Non-alcoholic fatty liver disease (NAFLD) is the main cause of liver diseases in Spain and the incidence is raising due to the outbreak of type 2 diabetes and obesity. This CPG suggests recommendation about diagnosis, mainly non-invasive biomarkers, and clinical management of this entity. Life-style modifications to achieve weight loss is the main target in the management of NAFLD. Low caloric Mediterranean diet and 200minutes/week of aerobic exercise are encouraged. In non-responders patients with morbid obesity, bariatric surgery or metabolic endoscopy could be indicated. Pharmacological therapy is indicated in patients with NASH and fibrosis and non-responders to weight loss measures. NAFLD could influence liver transplantation, as a growing indication, the impact of steatosis in the graft viability, de novo NAFLD rate after OLT and a raised cardiovascular risk that modify the management of this entity. The current CPG was the result of the First Spanish NAFLD meeting in Seville.
La enfermedad hepática grasa no alcohólica (EHGNA) es la causa más frecuente de hepatopatía crónica en nuestro medio y se prevé un incremento de su incidencia en los próximos años asociada al incremento de la obesidad y el síndrome metabólico. Esta guía de práctica clínica propone recomendaciones sobre el diagnóstico y en especial marcadores no invasivos, así como en el manejo y seguimiento de esta enfermedad. La intervención dietética basada en la dieta mediterránea y el cambio del estilo de vida constituyen el pilar del tratamiento de la EHGNA, pero aún falta por elucidar si la composición de la dieta puede influir en la mejoría de la enfermedad más allá de la pérdida de peso. El tratamiento con fármacos debe restringirse a los pacientes con esteatohepatitis y fibrosis significativa que no consiguen resolución de la esteatohepatitis después de una intervención con dieta y ejercicio físico durante un año. Nuevos fármacos aún en fases iniciales de desarrollo han demostrado ser superiores a placebo. Por último, el impacto de la EHGNA en la indicación de trasplante hepático, la viabilidad del injerto y la recidiva de EHGNA de novo tras el trasplante, así como el incrementado riesgo cardiovascular determinan todo el proceso peritrasplante hepático. Esta guía de práctica clínica se ha elaborado tras la I Reunión de Consenso sobre EHGNA con un panel de experto nacionales e internaciones en Sevilla y tienen como objetivo proponer recomendaciones basadas en la evidencia científica disponible para el manejo de estos pacientes.
Non-alcoholic fatty liver disease (NAFLD) has become the most common cause of chronic liver disease in children and adults. It is associated with the worldwide epidemic of obesity and metabolic syndrome (MetSyn),1–3 and is a growing cause of advanced liver disease in Europe.4,5 In the coming years, NAFLD and alcoholic liver disease will become the most prevalent causes of chronic liver disease in Spain. The incidence of new cases of liver disease caused by hepatitis C and hepatitis B will decrease drastically as a result of the efficacy of the new direct-acting antiviral agents6,7 and the universal vaccination against hepatitis B. Factors associated with the increased risk of liver disease progression include alcohol consumption and genetic and environmental factors, such as age, gender, dietary habits and nutritional status.8–10 Dietary intervention and changes in lifestyle are the most important elements in the treatment of NAFLD.11 Applying measures to maintain these lifestyle changes over time is a huge challenge.12
These clinical practice guidelines were prepared after the 1st NAFLD Consensus Meeting held in May 2016 in Seville supported by the Asociación Española para el Estudio del Hígado (AEEH) [Spanish Association for the Study of the Liver] with the collaboration of a panel of national and international experts. The aim was to propose recommendations for the management of NAFLD and answer key questions for clinical practice in gastroenterology, hepatology, internal medicine and primary care.
The levels of evidence in these guidelines have been established as A (high), B (moderate) and C (low), and the grades of recommendation as strong (1) and weak (2).
The initial recommendations after the Consensus Meeting on the level of scientific evidence were later assessed by a panel of experts to ensure they were as objective as possible.
Non-alcoholic fatty liver diseasePrevalence and incidenceWhat is the prevalence of NAFLD in the general population?The prevalence of NAFLD varies according to the population (age, gender, race, comorbidities), geographical area and diagnostic methods. Most data on NAFLD prevalence come from studies that used non-invasive techniques, particularly ultrasonography or magnetic resonance imaging (MRI).2,3
In studies assessing NAFLD by ultrasound only, the prevalence ranges from 17% to 46%. In Spain, the prevalence estimated by population studies is 25.8%, and the degree of significant fibrosis, estimated by the sequential combination of transient elastography and liver biopsy, is 2.8% in the general population.13,14 The results of a very recent meta-analysis, which analysed 86 studies from 22 countries with a sample of more than 8 million individuals, show a worldwide prevalence of 25%.15,16
Recommendations- •
The worldwide prevalence of NAFLD is high. It is estimated at 25% in the adult population in Europe, and is currently the most common chronic liver disease in Western countries (A1).
The prevalence of NAFLD increases in parallel with the prevalence of MetSyn and its components, particularly obesity and diabetes mellitus (DM).17,18
All the population studies and a number of different meta-analyses coincide in showing that the prevalence of NAFLD and non-alcoholic steatohepatitis (NASH) is significantly higher in patients with MetSyn or any of its components, particularly obesity and DM, but also hypertension. In patients with obesity undergoing bariatric surgery, the prevalence of NAFLD has been estimated at 91% and the prevalence of NASH at 37%. Among patients with DM, the prevalence of NAFLD is estimated at 40–70%, and the prevalence of NASH, around 22%.19 As the prevalence of type 2 DM (DM2) in Spain is 13.8%,20 the estimated rate of NASH in this population would be 5.5–9.7%.
RecommendationsThe prevalence of NAFLD and NASH is significantly higher in patients with MetSyn and particularly in those with obesity and/or DM2, compared to the general population (A1).
Has the prevalence of NAFLD been increasing over the last twenty or thirty years?Information on the incidence of NAFLD is limited. As the prevalence of obesity has increased 2- to 3-fold over the last 30 years in Spain,21 it is assumed that the incidence of NAFLD has increased proportionally. The results of a recent study indicate that in the United States, patients with NASH included on waiting lists increased by 170% between 2004 and 2013, which indirectly suggests a marked increase in the incidence of NASH.3,19 In 2013 NASH was established as the second most common reason for inclusion of patients on the waiting list for liver transplantation (LT).16–22
RecommendationsThe prevalence of NAFLD has been increasing all over the world over the last twenty or thirty years in parallel with the progressive increase in obesity and diabetes in the general population, although specific data regarding the incidence in Spain remain limited (B1).
Is there evidence to support screening for NAFLD in the general population?Given the high prevalence of NAFLD and its potential progression to NASH and LC, it seems reasonable to propose screening strategies.1 Recent studies have investigated the utility of screening programmes for the detection of significant liver fibrosis in the general population and in the population with risk factors.
In summary, these studies show a significant liver fibrosis prevalence of 6% in the general population and 18% in the population with risk factors, with NAFLD being the most frequent cause of fibrosis in these cases.14,23 Although these data suggest that screening should be performed in the population with risk factors, cost-effectiveness studies are necessary on these programmes to confirm the best strategy to follow.24
Recommendations- •
The at-risk population (patients with obesity, DM or MetSyn) should be screened for NAFLD, with study of liver enzymes and ultrasound (B1).
- •
However, considering the large population the screening would affect, we do not have data on whether or not this strategy is sustainable and cost-effective (A2).
NAFLD includes a broad spectrum of lesions ranging from simple hepatic steatosis, which is usually remains stable, to NASH and fibrosis.25 With NASH and fibrosis, there is a risk of progression to cirrhosis and hepatocellular carcinoma (HCC).26 In 20% of cases, mainly in patients with NASH, fibrosis develops rapidly.24 The mortality rate from liver disease in patients with NASH is 18%, compared to 3% in patients without NASH, after 18.5 years of follow-up.27 At 10–15 years post-diagnosis, 5–10% of patients with NASH will develop decompensated LC while under follow-up, and 1–2%, HCC.28
Recommendations- •
The natural history of NAFLD without NASH is similar to the general population, while NAFLD can progress to advanced fibrosis, LC and HCC, with an estimated mortality rate of 10–12% at 10–15 years post-diagnosis (A2).
- •
The comorbidity patients with NASH may have determines their survival. The common denominator is insulin resistance (IR) and metabolic syndrome (MetSyn). The main cause of death in patients with NASH is coronary artery disease, followed by extrahepatic malignancies and cancer associated with cirrhosis (A2).
Population studies and retrospective analyses confirm that NASH is a very common cause of cryptogenic cirrhosis and may be the indication in 5–10% of LT.29 The patient profile is of females aged 50–60, with a history of obesity or DM2 with slightly elevated transaminases. Clinical presentation in 50% of cases is with complications of portal hypertension (PH). The prevalence of HCC is around 7% and seems to be higher than that described in cirrhosis related to haemochromatosis or primary biliary cholangitis, but lower than that found in viral or alcoholic cirrhosis.
Recommendations- •
NASH is a common cause of cryptogenic cirrhosis and clinical presentation is typically as a complication of PH in 50% of cases (A2).
NAFLD in children is a growing problem which is reaching epidemic proportions in some developed countries. It is the most common cause of liver disease in childhood, with a prevalence of 7.6–9.6%, increasing to 29.8–34.7% in cases of overweight and obesity.30 Although it covers the same spectrum of conditions (NAFLD, NASH, LC, and HCC), less is understood about the natural history of NAFLD in children than in adults.31 In 66 children with NAFLD followed up for 20 years, transplant-free survival was significantly worse than expected, with two dying and another two requiring a second transplant for decompensated LC. At diagnosis, 29% of the children had complete MetSyn, and 83% had at least one parameter of MetSyn: obesity, hypertension, dyslipidaemia and/or hyperglycaemia.32
Recommendations- •
NAFLD in children has the same clinical spectrum as in adults (A1).
- •
NASH and its progression to cirrhosis and liver cancer are well established and MetSyn is considered as a factor of progression (A1).
In the paediatric and adolescent population, NAFLD/NASH develops in parallel with the increase in IR which occurs in the prepuberty period and during puberty, so presentation in children under 10 years of age is very rare.33,34 Abdominal obesity and MetSyn increase the risk of steatosis and NASH in children who are overweight or obese.35,36 There is no established screening tool for the paediatric population. Despite the limitations of the measurement of transaminases (underdiagnosis of steatosis) and radiological techniques (only define degree of steatosis, not inflammatory activity or fibrosis), in view of their availability, cost and safety, determination of transaminases and abdominal ultrasound are useful tools for screening for NAFLD.37
RecommendationsChildren over the age of 10 should be screened if they are:
- a)
Obese (body mass index [BMI] percentile≥95).
- b)
Overweight (BMI percentile≥85) with associated risk factors (abdominal obesity, MetSyn, IR or family history) (B1).
- •
The diagnostic tools recommended in clinical practice are transaminase activity and abdominal ultrasound (B1).
- •
Liver biopsy is the gold standard for diagnosis and prognosis of NAFLD. Paediatric NASH (called type 2 NASH, or non-alcoholic steatohepatitis) has differential characteristics from adult NASH (type 1 NASH) with a predominance of portal involvement (inflammation and fibrosis) and absence of ballooning, although in a majority of affected children the two types overlap, so specific histological severity models are needed in children.38,39 Liver biopsies are invasive and costly. Non-invasive methods to determine NASH and fibrosis used in adults are not sufficiently validated in the paediatric population, although the determination of cytokeratin-18 (CK-18) has potential to be used as a surrogate marker for NASH in children.40
Recommendations- •
We only recommend liver biopsy in cases of suspected advanced disease in the paediatric population, as a last step in the differential diagnosis, or in the context of research projects or clinical trials (B1).
- •
Non-invasive methods to assess prognosis are not sufficiently validated in children to be used in clinical practice (B2).
Despite the limited evidence available, treatment should be based on health and dietary measures (low-calorie diet and planned exercise).31,41 The effect of these measures, however, is not very long-lasting because of poor adherence. Bariatric surgery has been shown to lead to histological improvement in adults, but there is very little evidence available for this in the paediatric population. Bariatric surgery is recommended in patients with morbid obesity who have a BMI≥40 associated with severe comorbidities, including NASH.42 The pharmacological measures studied in adults, such as insulin sensitisers (metformin), antioxidants (vitamin E), polyunsaturated fatty acids, obeticholic acid and probiotics, have not yielded results or have not been studied in the paediatric population.
Recommendations- •
Treatment of NAFLD/NASH in children should be based on dietary recommendations and physical exercise; pharmacological measures are not recommended because of the lack of available evidence (B1).
- •
Bariatric surgery should be performed to treat morbid obesity in obese children and adolescents with fatty liver disease (B1).
No, the accumulation of fat in the form of triglycerides does not lead to cell death in hepatocytes or the progression of liver disease per se.43 There are experimental studies in vitro and in animal models in which the accumulation of triglycerides caused by overexpression of diacylglycerol acyltransferase type 2 (DGAT2), or the administration of monounsaturated fatty acids such as oleic acid, increased the presence of triglycerides in animal liver, but they did not increase IR or liver damage.44
Recommendations- •
The accumulation of fat in the form of triglycerides does not lead to cell death in hepatocytes or the progression of liver disease (A2).
Yes, studies carried out on primary human hepatic cells, established liver tumour lines and experimental models suggest that the presence of saturated free fatty acids, such as caprylic (8:0) and palmitic (16:0) acids, generate reticulum stress and hepatocellular injury.45,46
The administration of monounsaturated fatty acids such as oleic acid (18:1), although it increases the content of triglycerides, reduces cell stress and hepatocellular death.
Recommendations- •
The presence of saturated free fatty acids (e.g. caprylic and palmitic) generates reticulum stress and hepatocellular injury. The administration of monounsaturated fatty acids such as oleic acid reduces cell stress and hepatocellular death (A1).
Yes. IR, defined as an abnormal biological response of its effector tissues (liver, muscle and adipose tissue) and reflected by plasma levels above 20μU/ml of insulin, is related to cardiovascular events and progression of the NAFLD. Numerous experimental studies show that IR is associated with reticulum stress and oxidative stress, which induce serine and threonine phosphorylation of the insulin receptor, which in turn reduces the effectiveness of the insulin signal cascade, as has been shown in patients with morbid obesity and NAFLD.47
Recommendations- •
IR (plasma levels above 20μU/ml of insulin) has been linked to cardiovascular events and liver disease progression due to fat deposition (B2).
It is estimated that only 2% of liver disease resulting from fatty deposits is drug-induced. The drugs involved are classified into those which can directly cause steatosis and phospholipidosis (amiodarone, tamoxifen, perhexiline maleate and diethylaminoethoxyhexestrol), those which can induce metabolic changes and precipitate or aggravate fatty liver disease (tamoxifen, raloxifene, corticosteroids, valproic acid, carbamazepine, methotrexate, irinotecan, oxaliplatin, HAART) and those which can sporadically cause steatohepatitis (carbamazepine).48,49
The histological pattern is variable. Macrovesicular steatosis can be caused by amiodarone, cytostatics, tamoxifen, corticosteroids and methotrexate.
Microvesicular steatosis is related to the inhibition of beta-oxidation of fatty acids and has been associated with valproic acid, tetracycline, acetylsalicylic acid, ibuprofen, zidovudine, corticosteroids and vitamin A. Development of NASH has been associated with amiodarone, irinotecan, tamoxifen and methotrexate.44 Lastly, phospholipidosis can be induced by amiodarone, fluoxetine, perhexiline maleate and diethylaminoethoxyhexestrol50 (Table 1). In the differential diagnosis of NAFLD, other causes of liver disease need to be ruled out, as well as other causes of hepatic steatosis such as Wilson's disease, and other secondary causes of hepatic steatosis, such as lysosomal acid lipase deficiency, whose differential diagnosis with “idiopathic” NAFLD is complex.51
List of steatogenic drugs.
| Microvesicular steatosis | Macrovesicular steatosis | Steatohepatitis | Phospholipidosis |
|---|---|---|---|
| Acetylsalicylic acid | Amiodarone | Amiodarone | Amiodarone |
| Valproic acid | Carbamazepine | Irinotecan | Fluoxetine |
| Amiodarone | Glucocorticoids | Methotrexate | Perhexeline maleate |
| Antiretrovirals (zidovudine and didanosine) | 5-FU, irinotecan, cisplatin | Tamoxifen | Diethylaminoethoxyhexestrol |
| NSAIDs (ibuprofen and naproxen) | Asparaginase | ||
| Cocaine | Methotrexate | ||
| Tetracycline | Tamoxifen | ||
| Vitamin A |
- •
Drug-induced fatty liver disease is rare and can be the result of direct toxic action, the main pathogenic mechanism of which is fatty acid mitochondrial fatty acid beta-oxidation dysfunction, or have an indirect cause (C2).
- •
The histological patterns of drug-induced fat deposition are macrovesicular steatosis, microvesicular steatosis, steatohepatitis and phospholipidosis (A2).
Yes. Agents that increase the beneficial capacity of reticulum stress mediated by the Unfolded Protein Response (UPR), such as 4-phenylbutyric acid and ursodeoxycholic acid conjugated with taurine, reduce the damage caused by saturated fatty acids.52 The administration of α-tocopherol has not shown a conclusive effect in patients with NASH. However, recent studies suggest that a diet enriched with γ-tocopherol, alone or in combination with α-tocopherol, reduces the inflammatory response and biomarkers of oxidative stress in patients with MetSyn, resulting in improvement in patients from a cardiovascular point of view.53
Recommendations- •
Administration of agents that increase the beneficial capacity of reticulum stress, such as 4-phenylbutyric and ursodeoxycholic acids conjugated with taurine, reduces the damage caused by saturated fatty acids (B2).
Regardless of whether an individual is obese, a high-fat diet modifies the gut microbiota,54 the most consistent alteration being the increase in phylum Firmicutes and a decrease in Bacteroidetes. This intestinal dysbiosis contributes to liver disease through metabolic and inflammatory mechanisms causing the deposition of fat. The metabolic mechanisms include changes in the dietary energy balance, decrease in choline synthesis and production of toxic metabolites. Liver inflammation is largely due to the increase in endotoxin and other bacterial products in the portal blood when intestinal inflammation resulting from the dysbiosis increases permeability.55,56
Recommendations- •
Intestinal dysbiosis resulting from a high-fat diet contributes to metabolic or non-alcoholic fatty liver disease by causing changes in the dietary energy balance, decreasing choline synthesis and promoting endotoxaemia as a consequence of increased intestinal permeability (B1).
The data are inconsistent and come from retrospective studies designed with other objectives, and are therefore affected by confounding factors. The data published both in the study by Zoller and Tilg57 and in the EASL-EASD-EASO guidelines58 are an example of the heterogeneity in the population and in the tools used to establish the diagnosis of HCC (computed tomography [CT], alpha-foetoprotein, ultrasound or review of medical records).58,59 The data on the prevalence of HCC in patients with NASH come from retrospective cohorts and from a highly selected population in which the fibrosis stage is not taken into account.60–63 Despite these limitations, it has been found in recent years that approximately half of cases of HCC occur in pre-cirrhotic stages of NAFLD. In addition, HCC on top of NASH frequently presents as a large tumour with worse prognosis than HCC associated with liver disease from other causes. In a retrospective analysis of the Medicare database registry, 61% of patients with HCC associated with NAFLD died within a year, compared to 50% of those associated with viral hepatitis. However, this could be explained by the fact that the patients with NAFLD were older, had more comorbidities and were not participating in HCC screening programmes.64,65 This suggests a very interesting area for research in the next few years. One added problem is the difficulty of ultrasound examination in the context of obesity which can complicate the monitoring and follow-up of these patients.
In view of the huge size of the at-risk population that would be subject to screening, risk factors should be identified to enable recommendations for prioritising screening in these patients. Childhood obesity (from 7 to 13 years old) has been identified as a risk factor in a retrospective Danish cohort of 285,884 individuals.66
Obesity in young people (aged 20–40) has also been identified as a risk factor (OR=2.6).67 In a prospective study to analyse the risk of malignancies in obesity, BMI>35kg/m2 was associated with a RR=4.25.68
An increased risk of HCC has also been observed in explanted livers of obese patients with cryptogenic cirrhosis (OR=11.1).
DM has been independently associated with greater risk in both retrospective and prospective studies.69–71
Metformin was associated with a reduction in the risk of HCC in a recent meta-analysis.72
Recommendations- •
Patients with cirrhosis due to NASH have an estimated annual risk of developing HCC greater than 1.5% (A1).
- •
Patients with NASH without cirrhosis can develop HCC, but we do not know what the best strategy is for screening. However, we recommend screening in non-cirrhotic patients with fibrosis and obesity (BMI>35kg/m2) or DM (B2).
- •
We recommend optimising blood-glucose control in patients with DM2; the use of metformin, alone or combined with other oral antidiabetic agents, is a suitable option (B2).
The aim of screening for HCC is early diagnosis to help reduce mortality rates. When single nodules smaller than 3cm, ideally ≤2cm, are detected, radical treatments are possible. Screening is indicated in LT candidates with an annual risk of developing HCC>1.5%.73 The recommended tool for HCC screening is hepatobiliary ultrasound every 6 months. Alpha-foetoprotein levels and/or the use of CT or MRI instead of ultrasound are not recommended.74
Recommendations- •
CT and MRI involve an increased risk of irradiation and a high cost and are not therefore recommended as screening tests for hepatocellular carcinoma (B2).
- •
Patients with NASH and morbid obesity or severe cardiovascular complications may not be candidates for specific radical treatments (surgery, percutaneous treatment or LT). The severity of the comorbidity associated with NASH therefore needs to be considered when deciding whether or not the patient is a candidate for HCC screening (C1).
The diagnostic criteria for HCC are: (1) non-invasive diagnostic criteria (hyperintense liver injury in arterial phase and lavage of the lesion in venous phase) in patients with cirrhosis, regardless of the aetiology; and (2) invasive criteria, by means of a liver biopsy, in patients with cirrhosis who do not meet the typical non-invasive criteria for HCC or in non-cirrhotic patients.73,74 Focal hepatic steatosis can be interpreted as hepatic nodules and require differential diagnosis of HCC. There are no data in the literature to modify these criteria or the diagnostic algorithm in patients with NASH.
Recommendations- •
The diagnostic criteria for HCC should not be modified in patients with NAFLD (A1).
In non-cirrhotic patients, the first treatment option is surgery. In patients with cirrhosis, the treatment options will depend on the Barcelona Clinic Liver Cancer (BCLC) staging of the HCC. In patients with NASH there are no special recommendations, but a comprehensive assessment of cardiovascular risk should be performed if offering surgical treatment or sorafenib, as the comorbidities associated with the underlying disease make these patients more vulnerable to contraindications for this type of treatment. Similarly, in candidates for chemoembolisation, assessment of their vascular systems is essential, in order to avoid potential side effects related to the technique.74,75
Recommendations- •
Treatment options should not be modified in this population, but comorbidities should be taken into account when deciding which treatment is indicated (A2).
The risk of patients with NASH developing cancer other than HCC could be affected by factors related to the underlying disease (NASH) or unrelated factors, such as environmental or family factors, and adverse effects of the medication the patient receives.74 The risk analysis is therefore complex and published information on the increased risk of extrahepatic cancer in NASH is subject to debate.
Recommendations- •
The risk of patients with NASH developing cancer other than HCC is not well defined (C2).
One sole prospective study has evaluated the risk of idiosyncratic hepatotoxicity in patients with NASH (n=74) and hepatitis C (n=174), and six patients in the NASH group developed hepatotoxicity (incidence 2.4% and OR of 3.95).76 Nguyen et al.77 found that development of liver toxicity was more common in patients with NASH, with an OR of 7.43. However, the limited number of events and the absence of a control group limit their conclusions. Other drugs, such as statins, have shown no increased risk of hepatotoxicity in patients with hypertransaminasaemia.78
Recommendations- •
Having NASH may increase the risk of hepatotoxicity (B2).
- •
Having liver disease does not increase the risk of statin-induced hepatotoxicity (B1).
Biopsy is the only means of assessing liver disease in patients with steatohepatitis, as liver function tests do not provide an accurate picture of ballooning and/or hepatocellular necrosis or fibrosis stage.79–82 In patients with chronic liver disease of other aetiologies, metabolic risk factors such as IR and steatosis should be assessed by ultrasound. If IR and steatosis are detected, a liver biopsy should be performed to confirm concomitant NAFLD.33
The pathologist should tick off the following items when reporting the histological study:
- •
Suggest and provide guidance on the aetiology of the chronic liver disease.
- •
Confirm the clinical diagnosis and rule out other possible chronic liver disease.
- •
Determine the follow-up study.
- •
Assess the degree of inflammatory activity at the time of diagnosis.
- •
Determine the prognosis.
- •
Assess disease progression.
- •
Liver biopsy should be performed exclusively on selected patients using non-invasive techniques (analytical and imaging tests) (A1).
- •
Liver biopsy is not a screening method for NAFLD (A1).
Steatohepatitis should preferably be diagnosed histologically by pathologists who are experts in liver pathology or by general pathologists with basic training in hepatology.83 The main histological characteristic of liver disease due to fatty deposits is the accumulation of fat in hepatocytes, called steatosis. An essential criterion for the histological diagnosis of hepatic steatosis is for more than 5% of hepatocytes to be steatotic. The minimum criteria for the histological diagnosis of steatohepatitis include the presence of steatosis, hepatocellular damage (in the form of ballooning degeneration, apoptosis or necrosis) and lobular inflammatory infiltrate.84,85 Mild fibrosis is common in steatohepatitis, but it is not a prerequisite for histological diagnosis.
Recommendations- •
Steatohepatitis should be diagnosed following histological examination of a liver biopsy by pathologists experienced in liver pathology or by general pathologists with basic training in hepatology and according to established and internationally validated histological criteria (A1).
The degree of NASH histological activity is obtained by combining the steatosis, lobular inflammation and hepatocyte ballooning scores according to Kleiner et al.86
Steatosis is scored as 0 when it is less than 5% of the liver tissue; 1 if ≥5–33%; 2 if ≥34–66% and 3 if >66%. The grade of lobular inflammation is scored as 0 if there are no inflammatory foci; 1 if there are <2 foci; 2 if there are 2–4 foci, and 3 if there are >4 foci. Hepatocyte ballooning should be estimated as 0 (no ballooning), 1 (few ballooning cells) or 2 (prominent ballooning).
The stage of fibrosis is scored as 0 for no fibrosis; 1 if there is perisinusoidal or portal/periportal fibrosis; 2 if there is perisinusoidal and portal/periportal fibrosis; 3 in the case of bridging fibrosis; and 4 in the case of cirrhosis.87
Recommendations- •
Using an internationally accepted diagnostic protocol, such as Kleiner's “NAFLD activity score” or “Steatosis, Activity and Fibrosis Score” (SAF), avoids inter-observer variability and enables a better histological classification (A1).
The protocolised histological reading of liver biopsies from patients with clinical suspicion of NAFLD is essential for optimising the diagnosis and helps to reduce inter-observer variations.88 It is also a suitable method for studying series of patients and for assessing the effects of treatments in clinical trials which require periodic biopsies. However, use of a histological reading protocol such as the SAF (steatosis, inflammatory activity and fibrosis) score or the FLIP (fatty liver inhibition of progression) algorithm89 risks reducing our ability to integrate the microscopic data into the overall data interpretation for the diagnosis. Its purpose is to complement, not replace, histopathological diagnosis.
Recommendations- •
For the histological reading of liver biopsies from patients with clinical suspicion of NASH, we recommend systematic use of the new SAF score classification and staging system, developed in conjunction with the FLIP diagnostic algorithm (B1).
There are several validated panels of biomarkers capable of detecting steatosis with high specificity, although they are unable to determine the severity. They include the independently validated33 Hepatic Steatosis Index90 and Fatty Liver Index (FLI)91 (Table 2). These biomarker panels may be useful in screening for NAFLD in the general population and in at-risk patients, such as people with type 2 diabetes or obesity.92
Non-invasive methods for diagnosis of NAFLD.
| Variables | Formula | |
|---|---|---|
| Hepatic Steatosis Index (HSI) | AST ALT BMI Gender DM2 | 8×ALT/AST ratio)+BMI (+2, if female; +2, if DM) |
| Fatty Liver Index (FLI) | Triglycerides BMI GGT Waist (cm) | FLI=(e0.953*loge(triglycerides)+0.139*BMI+0.718*loge(GGT)+0.053*waist circumference−15.745)/(1+e0.953*loge(triglycerides)+0.139*BMI+0.718*loge(GGT)+0.053*waist circumference - 15.745)×100 |
- •
Biomarker panels are useful in the screening of NAFLD in at-risk populations such as people with type 2 diabetes. However, their use should be individualised and widespread use as screening tools cannot be recommended at present (A2).
To date, no biochemical marker has been able to displace liver biopsy as the gold standard for the diagnosis of steatohepatitis. Histological analysis remains necessary to confirm diagnosis and assess the severity of both alcohol-induced disease and NASH. However, biochemical markers of cell death, such as the M65 fragments of CK-18,93 have shown modest results in terms of diagnostic accuracy for steatohepatitis. Methods based on lipidomic or proteomic analysis,94 although promising, still require external validation studies to support them as standardised diagnostic tools.
Recommendations- •
The histological analysis of the liver biopsy continues to be necessary for confirming the diagnosis and assessing the severity of the steatohepatitis (A1).
The NAFLD fibrosis score and the FIB-4 are validated tests in NAFLD and their use can avoid a liver biopsy.95 In addition, they provide prognostic value, as they are able to predict overall mortality, cardiovascular mortality and mortality of hepatic origin.
Serological panels for fibrosis generally have a high negative predictive value for the diagnosis of advanced fibrosis and are useful for excluding advanced disease, while having less utility in early stages of fibrosis.96–98
Recommendations- •
Biomarker-based serological panels are useful for excluding advanced fibrosis, and can at times help avoid having to perform a liver biopsy (B2).
- •
These panels in turn provide information on the prognosis of the liver disease, as they are capable of predicting mortality of hepatic origin and, in the case of non-alcoholic aetiology, also of cardiovascular origin (B1).
Ultrasound is a simple and inexpensive technique, but has low sensitivity for detecting mild steatosis. CT is similar to ultrasound, but more costly.
The controlled attenuation parameter (CAP) is a non-invasive method for assessing hepatic steatosis based on measurement of the liver's viscoelasticity. It has the advantage of being used in combination with transient elastography and can therefore be measured at the same time as fibrosis. A CAP>248dB/m signifies hepatic steatosis.99
Both magnetic resonance spectroscopy (1H-RME) and the MRI techniques with different methods that analyse the proton density fat fraction have a high sensitivity and capacity for diagnosis.100,101
Recommendations- •
Abdominal ultrasound is a good imaging technique for the initial assessment of patients with suspected hepatic steatosis (A1).
- •
CAP can be used simultaneously with transient elastography to assess steatosis (detection and quantification), especially in high-prevalence populations (obese, diabetic). (B1).
- •
Magnetic resonance (imaging and spectroscopy) techniques have a very good capacity for diagnosis and enable accurate quantification of liver fat content. They are very useful for clinical studies and therapeutic trials (A1).
Detecting inflammation associated with the steatosis identifies patients with NAFLD with a higher risk of fibrosis and disease progression. Identifying fibrosis is an indirect sign that there will be inflammation.
There are some imaging techniques, such as ultrasound with special contrasts, MRI with the DeMILI technique102 and positron emission tomography (PET), with which preliminary studies suggest possible future utility for detecting inflammation in patients with NASH.103
A simple steatosis cannot be distinguished from steatohepatitis with the usual imaging techniques (ultrasound, CT, MRI, elastography).104
Recommendations- •
There are no imaging techniques capable of identifying and monitoring patients with NAFLD or NASH who have inflammation or steatohepatitis (C2).
Conventional imaging techniques (ultrasound, CT, MRI) are not capable of quantifying the stage of fibrosis. The stage of fibrosis can be estimated by elastography. In general, elastography has a high degree of diagnostic accuracy for advanced stages of fibrosis (F3–F4) and for ruling out fibrosis (negative predictive value).105,106 In patients with significant obesity, the addition of the XL probe will improve the reliability of the diagnosis.106 Elastography is therefore a useful tool for monitoring the progression of liver fibrosis in clinical practice.105
The frequency of the determinations has not been not defined, but it seems advisable to measure liver stiffness periodically; testing could be recommended annually to patients in more advanced stages (≥F3) and every three years to patients in more initial phases.
Recommendations- •
Transient elastography is useful for identifying patients with fibrosis due to NAFLD, and to monitor its progression (B1), repeating the measurements at intervals of one to three years depending on the degree of baseline fibrosis (C2).
- •
In patients with significant obesity, it is important to use transient elastography with the two available probes (M and XL) to improve the diagnostic performance and the reliability of the results (B2).
The finding of LC with PH or compensated advanced chronic liver disease in patients with NAFLD has important implications in terms of prognosis and recommendations for screening. Imaging tests (ultrasound, CT and MRI) may suggest the presence of LC on detecting a nodular liver surface, splenomegaly and collateral circulation.107 Collateral circulation is a sign of clinically significant PH.108
In patients with advanced chronic liver disease who have platelets >150,000 and liver stiffness <20kPa, diagnostic endoscopy can be avoided because of the low risk of having large varices. This criterion has been validated more for advanced liver disease of viral aetiology, but it is also applicable to patients with NAFLD.107
Recommendations- •
Imaging techniques (ultrasound, CT and MRI) (B2) can provide information to suggest LC with PH in patients with NAFLD.
- •
Endoscopy to screen for varices can be avoided in patients with NAFLD-related advanced chronic liver disease who have platelets >150,000 and liver stiffness <20kPa (B1).
In recent years there have been attempts to find the genetic involvement in both diseases, in order to improve the stratification of patients and contribute to so-called personalised medicine.109 The evidence gathered so far indicates that particular single nucleotide polymorphisms (SNPs) located in the PNPLA3 and TM6SF2 genes exert the greatest influence.110 Numerous studies confirm that being a carrier of the risk variant of PNPLA3111 and TM6SF2112 confers susceptibility to develop the disease, increasing the risk of progression. Moreover, several clinical trials have reported that the risk variant of PNPLA3 may be associated with the response to pharmacological treatment and to lifestyle interventions113.
Recommendations- •
In view of the fact that carriers of the risk variant of SNPs located in the PNPLA3 and TM6SF2 genes have an increased likelihood of developing more aggressive stages of the disease, it would be of interest to perform screening in at-risk populations, as a tool to further support physicians in their decision making (B2).
Inheritance of genetic markers is very stable and not influenced by other external factors. They have a predictive capacity, but as this is a multifactorial disease, their influence is limited. They are considered cost-effective to perform, as the technique is simple and inexpensive. Numerous algorithms have been described that include clinical, epidemiological and biochemical variables which provide moderate diagnostic certainty for the prediction of NAFLD and fibrosis in these patients.114–116
However, the inclusion of genetic information could significantly increase the capacity for diagnosis, although validation studies would first be required in at-risk populations.
A number of studies have shown that certain SNPs can modulate the response of patients with NAFLD to the dietary intervention.117–119
Recommendations- •
Determination of the patient's genetic profile is a novel strategy that should be considered as part of the full assessment of the patient (B2).
A low-calorie diet is recommended. In general, the diet's energy intake in the form of calories is the most important factor affecting the amount of fat in the liver, regardless of whether it comes from a high intake of fats or carbohydrates.120 With respect to the qualitative composition of the diet, the recommended proportions are 50–60% of carbohydrates and 20–25% of lipids. The Mediterranean diet respects these proportions and may be the recommended diet for the control of NAFLD. However, it is not associated with significant weight loss, as that depends on the diet's calorie content. Particular care has to be taken with insulin-dependent patients, as a significant association has been found between a diet with a glycaemic index ≥58 and the development of hepatic steatosis121 (Fig. 1).
Recommendations- •
We recommended that all patients with NAFLD who are overweight/obese should reduce their calorie intake (A1).
- •
The choice of diet should be personalised and based on the patient's comorbidities and preferences (B2).
The Mediterranean diet is currently considered to be a pattern of healthy eating for many diseases, including MetSyn, cardiovascular disease and cancer. It improves insulin sensitivity and has been shown to achieve a significant reduction in steatosis of up to 39%, compared with 7% with a low-fat, high-carbohydrate diet.122 This benefit is thought to come from the diet's olive oil content (as monounsaturated fatty acid) regardless of the calorie content, in combination with the increase in omega-3, fruit, vegetables, fibre and reduction in saturated fats, simple carbohydrates, sugary drinks and processed foods rich in fructose and trans fats, and alcohol.123 The benefits of olive oil have also been reported for other diseases, such as DM2.124
Recommendations- •
The recommended diet is the Mediterranean diet (B1).
- •
Consumption of olive oil may be indicated for patients with NAFLD when used as part of a Mediterranean diet (B2) as well as for the primary prevention of NAFLD (C2).
- •
It is advisable to cut out all processed drinks and foods containing fructose (B2).
It is suspected that many of the antioxidant and cytoprotective effects of coffee are independent of caffeine and are due to other components, such as chlorogenic compounds. A recent meta-analysis showed that although total caffeine consumption is not associated with either the prevalence or the degree of liver fibrosis in patients with NAFLD, regular consumption of coffee with caffeine can significantly reduce hepatic fibrosis in patients with NAFLD. Taking the potential benefits of coffee into account, we can recommend regular consumption in patients with NAFLD, while pointing out that the recommendation is to drink coffee with caffeine, not take caffeine on its own. No recommended dose has been established.125
Recommendations- •
Regularly drinking coffee with caffeine is recommended in patients with NAFLD. Drinking coffee has been associated with histological improvement of liver damage also from other causes (A2).
Several clinical trials and a meta-analysis have shown that supplements of omega-3 fatty acids, starting at a minimum of 0.83g/day of omega-3, reduce total fat in the liver. However, this has not been demonstrated with paired liver biopsies.126 The mechanisms that may explain the benefits of omega-3 in NAFLD seem to stem from its participation in the regulation of gene expression of insulin sensitisers and in the reduction of inflammation, as well as its inhibitory effects on nuclear factor kappa B.
Recommendations- •
Diets rich in omega-3 fatty acids seem to improve hepatic steatosis. There are no data on the optimal dose for recommending the use of omega-3 supplements. However, eating foods rich in omega-3 (B1) is considered advisable.
In epidemiological studies, moderate consumption of alcohol (particularly red wine) is associated with a lower prevalence of non-alcoholic fatty liver, NASH and fibrosis, and it is even thought to have a protective effect on DM, MetSyn and IR.127 Total abstinence is mandatory in cirrhosis resulting from NASH in order to reduce the risk of HCC, as alcohol is thought to be a predisposing factor.128 Moreover, it has been shown that even a small amount of alcohol is a significant risk factor for the development impaired glucose metabolism in patients with NAFLD and obesity, although the risk of varies according to the amount of alcohol consumed.129 Occasionally, moderate consumption (<21units of alcohol [UA] per week in males and <14 UA per week in females), particularly of red wine, may be recommended.130
Recommendations- •
Data are contradictory and there have been no conclusive studies. Until such studies are available, absolute abstinence from alcoholic beverages is recommended in patients with NASH (B1).
- •
Occasionally, patients with NAFLD without NASH or fibrosis may be permitted moderate consumption (<21 UA per week in males and <14 UA per week in females), particularly of red wine (B1).
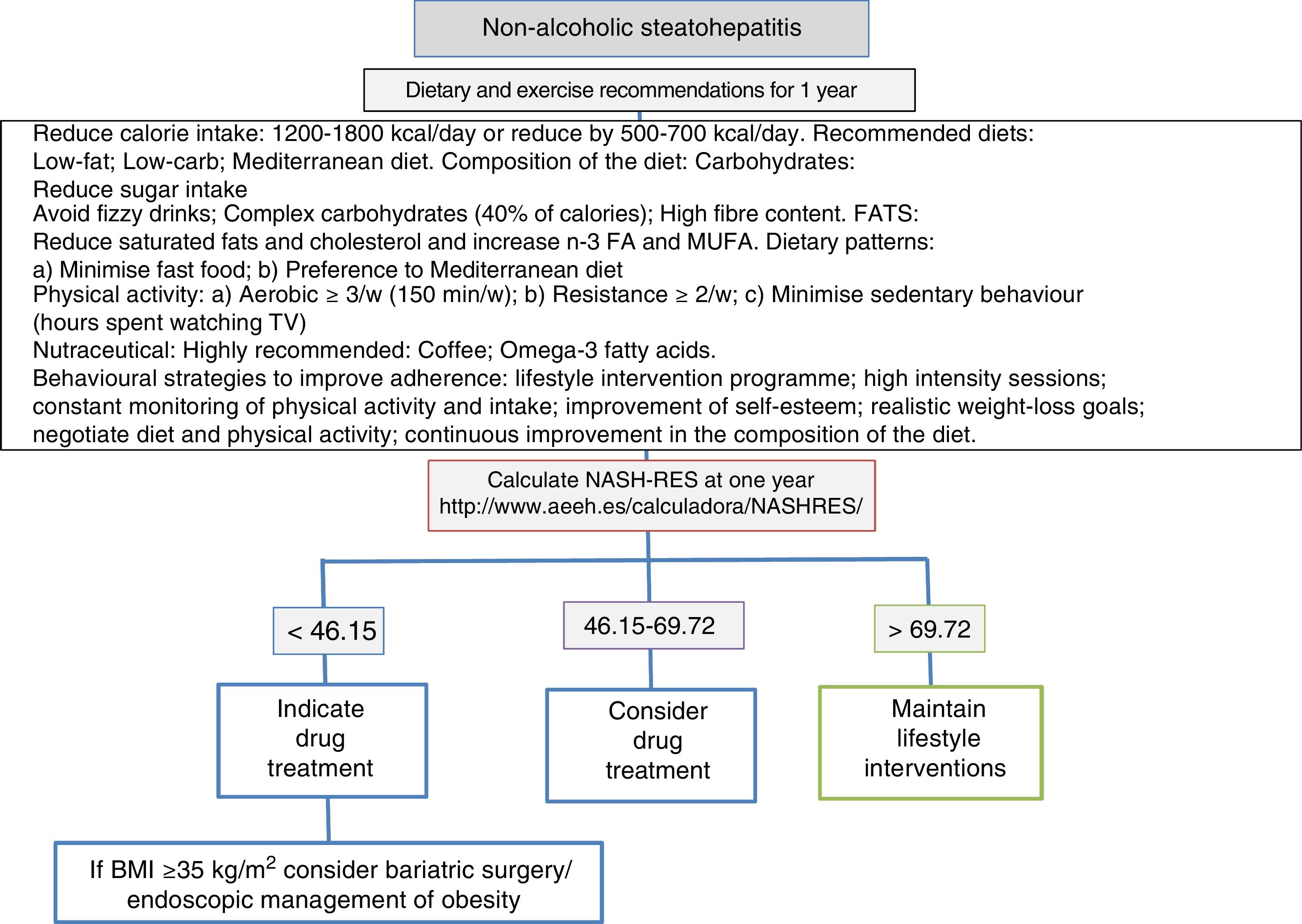
Weight loss of at least 7% through changes in lifestyle131 or bariatric surgery132 over one year, depending on the BMI and comorbidities, lead to beneficial effects on NAFLD and its comorbidities. A low-calorie Mediterranean diet and increase in physical activity for the purposes of losing weight should be recommended to all patients with NAFLD, as weight loss has been related to histological improvement in the tissue and improvement in the course of the disease.133 Bariatric surgery should be considered in patients with BMI>40kg/m2 or patients with BMI>35kg/m2 and significant comorbidities who have made failed attempts to change their lifestyle; NASH itself is not an indication for bariatric surgery.
Recommendations- •
Weight loss through changes in lifestyle or bariatric surgery when indicated should be recommended as a first therapeutic option in patients with NAFLD (A1).
The extent of weight loss is directly and proportionally related to the improvement of histological lesions in patients with NAFLD, regardless of the type of intervention, whether lifestyle changes or bariatric surgery. Weight loss of 10% or more leads to high rates of improvement (>80%), not only of comorbidities but of all the histological lesions associated with NAFLD. We should therefore recommend weight loss greater than 10% to achieve maximum benefit. Weight loss of 7–10% in patients with few risk factors also improves metabolic alterations and significantly reduces steatosis, inflammation and ballooning.133
Recommendations- •
Interventions in terms of lifestyle changes or bariatric surgery should be designed to achieve weight loss of over 10%. However, rates of 7–10% also produce benefits in the liver and in cardiometabolic risk factors (A1).
Regardless of the type of diet or physical activity, weight loss is the most important factor related to improvement of the histological features of patients with NAFLD. However, further studies are necessary to confirm these observations. Studies show that physical activity (150–200min per week) leads to a significant improvement in metabolic alterations and hepatic steatosis determined by histology or non-invasive methods.134 Transaminase levels are found to decrease with weight loss≥5%.131,135 There are no studies in the literature to confirm the benefits of the type, intensity and duration of exercise.134–136
Recommendations- •
Weight loss should be recommended to all patients with NAFLD (A1).
- •
Walking or running for 150–200min a week is recommended to obtain the maximum benefit (B2).
Bariatric surgery provides positive and sustainable effects in terms of weight loss.137 The efficacy and safety of bariatric surgery depend fundamentally on the type of technique used and the experience of the hospital.138 Vertical gastrectomy and gastric bypass provide higher rates of weight loss than gastric band placement; however, they also have higher complication and mortality rates.137
The severity of the liver disease should be considered in all patients who are candidates for bariatric surgery, as LC is associated with a higher risk of complications and mortality. The techniques of tubular gastrectomy and gastrointestinal bypass are those recommended to treat Child–Pugh class A compensated cirrhosis, without PH, although few studies have assessed the safety of the different techniques in patients with LC and more research is required in this area.139 The effectiveness and long-term safety of different surgical techniques in patients with NAFLD needs to be explored in future studies.
Recommendations- •
Bariatric surgery is a safe and effective procedure for treating patients with morbid obesity and NAFLD (A1).
- •
In patients with LC, the decisions about treatment should be dictated by the severity assessed by the Child–Pugh and/or MELD scores. Patients with decompensated cirrhosis should not undergo bariatric surgery (B1).
Patients with NASH and advanced fibrosis have increased risk for all-cause mortality compared to the general population; in fact, the presence and severity of fibrosis is the determining factor associated with increased mortality from any cause, including cardiovascular disease (CVD).140 Most of the increase in the mortality rate is accounted for by cardiovascular causes. A recent meta-analysis shows a strong association between NASH diagnosed by imaging or biopsy and various markers of subclinical atherosclerosis.141 Moreover, several studies show NASH to be associated with an increased prevalence of alterations in cardiac structure and function and the development of arrhythmias.142 Patients with NASH have a two-fold increased risk of developing CVD in relation to the general population.143,144
Recommendations- •
Cardiovascular risk should be assessed in all patients with NAFLD, following the European guidelines (A1).
The main causes of the increase in cardiovascular morbidity and mortality in patients with NASH are coronary disease, myocardial dysfunction and hypertrophy, aortic valve sclerosis and cardiac arrhythmias.142,145 Patients with NASH have:
- a)
An increase in overall and hepatic IR that causes atherogenic dyslipidaemia.145
- b)
Early changes in the energy metabolism of the myocardium inducing structural and functional changes in the heart muscle (left ventricular dysfunction and hypertrophy in children146 and adults147), which may be associated with the increased risk of congestive heart failure in this group of patients.140
- c)
A five-fold higher risk of developing atrial fibrillation independent of the presence of MetSyn and other risk factors, as has been demonstrated in obese children146 and in patients with DM2.148
- •
All patients with NASH should be tested for cardiovascular risk factors, including: (1) lipoprotein profile (total cholesterol, triglycerides, HDL-C, LDL-C, apoB); (2) glucose, insulin and HOMA index; (3) determination of systolic and diastolic blood pressure, and (4) electrocardiogram (A1).
Three therapeutic tools are essential for managing the high degree of cardiovascular risk in these patients:
- a)
Modification of diet and other lifestyle elements.
- b)
Treatment of atherogenic hyperlipidaemia. Management and treatment of hyperlipidaemia in patients with NAFLD are recommended and may lead to improved liver function tests.149 The criteria and recommendations established in the European guidelines for the general population should be followed. Statins appear to be safe and have low hepatic toxicity. A reduction of 2–3mmol/l in LDL-C reduces the incidence of cardiovascular events by 40%.150,151
- c)
Treatment of hypertension following the recommendations established for the general population.151 When choosing the type of antihypertensive drugs, we should take into account that angiotensin II receptor blockers (ARBs) are anti-inflammatory and antifibrotic in the liver, although few studies have demonstrated these actions in patients with NASH.
- •
The management and treatment of hyperlipidaemia in patients with NASH should follow the criteria and recommendations established in the European guidelines for the general population (B1).
- •
Hypertension should be treated as per the recommendations for the general population (A1).
In addition to the control of the aforementioned cardiovascular risk factors, treatment should focus on treating the inflammatory lesions (NASH resolution), ballooning degeneration and fibrosis,152 which are the histological factors associated with disease progression, whether hepatic, cancer-related or cardiovascular. The presence of simple steatosis was not associated with disease progression, and nor was the NAS score, which combines steatosis, ballooning and ballooning degeneration, or the tissue pathology SAF score.153 Steatosis is therefore more a confounding factor than a therapeutic target. Fibrosis is by far the most important prognostic factor in this disease and regression of fibrosis would therefore be the main goal.154
Recommendations- •
The therapeutic targets should be the resolution of steatohepatitis and the regression of fibrosis (B1).
Weight loss is accompanied by an improvement in all histological parameters.131 The impact of weight loss depends on the disease severity at baseline, whether or not the individual has DM2 and their age.155 The normalisation of transaminases and the amount of weight loss, along with the above variables and Kleiner's NAS score,86 enable us to calculate the likelihood of achieving resolution of NASH through a non-invasive prediction model.156 In patients treated with liraglutide, a glucagon-like peptide 1 (GLP-1) agonist that promotes weight loss, the “NASH resolution” formula156 predicted the lack of resolution of NASH with a specificity of 94%, this being very useful for defining stop rules for the intervention.157 Several studies have stressed the need to investigate biomarkers which would help monitor the progression of the disease.158
Recommendations- •
In patients with NASH prescribed diet and physical exercise or treatment with liraglutide, non-invasive methods should be used to confirm the absence of response and the need for drug treatment (B1).
The drug treatment has to achieve the goals of resolution of steatohepatitis and regression of fibrosis with an excellent safety profile. Drug treatment is therefore indicated in patients with steatohepatitis and fibrosis, or in cases prescribed diet and physical exercise with no therapeutic response after one year158. Drug treatment is not indicated in patients with simple steatosis.
Recommendations- •
Drug treatment should be restricted to patients with steatohepatitis and significant fibrosis (B1).
- •
Drug treatment should be prescribed to patients who do not achieve resolution of steatohepatitis after intervention involving diet and physical exercise for one year (B1).
Both vitamin E (800IU/day) and pioglitazone have shown efficacy with respect to improving histological features in patients diagnosed with NAFLD (without LC or DM2), with no significant effect on fibrosis, but with an effect on portal inflammation and steatosis.159 However, both drugs have been associated with long-term adverse effects, including higher mortality rates and development of malignant disease such as prostate cancer, and the risk of haemorrhagic stroke in the case of vitamin E and weight gain, changes in bone metabolism and heart failure in the case of pioglitazone. Vitamin E is only recommended in short courses and in patients with advanced disease; not, however, including those with cirrhosis or diabetes. The choice of one or the other should take into account both the efficacy and the adverse effects of each option.160
Recommendations- •
Vitamin E (800IU/day) improves liver histology in patients with NASH (B1), but it is not indicated in patients with diabetes, patients without histological diagnosis or patients with LC (C1).
- •
Pioglitazone can be used in short courses in the treatment of non-diabetic patients with steatohepatitis demonstrated by biopsy (off-label) (B2).
Three published phase ii studies show that both obeticholic acid (25mg/day),161 elafibranor (120mg/day)162 and liraglutide (1.8mg/day)163 are agents capable of improving histological lesions of NASH with an acceptable safety profile. Mild-to-moderate gastrointestinal symptoms have been reported with the use of liraglutide, mild elevation of creatinine in patients treated with elafibranor, and pruritus in patients taking obeticholic acid. In addition to the above-mentioned ability of GLP-1 agonists to improve liver histological lesions, in view of their incretin action, enhancing insulin sensitivity, and their ability to induce weight loss, they could be a suitable therapeutic option in patients with DM2 and NASH. However, phase iii studies are necessary to establish safety and efficacy in patients with more advanced liver disease.163
Recommendations- •
The identification of molecular targets in the pathogenesis of NAFLD has made it possible to develop new drugs which have been shown to be superior to placebo in phase ii. However, the development of these drugs has to be completed (in phase iii clinical trials) before evidence-based recommendations can be made (A1).
The significant increase in the prevalence of liver disease caused by fatty deposits is a direct consequence of the world's obesity epidemic along with the concomitant increase in diabetes and MetSyn. Two recent studies conducted in the United States show that NAFLD-related LC, with or without associated HCC, is the indication for LT that has increased most since 2002 (both isolated LT and combined liver and kidney transplantation),164 to now be the second leading indication in the new registries for LT165,166 and the leading indication for combined liver and kidney transplantation. There are no similar data published in Spain.
Recommendations- •
NASH should be considered as the main or concomitant cause of cirrhosis in the LT candidate (A1).
Patients with NAFLD-related LC have comorbidities which affect their prognosis. They also tend to be older individuals, often female and with a history of cardiovascular disease, with a higher prevalence of associated chronic kidney disease. The few available studies have found that although the rate of contraindication for receiving LT is similar to that reported in other indications, the reasons are different. In patients with NASH, contraindication is predominantly because of cardiovascular comorbidities, whereas in LC caused by hepatitis C virus, socio-psychological causes predominate.167,168
Recommendations- •
In view of the high rate of association between NASH and DM, obesity, hypertension, CVD and peri-transplant morbidity, a thorough investigation of cardiovascular risk is recommended in patients with NASH who become candidates for LT (A1).
- •
The accumulation of cardiovascular risk factors should be carefully assessed by a multidisciplinary team, and should be a reason for not including a patient on the waiting list if the risk is considered high (B1).
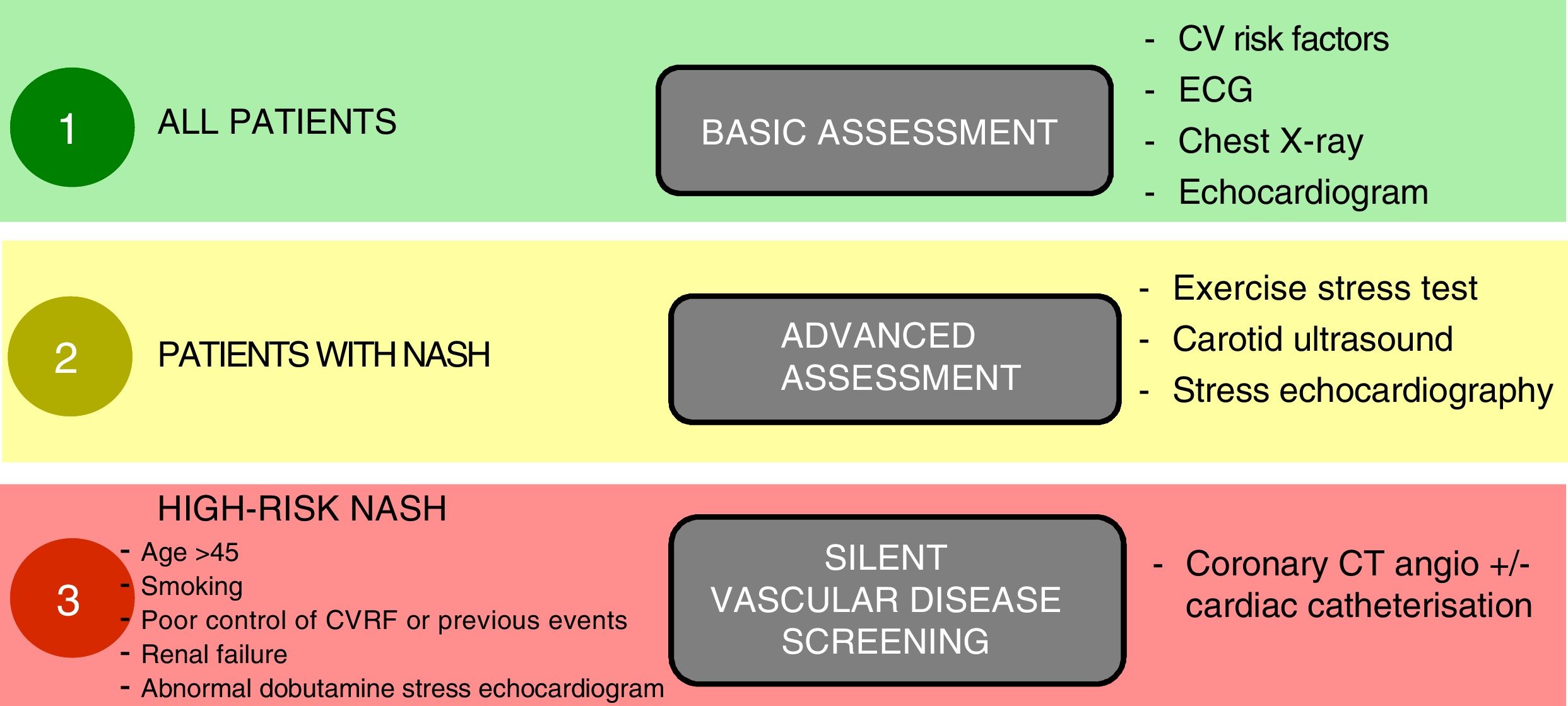
Yes, patients with NASH tend to have a high cardiovascular-risk metabolic profile, which makes it more likely that they will have silent vascular disease.164,165,168 The physical stress inherent to the transplant, including the surgery itself and the potential postoperative complications, can transform silent vascular disease into severe CVD that eventually increases the mortality rate. It is therefore essential that the pre-transplant assessment includes full cardiology screening, both structural and functional, under resting and stress conditions, including stress echocardiography (physical or pharmacological) and CT-coronary angiography or even catheterisation in high-risk patients, in order to detect and, if possible, correct any significant vascular disease169 (Fig. 2). Moreover, the presence and severity of NAFLD are associated with chronic kidney disease regardless of whether or not the patient has diabetes.170
Proposed plan for pre-transplant cardiological assessment according to cardiovascular risk.
Notes: Cardiovascular risk factors should be suitably under control before transplant.
Clinically significant vascular disease should be treated and stabilised before transplant.
Adapted from Malhi et al.169
- •
The pre-transplant assessment should include in-depth cardiovascular screening in order to detect silent vascular disease, and patients should be investigated for associated chronic kidney disease (B1).
Patients with morbid obesity have more infectious and postoperative complications after LT. Several short series have been published showing that performing tubular gastrectomy at the same time as the LT helps these patients to lower their BMI quickly post-transplant.171 However, it has to be remembered that bariatric surgery adds extra morbidity and mortality to LT, and could at the same time undermine nutritional status in the immediate post-transplant period which would in turn impede the patient's recovery. Therefore, although bariatric surgery could help improve the metabolic profile in patients transplanted for NASH-related cirrhosis, there is no defined optimal time for performing it, so the indication for LT and the type of combined bariatric surgery in patients with BMI>35 and DM has to be individualised.171
Recommendations- •
Because of the comorbidity associated with morbid obesity post-transplant, performing bariatric surgery should be considered in these patients. There is no consensus on the best time for this intervention and it therefore has to be individualised (C2).
- •
The indication for LT and the type of bariatric surgery in obese individuals with a BMI>35 and DM should be individualised (A1).
The various published series, including systematic reviews and meta-analyses, show that the post-transplant survival of patients transplanted for NASH-induced cirrhosis is similar to that for other indications.172 In a recent meta-analysis that included 717 patients transplanted because of NASH compared to 3520 transplanted for other indications, there was no difference between groups in one-year survival (OR: 0.77; 95% CI: 0.59–1.00; p=0.05), three-year survival (OR: 0.97; 95% CI: 0.67–1.40; p=0.86) or five-year survival (OR: 1.09; 95% CI: 0.77–1.56; p=0.63). However, the causes of death were different, with cardiovascular origin (OR: 1.65; 95% CI: 1.01–2.70; p=0.05) and sepsis (OR: 1.71; 95% CI: 1.17–2.50; p=0.006) being more common among the patients transplanted because of NASH.168
Recommendations- •
Post-LT survival is similar in patients with NAFLD and LT for other indications, but death from a CV cause and sepsis is more common (B1).
According to the studies published to date, it is common to detect fatty disease within the first 12 months in livers transplanted because of NASH-related LC, and it is usually mild.173 However, at least in the short-to-medium term, there are still no data to show that recurrence of NASH leads to significant fibrosis or LC.174 Overall it is estimated that 8% progress to significant fibrosis in the first five years, while that figure is only 5% in the case of LC. Both hepatic steatosis and recurrent steatohepatitis, including those detected de novo after transplantation, may present with elevated transaminases and/or similar ultrasound findings. Liver biopsy is therefore required to confirm the diagnosis and/or establish a differential diagnosis with other causes of transaminase elevation.174,175
Recommendations- •
It may be necessary to perform a liver biopsy to confirm the diagnosis of recurrent or de novo steatosis/NASH post-transplant or to exclude other causes of abnormal liver biochemistry (A1).
There have been no clinical trials carried out specifically in patients transplanted for NASH-related cirrhosis. The available evidence needs to be extrapolated from clinical trials conducted on LT for any aetiology and indication. It seems reasonable to presume that immunosuppression protocols associated with less negative impact on the metabolic profile will be the most suitable for patients with NASH. First choice should be strategies minimising calcineurin inhibitors, as well as to steroid-free protocols.176 Moreover, everolimus is closely related to the development and/or aggravation of hypertriglyceridaemia, and should therefore be avoided in patients with NASH, unless the benefit significantly outweighs the risk.177
Recommendations- •
Protocols for steroid-free immunosuppression and calcineurin inhibitor minimisation (supported by induction and/or mycophenolate therapies) could be beneficial in patients with NASH undergoing LT (B1).
- •
The use of mTOR inhibitors is not the first choice in patients with NASH due to their negative effect on the lipid profile. However, they may be administered when there is a significant expected clinical benefit (B2).
The risk factors for MetSyn after LT are the same as those for non-transplant patients, so the control measures should be similar: avoid weight gain and control hypertension, DM and dyslipidaemia. Unfortunately, several series have highlighted that the control of these metabolic complications is inadequate in a significant percentage of patients due to the metabolic side effects of the immunosuppressive treatment (hypertension, DM, dyslipidaemia, weight gain, renal failure).178 From a surgical point of view, the studies on surgery for obesity after transplantation show that it is a complex intervention with a high complication rate.179
Recommendations- •
No specific recommendations can be given in relation to the prevention and/or treatment of post-LT steatosis/NASH, except for the general advice to avoid weight gain and keep metabolic comorbidities adequately under control (B1).
- •
Close monitoring of renal function is recommended for early diagnosis and treatment of chronic kidney disease (A1).
- •
Individualised immunosuppression is recommended to prevent metabolic complications and protect renal function (B1).
NAFLD includes a broad spectrum of disorders ranging from simple hepatic steatosis to NASH with fibrosis.2,15 NASH can progress to advanced fibrosis, cirrhosis and HCC, with a mortality rate of 10–12% at 10–15 years post-diagnosis.25 The survival of patients with NAFLD depends on the comorbidities they have, with IR and MetSyn43–47 as the common denominator. Consequently, the main cause of death in these patients is coronary artery disease,142–148 followed by extrahepatic malignancies and cancer associated with LC. NAFLD is the most common cause of fibrosis and cirrhosis in patients with unexplained elevation of transaminases.4,5 NAFLD in children is a growing problem which is reaching epidemic proportions in some developed countries; it is the most common cause of liver disease in childhood.32 While less is understood about the natural history of NAFLD in children than in adults, it covers the same spectrum of conditions.32–39 The definitive diagnosis continues to be based on liver biopsy,79,84,85 and using an internationally accepted diagnostic protocol, such as the Kleiner score,86 or the more recent SAF score,89 reduces inter-observer variability88 and allows better histological classification for therapeutic purposes and prognosis. Liver biopsy is not recommended as a screening tool. Biomarkers,94 non-invasive indices95 or controlled attenuation parameter (CAP)99 are useful, as well as methods based on elastography to detect fibrosis and steatosis.105,106 Certain genetic markers, such as the SNPs located in the PNPLA3 and TM6SF2 genes, have an increased likelihood of progressing to more aggressive stages of the disease.111,112 We need studies to validate non-invasive diagnostic methods, such as radiological biomarkers based on MRI.81,102 The lifestyle changes based on the Mediterranean diet and physical exercise are the most important elements in the treatment of these patients and should be designed to encourage weight loss of more than 10%.131 Losing 7–10%, however, should also produce benefits both for the liver and cardiometabolic risk factors.131,133 All the associated cardiovascular risk factors, such as atherogenic dyslipidaemia, hypertension and DM,142–151 also need to be assessed and treated.
Pharmacological treatment should achieve the goals of resolving steatohepatitis and regression of fibrosis, and should be used in cases with no therapeutic response after a year on diet and physical exercise.156–158 Drug treatment is not indicated in patients with simple steatosis. The development of new drugs, some in clinical trials in phase iii,161–163 should in future define the evidence-based recommendations. In view of the close association between NAFLD and DM, obesity, hypertension, cardiovascular disease and peri-transplant morbidity, a thorough analysis of cardiovascular risk is recommended in patients with NASH who are candidates for LT.164,169 The use of mTOR inhibitors is not the first choice in patients with NASH due to their negative effect on the lipid profile.165 In conclusion, new epidemiological studies are necessary to assess the actual prevalence of NAFLD in our area. We also need to develop reliable and reproducible non-invasive diagnostic methods, and complete the ongoing clinical trials with drugs (such as elafibranor, obeticholic acid and semaglutide) which act on different therapeutic targets involved in the pathogenesis of NAFLD. Patients with NASH and fibrosis could benefit from these drugs in terms of changing the natural history and improving the prognosis of NAFLD.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Aller R, Fernández-Rodríguez C, lo Iacono O, Bañares R, Abad J, Carrión JA, et al. Documento de consenso. Manejo de la enfermedad hepática grasa no alcohólica (EHGNA). Guía de práctica clínica. Gastroenterol Hepatol. 2018;41:328–349.