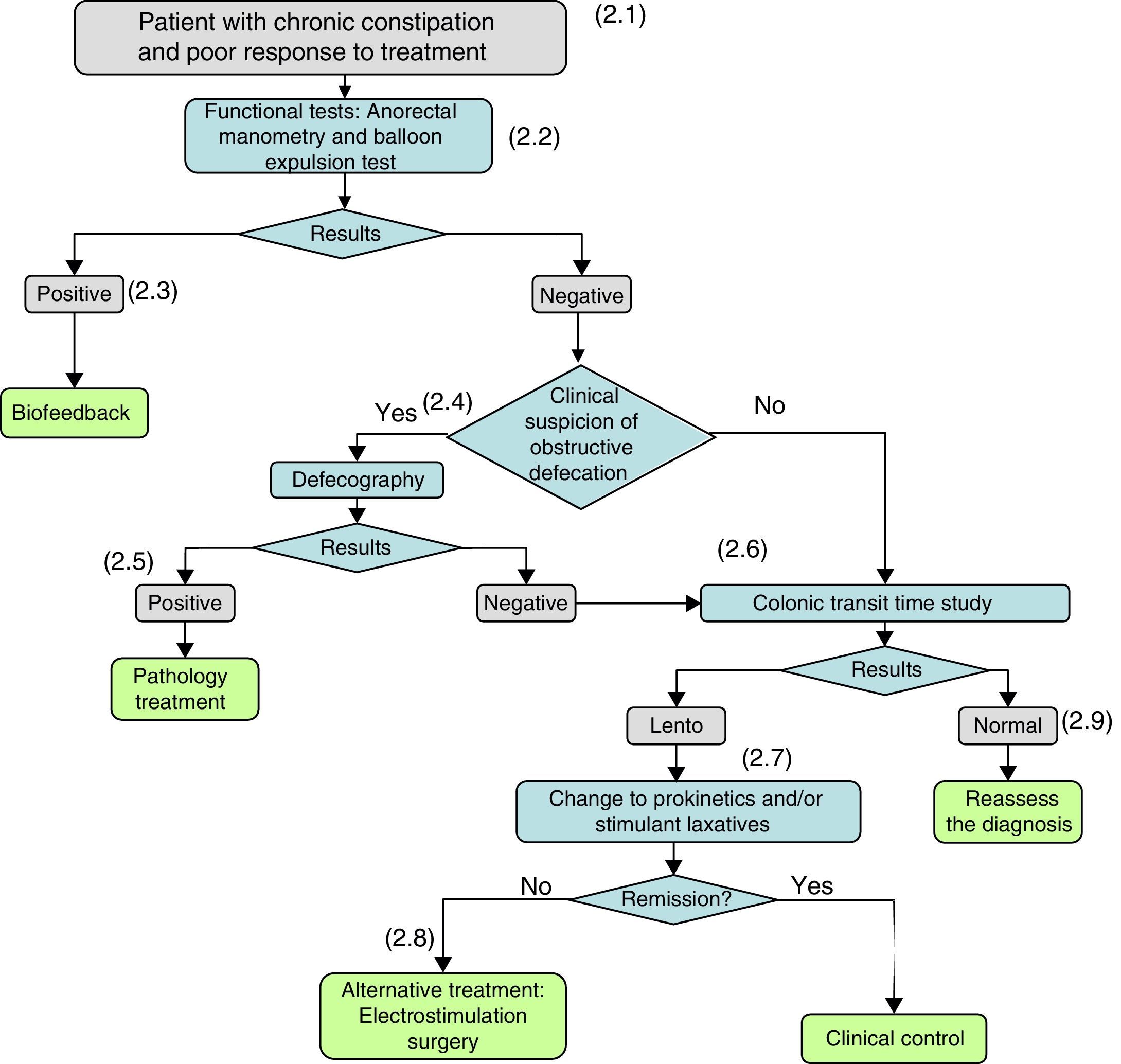
Diagnosis and treatment of the patient who does not respond to bulk-forming or osmotic laxatives.
(2.1) When organic disease and/or the intake of drugs that cause constipation have been ruled out and there is no response to non-pharmacological measures (lifestyle and dietary and hygiene measures) or treatments with bulk-forming and/or osmotic laxatives or rescue therapies do not give satisfactory results, it must be suspected that the constipation is caused by a defecation disorder and the patient should be referred to a gastroenterologist for investigation.
(2.2) When a defecation disorder is suspected, a functional study with anorectal manometry (on those patients on whom it has not been performed previously) and a balloon expulsion test should be performed.
(2.3) If the manometry and/or balloon expulsion test demonstrate a defecation disorder, it will be treated by anorectal biofeedback.
(2.4) If the results of the manometry and/or balloon expulsion test are negative, or the patient persists with constipation after his/her dyssynergic defecation has been corrected, the clinical suspicion of persistent obstructive defecation (rectocele, enterocele) shall be considered again and, if positive, video defecography will be performed.
(2.5) If the defecography identifies the cause, aetiological treatment will be administered.
(2.6) If treatment-refractory constipation persists, and/or if the tests performed to date remain negative, the colonic transit time (CTT) will be determined. A slow CTT may be a consequence of an underlying condition (metabolic, endocrine, systemic disease) or associated with neuropathic or myopathic alterations of the colonic wall.
(2.7) If the CTT is slow, treatment with laxatives should be optimized by adding prokinetics and/or stimulant or secretory laxatives. Cleansing enemas or suppositories may be used as rescue medication.
(2.8) If there is no response to prokinetic laxatives and stimulant and secretory laxatives, other treatments will be considered: electrostimulation, surgery.
(2.9) If the CTT is negative, the patient will be reassessed and the possibility of alternative diagnoses will be reconsidered.
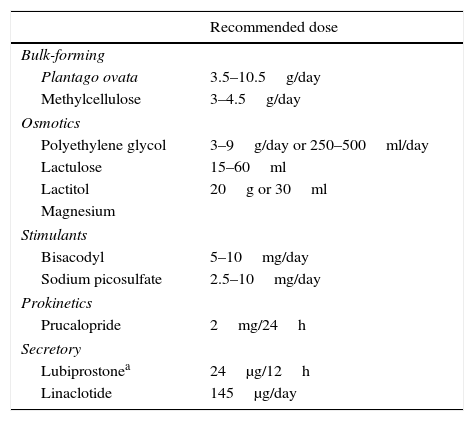
Classification of different types of laxatives.
| Recommended dose | |
|---|---|
| Bulk-forming | |
| Plantago ovata | 3.5–10.5g/day |
| Methylcellulose | 3–4.5g/day |
| Osmotics | |
| Polyethylene glycol | 3–9g/day or 250–500ml/day |
| Lactulose | 15–60ml |
| Lactitol | 20g or 30ml |
| Magnesium | |
| Stimulants | |
| Bisacodyl | 5–10mg/day |
| Sodium picosulfate | 2.5–10mg/day |
| Prokinetics | |
| Prucalopride | 2mg/24h |
| Secretory | |
| Lubiprostonea | 24μg/12h |
| Linaclotide | 145μg/day |
In the vast majority of patients, the diagnosis of chronic idiopathic constipation is based on the description of symptoms and/or signs recorded in the medical history, as well as the findings of the physical examination. Three aspects must be taken into account: (1) compliance with the diagnostic criteria for chronic constipation, (2) determination of the causes of constipation and (3) detection of warning signs (see Part 1). Physical examination includes an abdominal examination, a visual inspection of the perianal and rectal regions, and a rectal examination to assess the tone of the anal sphincter, both at rest and during a bowel movement, whether the rectal bladder is occupied and the presence of masses, prolapse, haemorrhoids, fissures, rectocele, etc.
Additional testsBlood testsA systematic review concluded that there is no evidence that a general blood test is effective as a routine test in patients with constipation1 and that the cost-utility of these tests is likely to be very low.2 Blood tests will be indicated in patients who present with warning signs and in those whose medical history leads us to suspect a possible condition causing the constipation.3 Some guidelines4,5 recommend a CBC as part of the diagnostic study, determining serum iron, ionogram, calcium and phosphorus, glucose and thyroid hormones, in addition to any specific tests deemed necessary in accordance with the clinical suspicion of the disease causing constipation.
Imaging testsColonoscopy. Several studies have shown that the diagnostic performance of colonoscopies in patients presenting constipation as the sole indication is similar to that of the asymptomatic population.6–9
The different clinical practice guidelines indicate performing a colonoscopy on all patients who present with constipation and warning signs and/or symptoms4,5,10 (see Part 1). Some of these guidelines also indicate colonoscopy in constipation patients who do not respond to treatment and in those over 50 years of age or younger, in case of a family history of colorectal cancer, who were not assessed by means of colonoscopy after the onset of constipation.4
Barium enema. There is no evidence (2 studies and 122 patients) that the barium enema is effective as a routine test in all patients with constipation.1 The barium enema identifies the presence of megacolon and megarectum, and in children with Hirschsprung's disease shows the extension of the aganglionic segment. However, since the barium enema does not allow for direct visualization or biopsies, its use should be reserved for cases in which colonoscopy cannot be performed or generates diagnostic doubts.
Functional testsAnorectal manometry. Manometry consists of measuring the pressures in the anal canal and rectum at rest, and during bowel movements. It also allows for the assessment of both intrinsic and extrinsic anorectal innervation,11 as well as the volume and sensitivity of the rectum.
It is considered to be indicated in patients who do not respond to treatment with lifestyle changes, bulk-forming agents or osmotic and prokinetic laxatives. Anorectal manometry is useful in patients with functional constipation who also have faecal incontinence, anal fissure, haemorrhoidal disease and rectocele, etc., since it would prove useful in diagnosing dyssynergia.12
Manometry allows for the identification of different types of pelvic floor dyssynergia (see Part 1),13 the presence of rectal sensory alterations and neurological pathologies, such as Hirschsprung's disease, or the involvement of innervation secondary to neurological diseases. Likewise, manometry allows us to identify patients who could benefit from constipation treatment by means of anorectal biofeedback.14
Balloon expulsion test. The balloon expulsion test is easy to perform and widely available. It involves inserting a balloon into the rectum which is inflated with air or fluid, and the patient is asked to evacuate it. Ideally, the balloon should be inflated with a volume that produces a desire to defecate, and the patient will expel it in a bathroom in private. The methodology for this test is not standardized.15 The preferred approach is to quantify the time needed to expel a rectal balloon in seated position; depending on the technique, the recommended normal values vary.4
The expulsion test is indicated in patients who do not respond to treatment with lifestyle changes, bulk-forming agents or osmotic and prokinetic laxatives, in whom we may suspect that constipation is caused by a defecation disorder.11
An uncontrolled study (106 patients with constipation and 24 patients with defecation disorders) assessed the usefulness of the balloon expulsion test in chronic constipation to identify defecation dyssynergia, showing a sensitivity and specificity of 87.5% and 89%, and positive and negative predictive values of 64% and 97%, respectively.16 The authors conclude that the expulsion test allows for a very simple identification of patients who have a defecation disorder and who could benefit from a comprehensive anorectal function manometry study to assess the possibility of treatment with biofeedback.16
Determination of colonic transit time. Colonic transit time is defined as the time taken by the faeces to pass through the colon. It is determined through the ingestion of small radiopaque markers made from a light plastic substance (generally polyethylene) and the subsequent performance of a series of simple abdominal radiology tests to quantify the colonic transit time. Several different protocols exist; the most widely used in Spain consists of taking X-rays on days 4 and 7, sometimes even on the tenth day.17
Colonic transit time is indicated in patients who do not respond to treatment with lifestyle changes, bulk-forming agents or osmotic and prokinetic laxatives.11
The test allows for the identification of patients who are constipated due to a slow colonic transit. Prior to considering that the patient may have constipation due to a slow colonic transit (colonic inertia), defecation disorders (dyssynergia), which may also cause a slow-down of the colonic transit, must be ruled out, and if the diagnosis is confirmed it must be treated.18
Other tests used to evaluate colonic transit times are the wireless capsule (smart pill) and scintigraphy studies. The results of a systematic review (9 studies) show that, in terms of sensitivity, specificity and ROC curves, the capsule presents similar results to colonic transit time measured with radiopaque markers and scintigraphy studies. The capsule is well tolerated, has good compliance and avoids the risks of radiation exposure. However, it is also more expensive and in the majority of patients it is not clear as to whether it provides any added value.15
Defecography. Two techniques are used to identify anatomical alterations: (a) fluoroscopy (radioscopy), which is a very accessible, fast technique that allows the patient to be comfortably seated, but irradiates, and (b) magnetic resonance imaging (MRI), which has the advantage of observing all of the organs of the pelvic floor during non-irradiated defecation, but which is expensive and less accessible than fluoroscopy.
The main indication of these techniques is in patients with a defecation disorder where rectocele, enterocele or intussusception is suspected as the underlying cause. They may also be used to diagnose pelvic floor dyssynergia, although adjuvant tests are considered, which should not be used alone in the diagnosis of constipation.18 Moreover, the techniques allow us to assess the correct rectification of the anorectal angle as well as sphincter relaxation during bowel movements.19
TreatmentTreatment not only seeks to increase the number of stools, but also aims for complete evacuation, performed effortlessly without anorectal obstruction problems, striving for the disappearance of pain and associated abdominal distension. The main objective is to improve the consistency and volume of the faeces. The choice of treatment and the order of its introduction will depend on the aetiology of the chronic constipation.
Non-pharmacological measuresMost patients seeking help for chronic constipation have potentially modifiable risk factors related to their lifestyle (low fibre diet, poor hydration and/or sedentary lifestyle) (see Part 1).
Health educationPromoting lifestyles and reducing laxative dependence. Adopting a regular schedule for defecation, taking advantage of moments of greater colonic motility.15 Having enough time to defecate and using a suitable position (squatting), training the pelvic muscles and anal sphincters to achieve the expulsion manoeuvre without undue stress.15
High-fibre diet. Several studies have shown that fibre improves functional constipation.20–22 In a cross-over randomized clinical trial (RCT) (40 patients), it was shown that 50g of prunes consumed twice a day for six weeks increases the number of weekly defecations, the score on the Bristol stool chart and improves the symptom score associated with constipation and defecation effort.23 The results of this study prove that consuming prunes is safe, palatable and, compared to psyllium, more effective in the treatment of mild to moderate constipation (see “Bulk-forming laxatives” section).
In some patients, especially those with slow transit constipation, a high-fibre diet causes abdominal swelling and flatulence.24 These effects can be modulated by starting with small amounts and slowly increasing the fibre intake according to tolerance and efficacy.25,26
Many of the available studies present methodological biases in relation to the design, size, subjects, constipation severity, interventions and efficacy variables evaluated. New, well-designed studies are needed to evaluate the effect of a high-fibre diet on treating constipation, taking into account adverse events.
Water intake. A clinical trial (117 patients) compared daily fibre intake (25g) and water (1.5–2L) with fibre intake alone.27 In the fibre and water group, a higher number of weekly stools and a greater decrease in the number of laxative doses used per week were observed.
There are no studies assessing the undesirable effects of fluid intake in patients with constipation. An increase in fluid is more important in patients who are dehydrated or who drink less fluids than in patients who are correctly hydrated.
Physical exercise. Several studies show that physical exercise (walking, running, resistance exercises) reduces the intestinal transit time,28–31 while physical inactivity increases intestinal transit time.32 Other studies do not show the effects of physical exercise on intestinal transit time.33,34
An RCT on middle-aged people with a sedentary lifestyle and chronic constipation (43 people)30 did not show changes in the number of weekly stools but a statistically significant decrease in the percentage of hard, incomplete or forced stools was observed.
The available studies are scant and methodologically biased. Well-designed RCTs are required in order to assess the benefits and risks of physical activity. However, physical activity does have a positive effect on health.
Pharmacological treatmentMedical treatment with laxatives usually begins after the failure of diet and lifestyle modifications.35 Currently, there is a wide variety of therapeutic options (Annex 3). Most laxatives produce side effects, especially cramping abdominal pain, watery diarrhoea and distension. Frequently, the different laxatives produce accommodation and tachyphylaxis and it is necessary to increase the dose progressively and/or to establish periods of rotation. When choosing the initial treatment, consideration should be given to efficacy, safety, convenience, cost and clinical response.15
According to data from a European survey conducted in 2009, the use of laxatives among patients with chronic constipation is very high, at 68%.5 The type of laxative used varies widely between the participating countries. Regardless of the treatment used, the degree of laxative satisfaction among the European population is low, averaging at 28%. According to the survey data, in Spain satisfaction stands at 19%, the lowest percentage of all countries participating in the survey.
Bulk-forming laxativesFibre can be taken as a supplement. It comes in the form of non-absorbable polysaccharides that, once adequately hydrated, increase the faecal volume and favour peristalsis. These laxatives improve intestinal transit and stool expulsion, as observed with dietary fibre. In this category we have psyllium (or ispaghula, which is derived from the husks of plantago ovata) and various semi-synthetic agents such as methylcellulose, calcium polycarbophil and dextran (see Annex 3).
A systematic review identified nine RCTs conducted with psyllium (3 of them randomized and compared to placebo) and concluded that there is moderate evidence favouring its use.36 A systematic review evaluating the effect of soluble fibre on patients with constipation (3 RCTs with psyllium and 1 RCT with inulin and maltodextrim37) shows that, compared to placebo, soluble fibre improves overall symptoms, defecation effort, pain during defecation and stool consistency, with an increase in the mean number of stools per week and a reduction in the number of days between stools. The different RCTs do not report adverse effects. However, the Summary of Product Characteristics for psyllium indicates abdominal cramps, constipation, diarrhoea, and oesophageal or intestinal obstruction.
There is no RCT assessing methylcellulose versus placebo in patients with chronic constipation. There is only one RCT comparing the use of methylcellulose versus psyllium.38 This study compared the effect of daily single doses of 1, 2 and 4g of methylcellulose with 3.4g of psyllium in 59 patients with chronic constipation. A significant increase was observed with respect to the pre-treatment figures in faecal frequency and in the solid and liquid contents of the daily stools for all doses of methylcellulose and psyllium tested. There is no RCT assessing calcium polycarbophil versus placebo in patients with chronic constipation. A systematic review36 identified one RCT comparing calcium polycarbophil versus psyllium and concluded that the evidence for the use of calcium polycarbophil is insufficient.
The most recent systematic review available39 does not identify new RCTs in relation to fibre supplements and chronic idiopathic constipation. This review also concludes that the quality of the evidence available on the efficacy of treating chronic idiopathic constipation with bulk-forming laxatives is moderate for natural agents and low for semi-synthetics, and the response to these laxatives does not appear to be superior to dietetic and hygiene measures. Moreover, bulk-forming laxatives can cause abdominal discomfort and other adverse effects, such as fluid overload. Proper use involves increasing the dose gradually.
Osmotic laxativesOsmotic laxatives contain non-absorbable ions or molecules, thus creating an osmotic gradient that promotes luminal fluid secretion in the intestine. The hydration of the faeces and the increase in volume improve peristalsis. Included in this group of laxatives are polyethylene glycol (macrogol), lactulose, sorbitol, glycerol and magnesium salts.
Two systematic reviews compared osmotic laxatives versus placebo. The first review (6 RCTs) includes 5 RCTs assessing polyethylene glycol and one RCT assessing lactulose.40 Overall, 149 of the 396 patients (37.6%) assigned to the osmotic laxative group did not respond to therapy, compared to 193 of the 280 patients (68.9%) assigned to the placebo group (RR=0.50; CI 95%: 0.39–0.63) with a number needed to treat (NNT) of 3 (CI 95%: 2–4).40 No significant heterogeneity was found between the studies (I2=36%; p=0.18). Three of the RCTs reported on symptoms, two of them on defecation effort (118 patients) and three on faecal hardness (269 patients).40 The results indicate a significant reduction in the risk of these symptoms (RR=0.37; CI 95%: 0.19–0.71, and RR=0.26; CI 95%: 0.16–0.44, respectively).40 These RCTs have also shown a beneficial effect on the number of weekly stools in the osmotic laxative group versus the placebo group (mean difference=2.51) (CI 95%: 1.30–3.71).40
The second most recent review available39 also identifies the five RCTs for polyethylene glycol, all of them with low risk of bias, and adds a second RCT for lactulose, placing the two RCTs at high risk of bias.
Only two of the RCTs assessed the adverse effects (abdominal pain, headache), and no statistically significant differences were found between the treatment and placebo groups.39,40 Excessive use of osmotic laxatives may lead to electrolyte and volume overloads in patients with renal and cardiac dysfunction.41
A Cochrane systematic review (10 RCTs and 868 patients) indicates that polyethylene glycol is better than lactulose in terms of weekly stool frequency, stool consistency, relief of abdominal pain and the need for additional products to combat constipation.42 The systematic review concludes that polyethylene glycol should be used in preference to lactulose in the treatment of chronic constipation.42
Other osmotic laxatives have not been adequately studied. Saline laxatives (magnesium salts) are often used in excess. Magnesium salts are useful when a quick stool is needed. Phosphate enemas are useful for bowel cleansing. Magnesium-containing laxatives should be avoided in patients with renal and hepatic impairment.
- -
Polyethylene glycol is recommended as a treatment option in people with chronic constipation (moderate evidence, strong recommendation in favour).
- -
Lactulose is recommended as a treatment option in people with chronic constipation (low evidence, strong recommendation in favour).
- -
The use of polyethylene glycol is preferred over lactulose (moderate evidence, weak recommendation in favour).
Paraffin oil and docusate sodium are marketed in Spain. In general, they are non-aggressive and well-tolerated substances that promote defecation by improving stool consistency through emulsifying intestinal water with oil fat. These laxatives can be used in a timely manner and should be administered with caution in patients at risk of aspiration, as lipoid pneumonia could occur. Paraffin oil is not commonly used in the adult population, and is most widely administered in children.43
Stimulant laxativesThese laxatives include sennosides, bisacodyl, horseradish and castor oil.39 Glycerin and docusate sodium are also considered to be stimulants. Bisacodyl and its derivative, sodium picosulfate, are stimulant laxatives derived from diphenylmethane. These laxatives are inactive glycosides that are not absorbed in the small intestine and are hydrolyzed in the colon by glucosidases, resulting in active molecules that increase propulsive waves and intestinal secretion.
A systematic review (2 RCTs) compared stimulant laxatives versus placebo.40 The review includes an RCT assessing bisacodyl44 and an RCT that evaluated the picosulfate for 4 weeks.45 Overall in these two RCTs, 202 of the 480 (42.1%) patients assigned to the active treatment did not respond to therapy, compared to 199 of the 255 (78%) patients assigned to the placebo (RR=0.54; CI 95%: 0.42–0.69). The NNT was 3 (CI 95%: 2–3.5).40 These RCTs have also shown a beneficial effect on the number of weekly stools in the osmotic laxative group versus the placebo group (mean difference=2.50) (CI 95%: 0.93–4.07).40
Only the bisacodyl study included all adverse effects, and the RR of experiencing some risk was 1.94 (CI 95%: 1.52–2.47). In the RCTs evaluating stimulant laxatives, diarrhoea occurs more frequently than in those that assessed osmotic laxatives (RR=13.75; CI 95%: 2.82–67.14). During the preparation of the colonoscopy, side effects such as pain and abdominal distension, thirst, vomiting and vertigo have been described.46 Moreover, cases of severe rhabdomyolysis and hyponatremia have been reported.46 However, these problems have not been associated with the use of laxatives in therapeutic doses.
The most recent systematic review available39 does not identify new RCTs in relation to stimulant laxatives. The authors of this review point out that other stimulant laxatives, although commonly used by patients, have not been adequately studied and cannot be recommended at this time.39
- -
The use of stimulant laxatives is recommended as a rescue treatment option (moderate evidence, strong recommendation in favour).
- -
The use of stimulant laxatives is suggested as a treatment option in people with chronic constipation who have not responded to bulk-forming and/or osmotic laxatives (moderate evidence, weak recommendation in favour).
Prucalopride is a prokinetic agent with selective activity by the serotonin 5-HT4 receptor, with a stimulant effect on intestinal transit.
A systematic review (7 RCTs, 2639 patients) compared prucalopride versus placebo40 in patients resistant to other laxatives or who were dissatisfied with treatment. Only three RCTs have low a risk of bias.40 Overall, 1288 of the 1796 patients (71.7%) assigned to the prucalopride group did not respond to therapy, compared to 731 of the 843 patients (86.7%) assigned to the placebo group (RR=0.82; CI 95%: 0.76–0.88) and a NNT of 6 (CI 95%: 5–9). No significant heterogeneity was found between the studies (I2=60%; p=0.02). The funnel plot showed statistically significant asymmetry (Egger test, p=0.04; Begg test, p=0.07), suggesting a risk of publication.40
In sensitivity analyses, heterogeneity ceased to be significant when the only studies analyzed were those with a low risk of bias, those that defined the response to treatment as at least three or more complete and spontaneous bowel movements per week and those RCTs that used the modified ROME II criteria.40 Analyses based on the prucalopride dose showed similar efficacy for daily doses of 2 and 4mg.
Six RCTs included all adverse effects, and the RR of experiencing some risk was 1.14 (CI 95%: 1.05–1.24), with a NNP of 10 (CI 95%: 6–29).40 The adverse effects of headache, nausea and diarrhoea were statistically significant for the prucalopride treatment group. No serious adverse events have been found, although sufficient information on its efficacy and long-term safety is lacking.40 Although, at the present time, specific studies with prucalopride in RCTs and follow-up studies have not shown cardiovascular alterations, it is important to take them into consideration in clinical practice due to its mechanism of action.
The most recent systematic review available39 identifies a new RCT assessing prucalopride, as well as an RCT that evaluated velusetrag. In the same vein, this review concludes that 5-HT4 agonists prucalopride and velusetrag are effective in chronic idiopathic constipation, with many more studies supporting prucalopride.
The European Medicines Agency (EMA) and the National Institute for Health and Care Excellence (NICE)47 suggest that prucalopride may only be a treatment option in women (>18 years) with chronic constipation who have previously been treated with dietary and hygiene measures and at least two different types of oral laxatives (at the maximum possible dose for at least 6 months) without achieving adequate relief, prior to considering the use of more invasive treatments. NICE recommends re-evaluating patients after 4 weeks of treatment with prucalopride and discontinuing it in case of ineffectiveness.
Secretory laxativesLubiprostone. Lubiprostone is a compound belonging to the family of prostones, which activates type-2 chloride channels (CIC-2) without affecting the cystic fibrosis transmembrane conductance regulator (CFTR), thus increasing the secretion of intestinal chloride, without altering the serum electrolyte balance.
A systematic review (3 RCTs, 610 patients) compared lubiprostone versus placebo.40 Of the three RCTs, two have a risk of bias.40 Overall, 151 of the 335 patients (45.1%) assigned to the lubiprostone group did not respond to therapy, compared to 184 of the 275 patients (66.9%) assigned to the placebo group (RR=0.67; CI 95%: 0.56–0.80), with a NNT of 4 (CI 95%: 3–7).40 When considering the twice daily 24μg dose, the results of the three RCTs were similar (RR=0.64; CI 95%: 0.55–0.76). There are not enough studies to show the funnel plot.
The three RCTs assessed the adverse effects, and the systematic review shows that lubiprostone has significantly higher adverse effects than the placebo (RR=1.79; CI 95%: 1.21–2.65), with a NNR of 4 (CI 95%: 3–6).40 Both diarrhoea (RR=4.46; CI 95%: 1.28–15.48) and nausea (RR=7.27; CI 95%: 3.76–14.06) were significantly more common in the group receiving lubiprostone.40
The most recent systematic review available39 updates the data from this previous review.40 It identifies two new RCTs and includes one of them in their meta-analysis. Of the five RCTs, two have a low risk of bias.
A prospective, multicenter, long-term, unblinded study that included 248 patients with chronic constipation compared lubiprostone (48μg/day) to placebo for 48 weeks. The results show that lubiprostone was more effective than the placebo in reducing the severity of constipation, abdominal distension and discomfort. To minimize the impact of side effects from withdrawing the treatment, the study allowed the lubiprostone dose to be reduced.48
Linaclotide. Linaclotide is a peptide agonist of the guanylate-cyclase C (GC-C) receptor present on the luminal surface of the enterocyte. GC-C activation leads to the activation of CFTR which results in increased chloride and bicarbonate secretion into the intestinal lumen, thus increasing secretion volume and accelerating intestinal transit.
Linaclotide has recently been approved by the US Food and Drug Administration (FDA) for the treatment of chronic idiopathic constipation at doses of 145μg/day.15 In Europe, linaclotide is not indicated in the treatment of constipation; it is only approved for IBS-C.
A systematic review (3 RCTs, 1582 patients) compared linaclotide versus placebo.40 The three RCTs have a low risk of bias.40 The dichotomous response variable in these studies required the presence of ≥3 CSBMs per week and an increase of at least one CSBM per week compared to the pretreatment period. Overall, 860 of the 1089 (79%) patients assigned to the linaclotide treatment did not respond to therapy, compared to 468 of the 493 (94.9%) patients assigned to the placebo (RR=0.84; CI 95%: 0.80–0.87), with a NNT of 6 (CI 95%: 5–8).40 There are not enough studies to show the funnel plot.40 The subanalysis based on doses of 133 and 266μg/day shows a similar efficacy. The quantitative variables—pain or discomfort, abdominal distension, faecal consistency assessed according to the Bristol stool chart and defecation effort—improved significantly with regard to the placebo in the three RCTs. The mean score for overall treatment satisfaction, and the proportion of patients who were quite or very satisfied with the treatment, were significantly higher with linaclotide.
The number of adverse effects was similar for the active treatment groups (33.6%) and placebo (31.9%). The occurrence of diarrhoea was more common with linaclotide (RR=3.08; CI 95%: 1.27–7.48).40
The most recent systematic review available39 does not identify new RCTs in relation to linaclotide.
Other treatmentsCleansing enemasWhen patients do not respond to oral laxatives and have not defecated for several days, treatments with cleansing enemas (1500ml of water in 25min), commercial enemas (140–250ml of saline or mineral enemas) and/or glycerol or bisacodyl suppositories may be considered.
Biofeedback (pelvic floor rehabilitation)Biofeedback is a learning or rehabilitation treatment technique to gain control of the physiological response system through training. It is used to correct inadequate contractions of the pelvic floor and external anal sphincter muscles during defecation, in patients with chronic constipation caused by dyssynergia or poor coordination during defecation (see Part 1). Two systematic reviews have included eight uncontrolled or poorly controlled clinical trials on patients with dyssynergia.49,50 Biofeedback with electromyogram (EMG) is compared with other biofeedback techniques (pressure balloon, verbal feedback) in four of the studies included, and the four most recent ones compare biofeedback with laxatives or other treatments (diazepam, botox, placebo). These studies were performed in highly specialized hospitals. The results of the meta-analysis show that biofeedback is more effective than treatment with laxatives or other treatments (OR=3.657; CI 95%: 2.127–6.290; p<0.001) and that the efficacy of biofeedback with EMG is just as effective as other biofeedback techniques (OR=1.436; CI 95%: 0.692–3.089; p=0.319).50 Both reviews agree on the poor quality of the studies.49,50
The most recent systematic review available39 identifies a new RCT. For the authors of the review, the RCTs are either unclear or present a high risk of bias due to their inability to blind participants because of the nature of the interventions or a lack of information on the methods used to generate the randomization programme or hide the assignment. Adverse effects were not reported in any of the RCTs.
A recent study shows that the response rates achieved outside of an RCT are only slightly lower.51 These results are explained by the fact that these techniques should always be performed by an experienced and specialized professional.
Sacral neuromodulationThis technique is based on the physiological principle that the presence of bioelectrical activity in a neural pathway can modulate a pre-existing activity in another pathway through synaptic interactions. It is performed by the percutaneous placement of one or more electrodes on the sacral roots (generally S2–S3) through the sacral holes and implantation of a stimulation device under the skin on the buttocks.52
Neuromodulation may be effective in patients with very individualized indications, but although several studies have been conducted,53–59 the available evidence is limited. Prospective studies are required to evaluate their exact function and safety in the management of patients with constipation,60 which also allow for the discovery of the adverse events associated with this technique.61
A recent systematic review shows that the efficacy of neurostimulation goes beyond a local effect on the anorectal area, and that it acts both through the central and autonomic nervous systems, increasing the appearance of large amplitude waves in the colon and visceral sensitivity.62 The authors of this review conclude that, despite available studies, it is unknown which patients could benefit from sacral nerve stimulation.62
SurgerySurgery is a last option treatment that should be reserved for very exceptional cases of patients with chronic constipation and a slow colonic transit time without defecation dyssynergia, who are refractory to all treatments and who, because of this health problem, endure a significant effect on their quality of life.
The technique used is subtotal colectomy, which has shown good short- and long-term results on constipation, abdominal pain and quality of life in different series over the years.60,63,64 Subtotal colectomy is not without complications. Apart from the complications related to the surgery itself (infections, adhesions, etc.), some patients may develop diarrhoea, and nocturnal leakage is not uncommon.64
It is important to note that these patients may have generalized neuropathy, not limited to the colon, so before indicating surgery it is necessary to conduct a study about the possible alteration of motility to other levels of the digestive tract, such as the small intestine, to prevent severe intestinal occlusion after surgery.
Other surgical techniques have been published, such as ileostomy with antegrade enemas, which would have the advantage of being less aggressive and reversible, but there are no controlled studies that support its efficacy.60
- -
Colectomy should only be considered as a treatment option in people with severe refractory chronic constipation that does not respond to other treatments and in whom extensive involvement of intestinal motility has been ruled out using special tests, including gastrointestinal manometry (low evidence, weak recommendation in favour).
These clinical practice guidelines have received external funding from Laboratorios Shire. The sponsors have not influenced any stage of their development.
Conflicts of interestDr Serra is a consultant for Norgine, and takes part in conferences and “advisory boards” for Allergan, Almirall and Reckit Benkiser.
The following people have acted as external reviewers:
Carmen Vela Vallespín. General practitioner. Riu Nord i Sud Primary Care Centre. Catalan Health Institute Santa Coloma, Barcelona.
Iván Villar Balboa. General practitioner. Florida Sud Primary Care Centre. Catalan Institute of Health, L’Hospitalet de Llobregat, Barcelona
Mercè Barenys de Lacha. Gastroenterologist, Viladecans Hospital. Professor of Gastroenterology. University of Barcelona. IDIBELL Scientific Committee.
Francisco Javier Amador Romero. General practitioner. Los Ángeles Health Centre. Madrid.
Miguel Mínguez Pérez. Gastroenterologist. Valencia Clinical University Hospital.
Please cite this article as: Serra J, Mascort-Roca J, Marzo-Castillejo M, Delgado Aros S, Ferrándiz Santos J, Diaz Rubio ER, et al. Guía de práctica clínica sobre el manejo del estreñimiento crónico en el paciente adulto. Parte 2: Diagnóstico y tratamiento. Gastroenterol Hepatol. 2017;40:303–316.
These clinical practice guidelines have the support of the Asociación Española de Gastroenterología (AEG) [Spanish Gastroenterology Association] and the Sociedad Española de Medicina Familiar y Comunitaria (semFYC) [Spanish Society of Family and Community Medicine].