Prevalence of hepatopulmonary syndrome (HPS) ranges from 4% to 47% in patients with cirrhosis. This study aimed to explore possible relationship between CX3CR1 and angiogenesis or macrophage accumulation in pathological process of HPS.
Material and methodsWide-type C57Bl/6 mice were divided into WT-sham, WT-common bile duct ligation (WT-CBDL), WT-CBDL plus antibody (WT-CBDL+Ab) and WT-CBDL plus Bevacizumab. The CX3CR1GFP/GFP mice were grouping into CX3CR1 GFP/GFP-sham, CX3CR1 GFP/GFP-CBDL and CX3CR1 GFP/GFP-CBDL+Bevacizumab group. Intrapulmonary expression of Akt, pAkt, ERK, pERK, iNOS, VEGF, PDGF was measured using biological technology. Hematoxylin-eosin (H&E) staining and immunohistochemical analysis were used to evaluate changes of pulmonary tissues including pathological abnormality, angiogenesis and macrophage accumulation.
ResultsBlockade CX3CR1 pathway inhibited angiogenesis, macrophage accumulation and pathological changes of lung tissues. Blockade of CX3CR1 pathway reduced pAkt, pERK, iNOS, PDGF and VEGF activation. CX3CR1 contributed to the process of angiogenesis and activate the pro-angiogenic factors. CX3CR1 deficiency obviously reduced the macrophage accumulation. Inhibition of VEGF by Bevacizumab improved intrapulmonary angiogenesis and pathological changes of lung tissues. Inhibition of VEGF by Bevacizumab retarded the production of pAKt, PDGF, and iNOS. Inhibition of VEGF by Bevacizumab reduced CX3CL1 production.
ConclusionCX3CR1 could regulate the angiogenesis and activation of pro-angiogenic factors, including pAKT, pERK, iNOS, VEGF and PDGF in the process of hepato-pulmonary syndrome. Moreover, CX3CR1 could also contribute to the macrophage accumulation.
La prevalencia del síndrome hepatopulmonar (SHP) oscila entre el 4 y el 47% en pacientes con cirrosis. El objetivo de este estudio fue estudiar la posible relación entre el CX3CR1 y la angiogénesis o acumulación de macrófagos en el proceso patológico del SHP.
Material y métodosLos ratones C57Bl/6 sin mutaciones se dividieron en WT-sham, WT-ligadura de conducto colédoco (WT-CBDL), WT-CBDL más anticuerpo (WT-CBDL+Ab) y WT-CBDL más bevacizumab. Los ratones CX3CR1GFP/GFP se clasificaron en los grupos CX3CR1 GFP/GFP-sham, CX3CR1 GFP/GFP-CBDL y CX3CR1 GFP/GFP-CBDL+bevacizumab. La expresión intrapulmonar de Akt, pAkt, ERK, pERK, iNOS, VEGF y PDGF se determinó utilizando tecnología biológica. Se utilizaron tinción de hematoxilina-eosina (H&E) y análisis inmunohistoquímico para evaluar los cambios de los tejidos pulmonares, incluidas las anomalías histopatológicas, la angiogénesis y la acumulación de macrófagos.
ResultadosEl bloqueo de la vía CX3CR1 inhibió la angiogénesis, la acumulación de macrófagos y los cambios histopatológicos de los tejidos pulmonares. El bloqueo de la vía CX3CR1 redujo la activación de pAkt, pERK, iNOS, PDGF y VEGF. CX3CR1 contribuyó al proceso de angiogénesis y a activar los factores proangiogénicos. Evidentemente, la deficiencia de CX3CR1 redujo la acumulación de macrófagos. La inhibición del VEGF por parte del bevacizumab mejoró la angiogénesis intrapulmonar y los cambios histopatológicos de los tejidos pulmonares. La inhibición del VEGF por parte del bevacizumab retrasó la producción de pAkt, PDGF e iNOS. La inhibición del VEGF por parte del bevacizumab redujo la producción de CX3CL1.
ConclusiónEl CX3CR1 podría regular la angiogénesis y la activación de los factores proangiogénicos, incluidos la pAkt, la pERK, la iNOS, el VEGF y el PDGF en el proceso del síndrome hepatopulmonar. Además, el CX3CR1 también podría contribuir a la acumulación de macrófagos.
Hepato-pulmonary syndrome (HPS) is an independent risk factor for the survival of patients with cirrhosis. The incidence of HPS ranges from 4% to 47% according to different diagnostic criteria in cirrhotic patients.1 Moreover, it is confirmed that 2–30% of patients with cirrhosis waiting for liver transplantation experience HPS. On the other hand, HPS, as the main cause of liver dysfunction and pulmonary infection during peri-operative transplantation, can increase mortality. The rate of HPS mortality within 2.5 years has been estimated at 41%.1–3 However, at present, there is no effective therapy for HPS, except for liver transplantation.4
Angiogenesis and macrophage accumulation in the lung are important hallmarks of HPS, which is a common pulmonary complication of advanced liver diseases.1,5,6 Akt, ERK, vascular endothelial growth factor (VEGF), inducible nitric oxide synthase (iNOS), and platelet-derived growth factor (PDGF) belong to angiogenesis-related mediators. Akt, as a serine/threonine kinase, is located on the PI3K/Akt signaling pathway and contributes to cell proliferation, transcription, and migration.7 On the other hand, ERK, an extra-cellular signal-regulated kinase and a member of MAPK family, plays a key role in cell growth and fission.8 VEGF-dependent angiogenesis had been demonstrated in many diseases.9 In addition, PDGF contributes to the proliferation of artery smooth muscle cells.10 Therefore, AKT, ERK, iNOS, VEGF and PDGF are related closely with the process of angiogenesis. Previous researches11–13 showed that CX3CR1 can regulate the process of angiogenesis in human umbilical vein endothelial cells. However, the relationship between CX3CR1 and angiogenesis and activation of various pro-angiogenic factors in the process of HPS remains unclear.
Previous studies suggest that accumulation of macrophages plays a key role in the development of HPS.10 Depletion of macrophages can prevent and reverse HPS by reducing the production of various cytokines in the activated macrophages.10 Chemokines, besides growth factors, cytokines, and adhesion molecules, are majorly involved in the accumulation of macrophages.14 Chemokines are divided into four families, including C, CC, CXC, and CX3C, based on the conserved cysteine motifs.15 Fractalkine (FKN)/CX3CL1 is the only member of CX3C subfamily with membrane-anchored and soluble types.16 Membrane-anchored FKN is mainly expressed by endothelial and epithelial cells.17 CX3CR1 is the only high-affinity receptor of FKN, which is primarily expressed by monocytes, macrophages, lymphocytes, dendritic cells, and small glial cells.18 Membrane-anchored CX3CL1, with both chemotactic and adhesive functions, can quickly capture CX3CR1 on the surface of macrophages to mediate the chemotaxis and adhesion of cells, without the involvement of integrins or selectins.19,20 Therefore, CX3CR1 may regulate macrophage recruitment in the process of HSP. Of course, it needs further work to be explored.
Based on the mentioned findings, we hypothesized that CX3CR1 affects the process of angiogenesis, accumulation of macrophage and activation of various pro-angiogenic factors including AKT, ERK, iNOS, VEGF and PDGF in the process of HPS.
Material and methodsAnimalsThree male homozygous CX3CR1 GFP/GFP C57BL/6 mice, aging from 6–8 weeks, weighing from 15g to 25g, were purchased from Jackson Lab. (West Grove, PA, USA), which was established as instructed by the former research.21 The mice were mated together to breed more homozygous male mice for the experiments in this study (preparing at least 10 homozygous male mice, weight from 15 to 25g for the following experiments). In addition, total of 9 normal male C57BL/6 mice (weighting from 15g to 25g, aging from 6–8 weeks) were purchased from the Animal Center of Guizhou Medical University (Guizhou, China). Both of the male CX3CR1 GFP/GFP C57BL/6 mice and male C57BL/6 mice were fed under the circumstances with 12h/12h of light/dark cycle, temperature of 22°C and 40% to 50% humidity. The mice were also fed with the standard-chow diet and the water ad libitum.
All experiments were conducted according to the National Institutes of Health (NIH) guidelines for the use of laboratory animals. The study protocol was also approved by the Ethics Committee of hospital, affiliated to Guizhou Medical University (Guiyang, China).
Trial groupingThe male CX3CR1 GFP/GFP C57BL/6 mice were divided into the following groups: Sham group (n=3), Common bile duct ligation (CBDL) group (n=3) and CBDL+Bevacizumab (a monoclonal antibody for VEGF) group (n=3). On the other hand, the male WT mice were divided into the following groups, including Sham group (n=3), CBDL+Ab (a CX3CR1 polyclonal antibody) group (n=3) and CBDL+Bevacizumab group (n=3).
Animal protocol and regents administrationCommon bile ducts (CBDs) were isolated and ligated in CBDL groups, while CBDs were only isolated in the Sham groups, as previously described.22 In all groups, surgery was performed under isoflurane-based inhalational anesthesia. The abdominal cavity was closed after surgery, and all animals were maintained in a laboratory for daily care. One day after surgery, all groups received normal saline or medicine once a day for two weeks.
At one week post CBDL surgery or Sham surgery, the rabbit CX3CR1 antibody (Cat. No. ab8021, Abcam Biotech., Cambridge, MA, USA) was intraperitoneally injected to mice at dosage of 80μg/kg/day for one week and the bevacizumab (Cat. No. A2006, Selleck Chemicals, Houston, TX, USA) was also intraperitoneally injected to mice at dosage of 5mg/kg/day for one week. All animals in different groups (3 CX3CR1 GFP/GFP and 3 normal C57BL/6 mice in each group) were sacrificed to collect samples for evaluating expressions or levels of cytokines, including Akt, p-Akt, ERK, p-ERK, PDGF, iNOS, VEGF.15 In the whole processes of experiments, no mice were dead (with mortality of 0).
Hematoxylin-eosin (H&E) staining of pulmonary tissuesAfter sampling, the tissues were washed in phosphate buffered saline (PBS) and fixed with 4% paraformaldehyde. Next, the paraffin-embedded sections were deparaffinized and rehydrated. For histological analysis, the tissues were stained with Hematoxylin (Nanjing Jiancheng BioTech., Nanjing, China) and Eosin (Beyotime Biotech., Shanghai, China). Subsequently, the dehydrated and sealed sections were evaluated in terms of the histological structure of the lungs and liver under a microscope (Mode: IX81, Olympus, Tokyo, Japan). Finally, the collected data were analyzed in Image Pro Software (version: Plus 6.0, Media Cybernetics Inc., Bethesda, MD, USA).
Immunohistochemical analysis of pulmonary angiogenesis and macrophage accumulationIn brief, paraffin-embedded sections (with the thickness of 5 um) were deparaffinized and dehydrated, and then, thermal antigen repair was performed in a temperature range of 92–98°C. Next, the sections were cooled down to room temperature, and peroxidase activity was inhibited using 3% hydrogen peroxide (H2O2, Beyotime Biotech). Before incubation with the primary CD31 antibody (1:100; Cat. No. 59818, Cell Signaling Technology, MA, USA) at 4°C overnight, a goat serum was used to block other ineffective antigens. The paraffin-embedded sections, labeled with the secondary antibody (1:50, Cat. No. ab6721, Abcam Biotech., Cambridge, Massachusetts, USA), were incubated with HRP at 37°C for 30min. DBA was also prepared according to the manufacturer's instructions before the dye turned blue again and became dehydrated, sealed, and transparent. Finally, changes in pulmonary angiogenesis or macrophage accumulation were observed under a fluorescence microscope (Mode: IX81, Olympus, Tokyo, Japan).
Western blottingTissues of mice were lysed with the RIPA lysis solution (Beyotime Biotech. Shanghai, China) supplementing with 1% PMSF and centrifuged at 4°C and 12,000r/min for 10min, to harvest total proteins, as described by manufacturer. The protein concentrations of lysates were examining using the BCA Protein Assay kit (Beyotime Biotech.) as instructed by the manufacturer. Then, total of 50μg protein lysates (about 5μg purify protein) were separated onto the 15% SDS-PAGE (Amresco Inc., USA) and transferred to a polyvinylidene difluoride (PVDF, Amresco Inc.). The PVDF membrane was blocked using the 5% non-fat milk (Beyotime Biotech.) in PBS supplementing with 0.05% Tween-20 (Beyotime Biotech.) for 1h at room temperature. Then, PVDF membrane was incubated using antibodies against CX3CL1 (1:1000, Cat. No. ab25091), CX3CR1 (1:1000, Cat. No. 13885-1-AP, Wuhan Sanying, Wuhan, China), Akt (1:1000, Cat. No. ab8805), pAkt (1:1000, Cat. No. ab38449), ERK (1:1000, Cat. No. ab17942), p-ERK (1:1000, Cat. No. ab201015), and β-actin (1:1000, Cat. No. ab8227) for 90min at room temperature and washed using Tris-buffered saline (TBST) in triplicate and 5min per time. Excepting for antibody against CX3CR1, all the primary antibodies were purchased from Abcam Biotech. (Cambridge, Massachusetts, USA). The PVDF membrane was then incubated with secondary antibody (1:1000, Cat. No. AP132, Sigma-Aldrich, St Louis, MI, USA) for another 90min at room temperature. Finally, the mixture was exposed and analyzed using a UVPTM gel scanning system (Model: GDS8000, UVP, Sacramento, CA, USA).
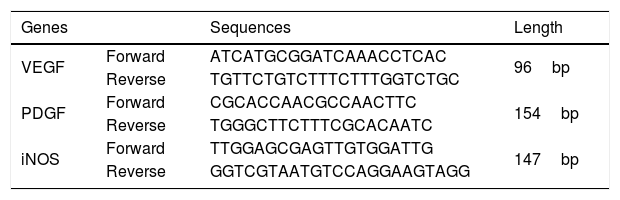
Quantitative real-time PCR (qRT-PCR) assayAll RNAs were extracted with TRIzol reagent (Beyotime Biotech) and used to analyze complementary DNAs (cDNAs) in a reverse transcription kit (Western Biotech., Chongqing, China). Next, the SYBR Green PCR Kit (Western Biotech) was employed to conduct qRT-PCR assay for evaluating the expression of VEGF, PDGF, and inducible nitric oxide synthase (iNOS) with specific primers (Table 1) in the apparatus (Model: FTC-3000P, Funglyn Biotech., Canada). Gel images for the amplified PCR products were analyzed using the GDS8000 UVP Gel Imaging Analysis System (UVP, Sacramento, CA, USA). Relative targeting gene expressions were calculated through normalizing with house gene (β-actin) using the comparative threshold cycle (2−△△CT) method as described by previous study.23
Statistical analysisIn this study, the professional SPSS software was used to analyzed the data. The data was represented as the mean±standard deviation (SD). The Tukey's test was applied to analyze the collected data. The statistical significance was set at P value less than 0.05.
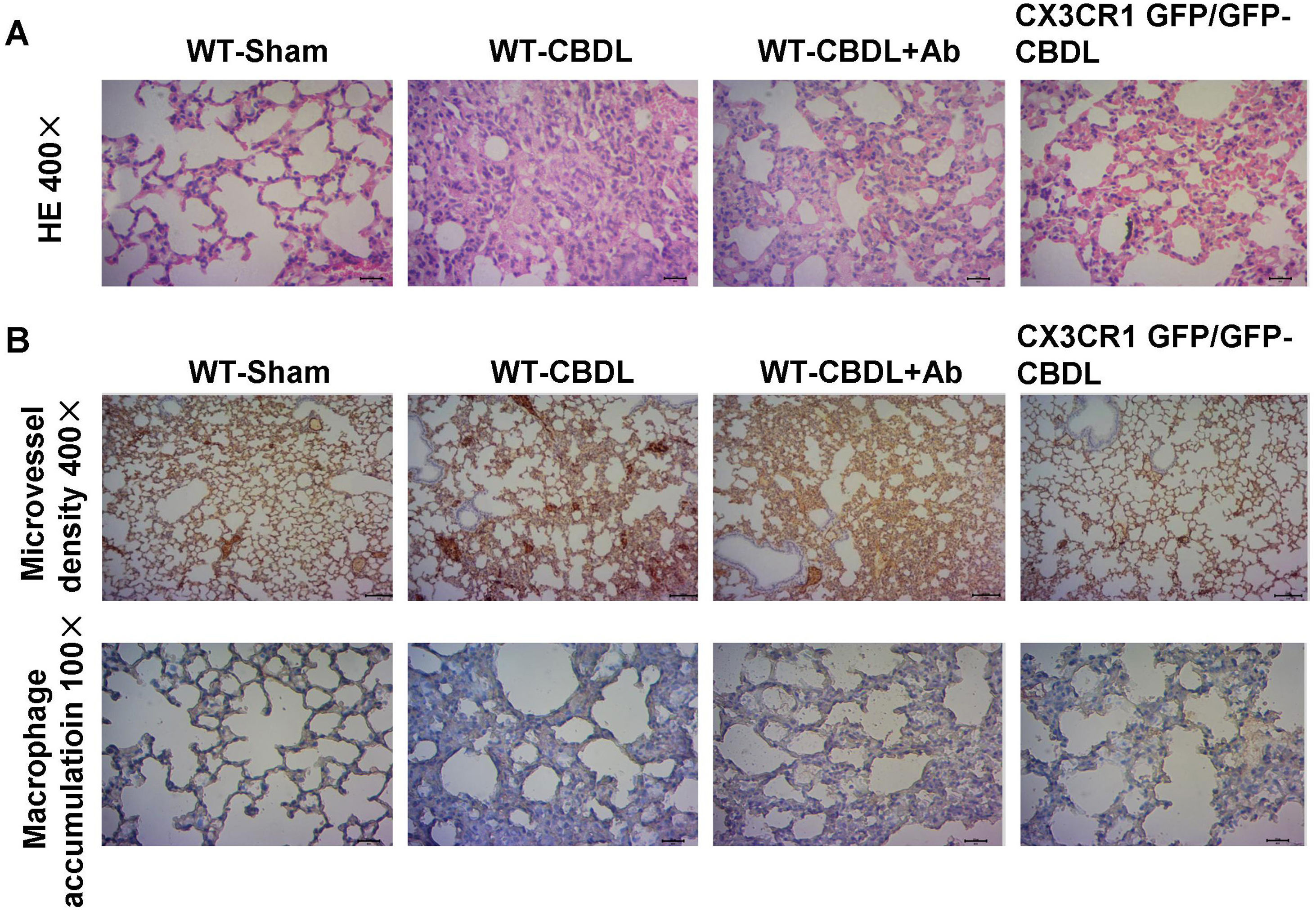
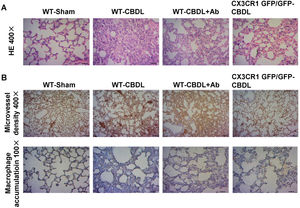
ResultsBlockade CX3CR1 pathway inhibited angiogenesis, macrophage accumulation and pathological changes of lung tissuesThe H&E staining of pulmonary tissues in CBDL mice showed a series of pathological abnormalities, including alveolar collapse, alveolar thickening, and infiltration of inflammatory cells into the alveolar wall (Fig. 1A). Aberrant vascular angiogenesis stained strongly positive for the tissue marker, CD31 (Fig. 1B). Also, accumulation of macrophages, stained for the macrophage-specific marker, CD68 (Fig. 1B), was observed in CBDL mice. To determine if the inhibition of CX3CR1 pathways could partly influence the pathological abnormalities of HPS, angiogenesis and macrophage accumulation, a CX3CR1 polyclonal antibody (Ab) and CX3CR1 GFP/GFP mice which is widely used in the research of microglial dysfunction, liver fibrosis, and immune inflammation were used.24 Less significant intrapulmonary changes (Fig. 1A), micro-vessel angiogenesis, and macrophage accumulation (Fig. 1B) were reported in the WT-CBDL+Ab group versus the WT-CBDL group. A similar finding was reported in the CX3CR1GFP/GFP CBDL group versus the WT-CBDL group.
The effects of CX3CR1 blockade on the pathological changes of the lungs, angiogenesis, and macrophage accumulation. A. H&E staining of lung tissues at 100× and 400× magnification in the WT-sham, WT-CBDL, WT-CBDL+Ab, and CX3CR1GFP/GFP groups. B. Immunohistochemical staining for intrapulmonary angiogenesis using the CD31 antibody in the groups and immunohistochemical staining for macrophage accumulation in the lungs using the CD68 antibody in four groups.
The activation of pAkt/pERK signaling pathway and increased iNOS, VEGF and PDGF mRNA contents were discovered in lungs of CBDL mice (Figs. 2 and 3, P<0.05).
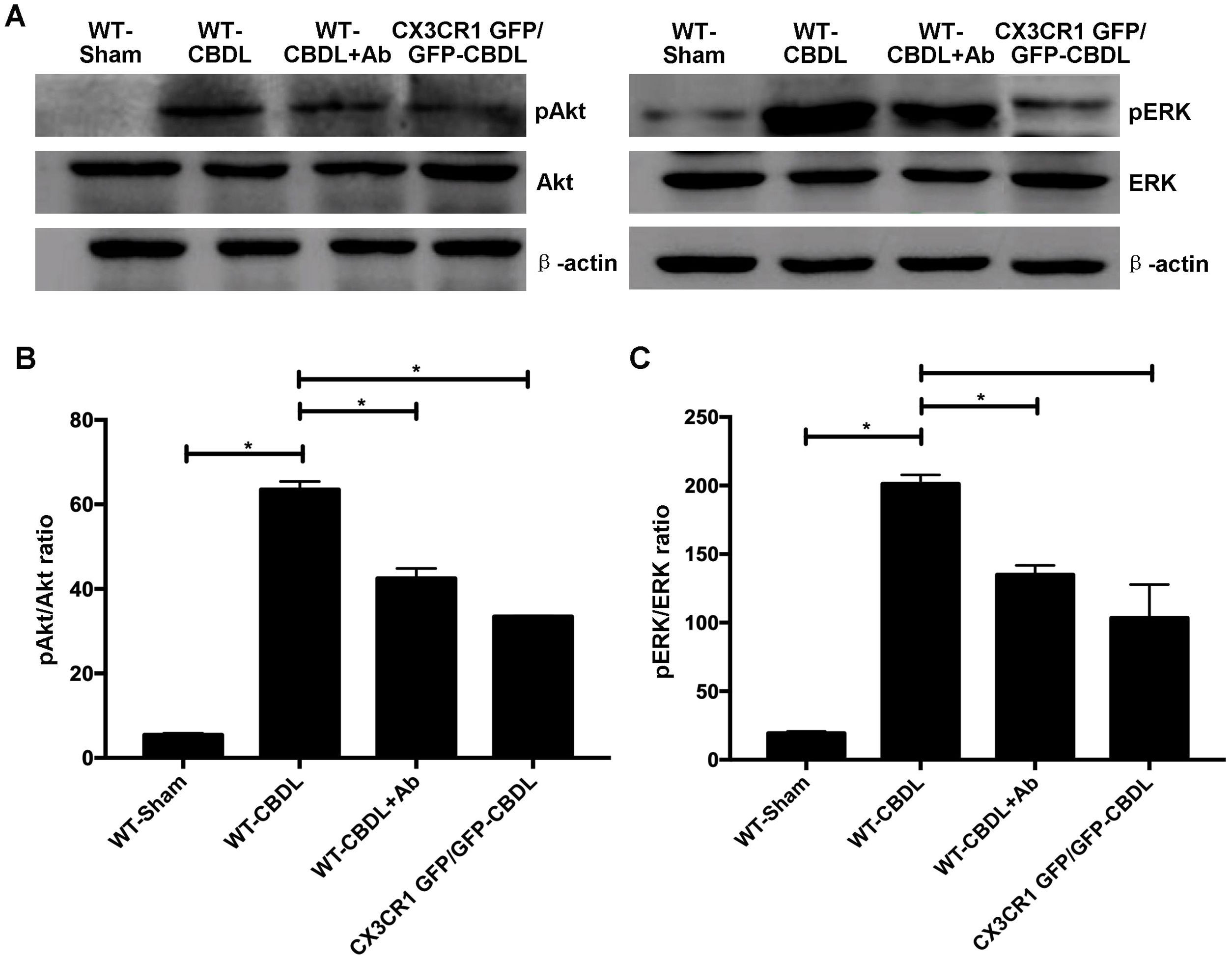
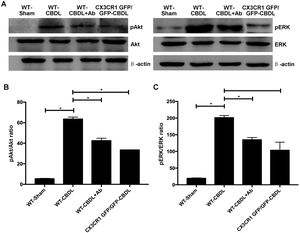
The effect of CX3CR1 blockade expression of molecules in Akt/ERK signaling pathway. A. Western blotting images for expressions of AKt, ERK, pAKt, and pERK in WT-Sham, WT-CBDL, WT-CBDL+Ab and CX3CR1GFP/GFP groups. B. Statistical analysis for the pAkt/Akt ratio. C. Statistical analysis for the pERK/ERK ratio. *P<0.05 represents the different value between two illustrated groups.
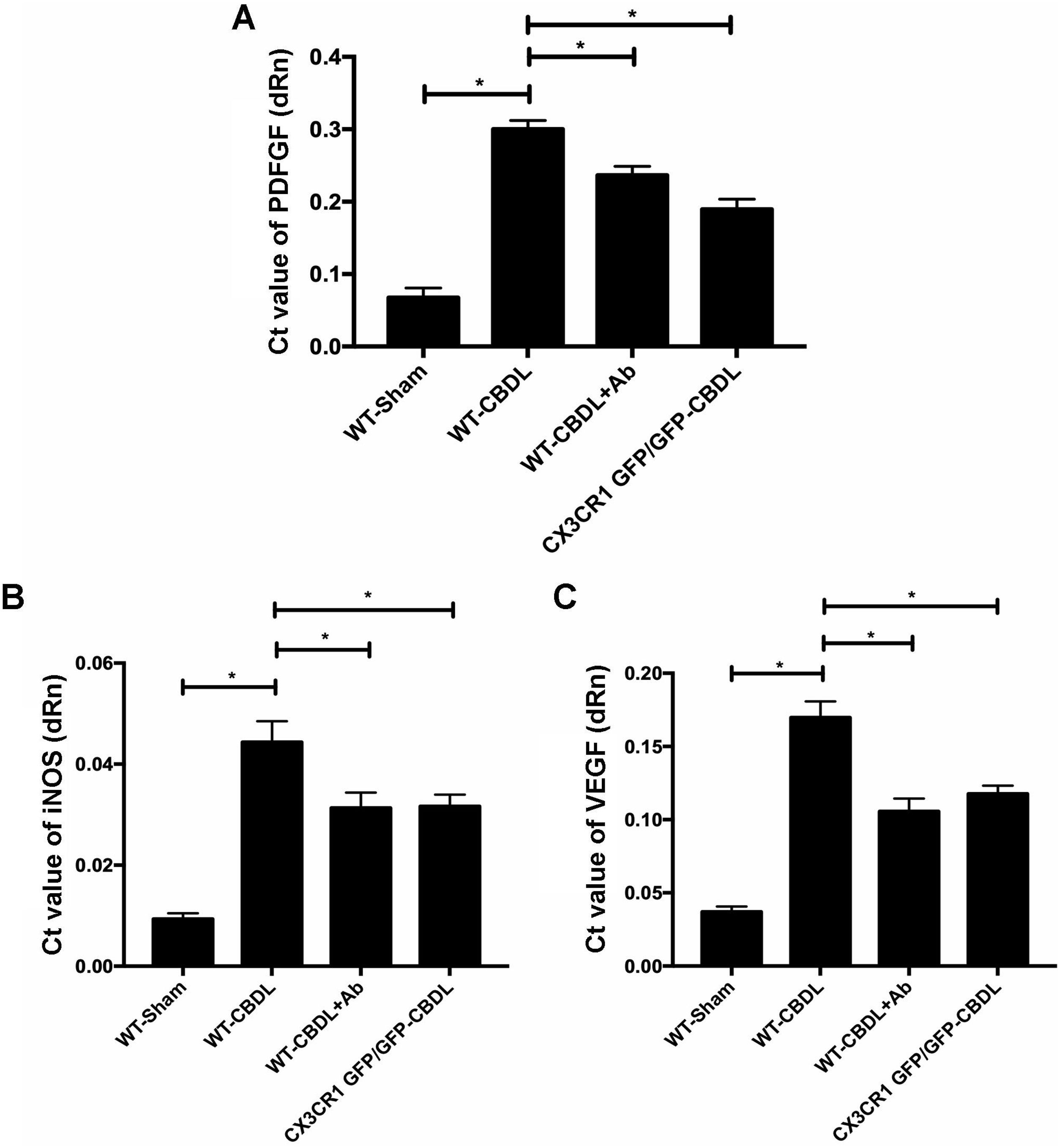
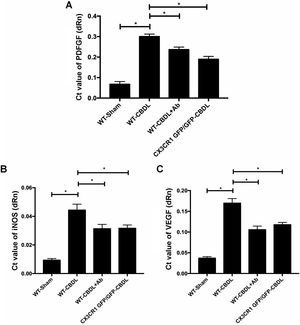
In order to determine the pro-angiogenic factors which CX3CR1 can regulate, we examined expression of pAkt and pERK in lungs with western blot assay (Fig. 2A). We found that both ratio of pAkt/Akt (Fig. 2B) and ratio of pERK/ERK (Fig. 2C) were significantly increased in WT-CBDL group comparing with that of WT-Sham group (P<0.05). The results showed that ratio of pAkt/Akt and ratio of pERK/ERK were significantly down-regulated in lungs of both WT-CBDL+Ab and CX3CR1 GFP/GFP CBDL group compared to that in WT-CBDL group (Fig. 2B, P<0.05). Meanwhile, iNOS, VEGF and PDGF mRNA expressions were also evaluated using RT-PCR assay. The results also indicated that mRNA levels of PDGF (Fig. 3A), iNOS (Fig. 3B) and VEGF (Fig. 3C) were remarkably down-regulated in lungs of both WT-CBDL+Ab group and CX3CR1 GFP/GFP CBDL group compared to that in WT-CBDL group (P<0.05).
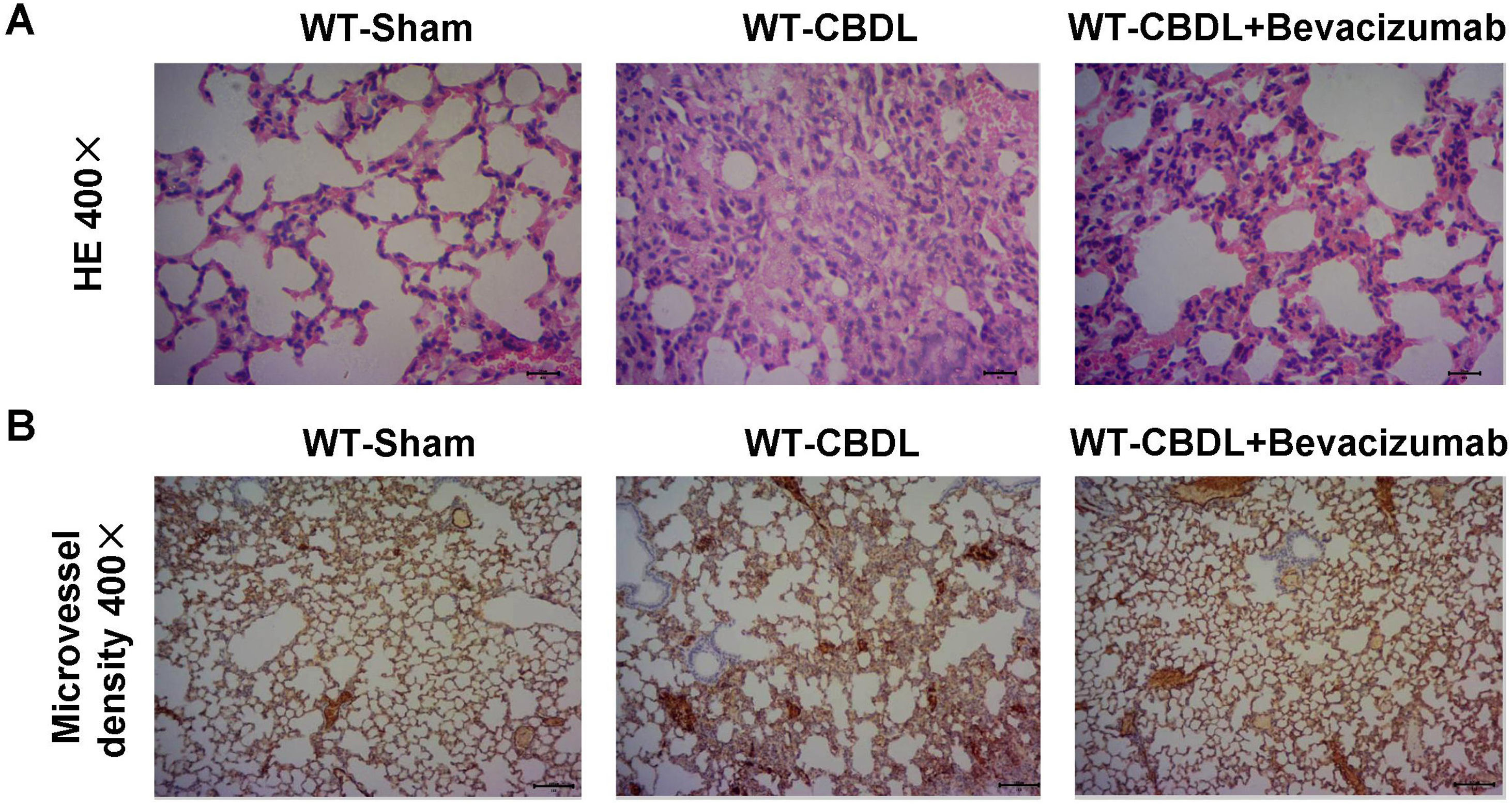
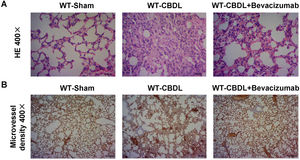
Inhibition of VEGF by Bevacizumab improved intrapulmonary angiogenesis and pathological changes of lung tissuesTo determine whether VEGF participates in hepatopulmonary process, we measured pulmonary changes by H&E staining (Fig. 4A) and the intrapulmonary angiogenesis by a specific CD31 antibody (Fig. 4B) in the WT-CBDL+Bevacizumab group. A lower level of intrapulmonary angiogenesis and smaller pulmonary lesions (Fig. 4B) were found in WT-CBDL+Bevacizumab group versus the WT-CBDL group.
Effect of VEGF blockade by Bevacizumab on pathological changes of the lungs and angiogenesis. A. H&E staining of lung tissues at 400× magnification in WT-sham, WT-CBDL, and WT-CBDL+bevacizumab groups. B. Immunohistochemical staining for intrapulmonary angiogenesis using the CD31 antibody among the groups.
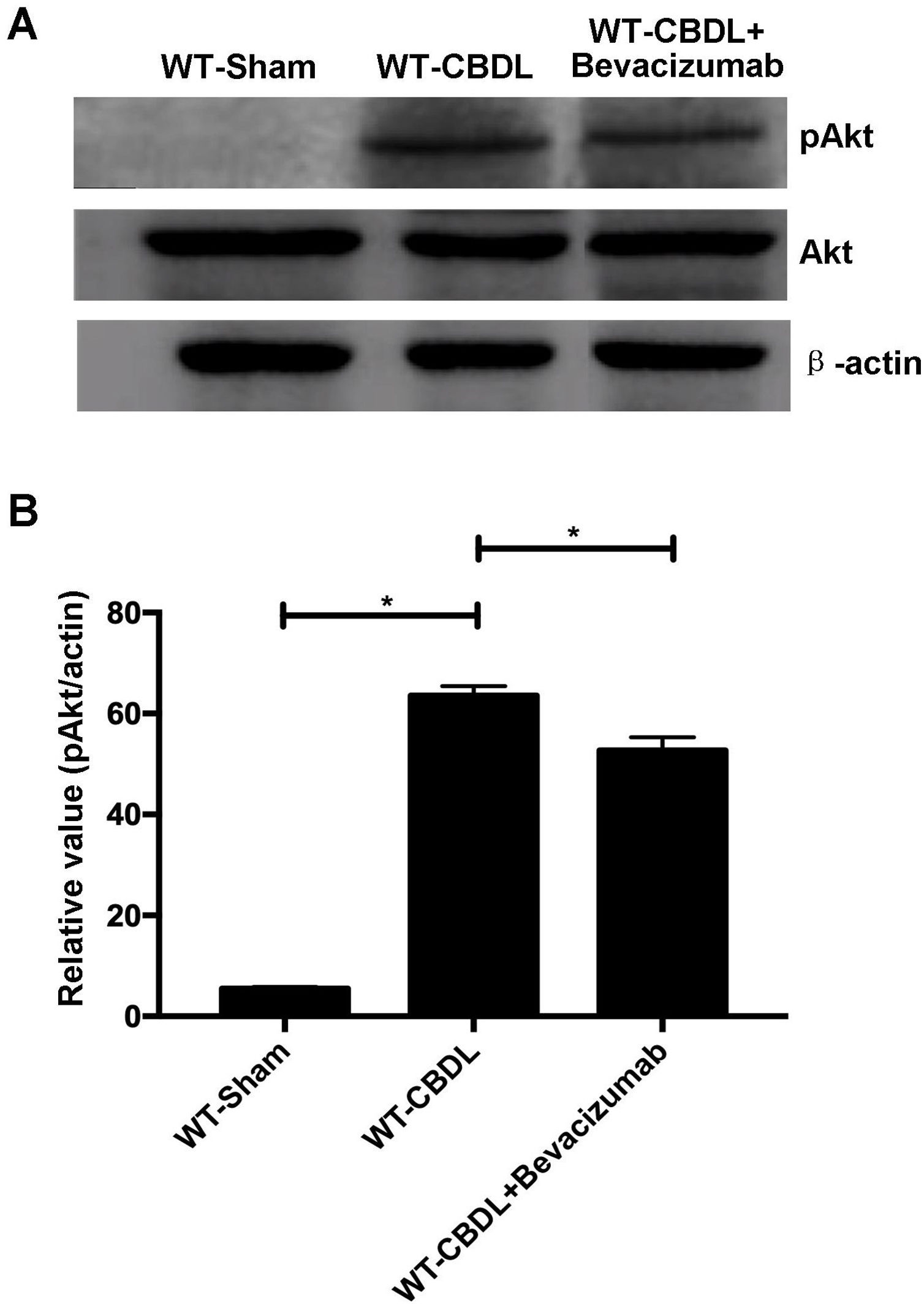
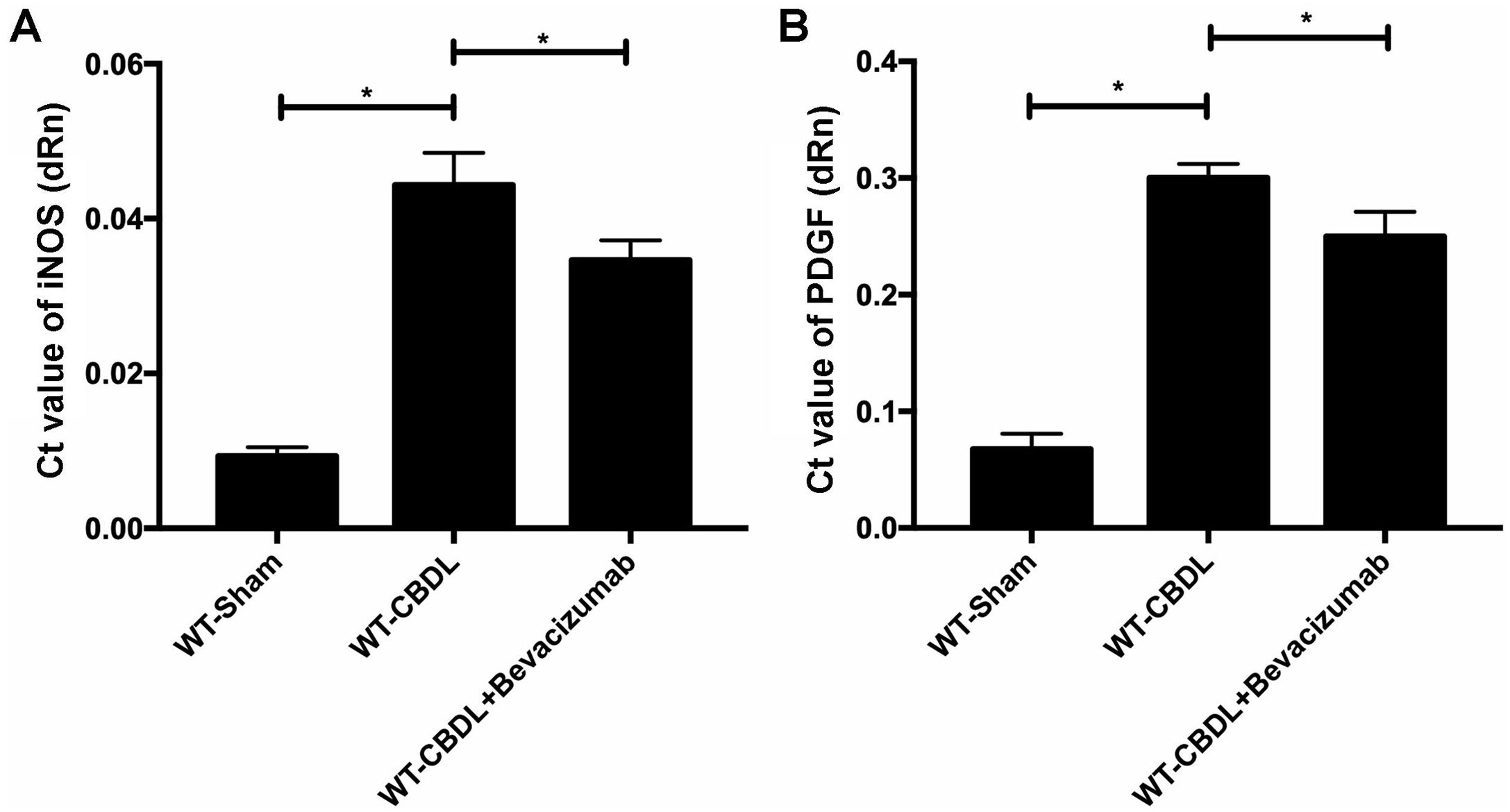
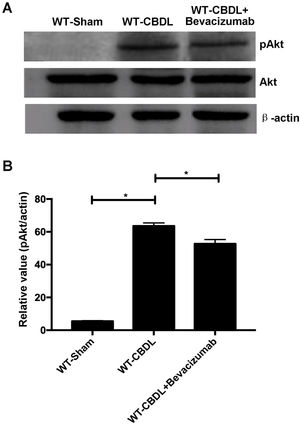
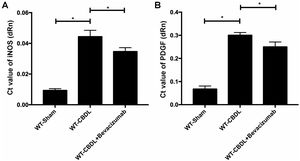
To further explore the pro-angiogenic factors which VEGF may regulate, the expression of pAkt and pERK were measured using western blotting assay (Fig. 5A) The findings showed that production of pAkt (ratio of pAkt/Akt) were significantly increased in WT-CBDL group compared to that in WT-Sham group (Fig. 5B, P<0.05). However, the ratio of pAkt/Akt was markedly alleviated in WT-CBDL+Bevacizumab group compared to that of WT-CBDL group (Fig. 5B, P<0.05). Meanwhile, the production iNOS mRNA and PDGF mRNA was also evaluated using RT-PCR assay. The results indicated that iNOS mRNA (Fig. 6A) and PDGF mRNA (Fig. 6B) were also remarkably alleviated in WT-CBDL+Bevacizumab group compared to that in WT-CBDL group (P<0.05). However, Bevacizumab demonstrated no significant effects on ERK phosphorylation (data not shown).
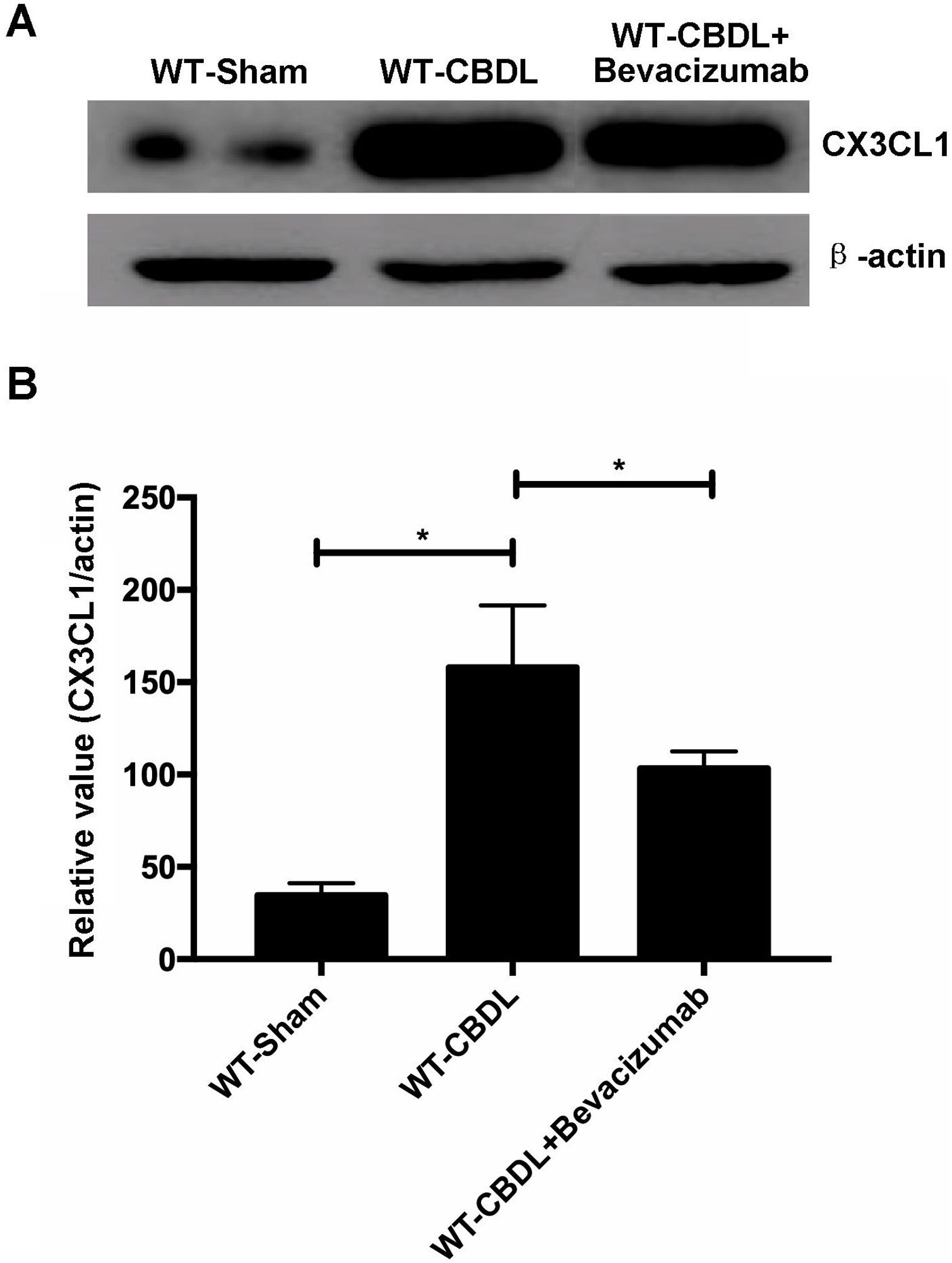
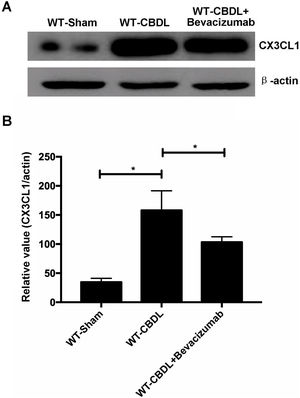
To determine if VEGF can regulate expression of CX3CR1 or its unique ligand CX3CL1, lung expression of CX3CL1 and CX3CR1 was measured using western blot assay (Fig. 7A), in WT-CBDL and WT-CBDL+Bevacizumab groups. We found that production of CX3CL1 was remarkably increased in WT-CBDL group compared to that in WT-Sham group (Fig. 7B, P<0.05). The data also showed that production of CX3CL1 was significantly reduced in WT-CBDL+Bevacizumab group comparing with that of WT-CBDL group (Fig. 7B, P<0.05).
DiscussionThe current study aimed at defining the relationship between CX3CR1 and angiogenesis, and macrophage accumulation in HPS. In the present study, CX3CR1 deficiency was induced by neutralizing antibody, or knockout of genes that regulate the angiogenesis and the activation of pro-angiogenic factors, including pAKT, pERK, inducible nitric oxide synthase (iNOS), vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF).
Angiogenesis is an important hallmark of HPS. Although the relationship between ERK or AKT/iNOS and the angiogenesis was not verified in our study, a previous study demonstrated that the phosphorylation of AKT/iNOS and ERK are important events in angiogenesis.11 Previous studies11,25,26 showed that CX3CR1 can activate AKT/iNOS and ERK signaling pathways in rheumatoid arthritis, osteoarthritis, and human umbilical vein endothelial cells. Angiogenesis and phosphorylation of AKT/iNOS and ERK could also be mediated by CX3CR1 deficiency in our study, which is consistent with previous discoveries.
VEGF, which is a commonly known angiogenic factor, regulates angiogenesis via phosphorylation of AKT and ERK, and the production of endothelial-derived NO through activation of iNOS.13,27 It can also induce the secretion of pro-angiogenic factors, such as the PDGF-BB.12 Our research also demonstrated that VEGF can regulate the angiogenesis and expression of pro-angiogenic factors, including pAKT, iNOS, and PDGF. Although the regulation of pERK by Bevacizumab had no statistical significance (P>0.05) in our research, there are previous studies supporting that VEGF can regulate the phosphorylation of ERK.12,13 Furthermore, the role of PDGF in causing the proliferation of pulmonary artery smooth muscle cells in HPS is closely related to angiogenesis.10 Additionally, in this study we found that CX3CR1 induced the expression of VEGF and PDGF.
In summary, our study suggested that CX3CR1 regulates the angiogenesis and expression of various pro-angiogenic factors including pAKT, pERK, iNOS, VEGF, and PDGF. In addition to angiogenesis, the intrapulmonary accumulation of macrophages is a crucial step in the development of HPS. Growth factors, cytokines, adhesion molecules, and chemokines seem to be involved in the accumulation of macrophages.1,10,15 CX3CL1, which is a member of CX3C family of chemokine, exists in a soluble or a membrane-anchored form. The former functions as a chemoattractant, while the latter as an adhesive molecule. CX3CR1, as a sole receptor of CX3CL1, can firmly bind to CX3CL1, accelerating the accumulation of macrophages in the endothelium.15,28,29 Accumulation of intrapulmonary macrophages was improved by CX3CR1 deficiency in our study, which implied that CX3CR1 may regulate the recruitment of macrophage in the lungs. Macrophage accumulation can lead to a series of cascade signal amplification responses and release of various inflammatory cytokines in the HPS, such as VEGF, iNOS, PDGF, and TNF-α.1,10 TNF-α released from the accumulated macrophage induced the production of CX3CL1, which is expressed in pulmonary microvascular endothelial cells. CX3CL1 contributed to angiogenesis via binding its unique receptors CX3CR1.11 Summarily, the accumulation of macrophages in the lungs in HPS may have a close relationship with angiogenesis, which is worthy of paying further attention. Interestingly, our study demonstrated that VEGF could induce the expression of the ligand of CX3CR1, CX3CL1, which is consistent with previous research showing that chemokine were modulated to a lower extent by VEGF.12
In the present study, CX3CR1 could regulate the angiogenesis and expression of various pro-angiogenic factors, including pAKT, pERK, iNOS, PDGF, and VEGF. In addition, CX3CR1 contributed to the accumulation of macrophage, which may prompt the development of angiogenesis in HPS. Our research is based on animal experiments for clarifying the effects of CX3CR1 on angiogenesis. Actually, effects of CX3CR1 on angiogenesis has been widely proven in vitro (cell) level in the previous studies,30–32 therefore, this study has not been repeated the experiments in vitro. Based on the previous findings and the present results, a close association between CX3CR1 and angiogenesis has been fully proven. Therefore, CX3CR1 may be regarded as a target for medical intervention of HPS in the future.
ConclusionThe present findings revealed that CX3CR1 regulates the angiogenesis and production of various pro-angiogenic factors including pAKT, pERK, iNOS, VEGF and PDGF in the HPS. Meanwhile, CX3CR1 could also contribute to the accumulation of macrophage in the lung.
Conflict of interestAuthors declare no competing financial or commercial interests in this manuscript.
This work was supported by the National Natural Science Foundation of China (No. 81560106) and the Basic Research Plan of Guizhou Science and Technology Program (No. [2017]1145).