The life cycle of the hepatitis C virus (HCV) is closely associated with lipid metabolism. Recently, NPC1L1 (a cholesterol transporter) has been reported to function as an HCV receptor. This receptor is expressed in the hepatocyte canalicular membrane and in the intestine; serving as a key transporter for the cholesterol enterohepatic cycle.
ObjectivesWe hypothesized that HCV might have a similar cycle, so we aimed to study the presence of HCV in bile and stools of infected patients.
Materials and methodsBlood, feces, and duodenal bile samples were collected from patients infected with HCV. The biliary viral load was normalized to the bile salt concentration of each sample and the presence of HCV core protein was also evaluated. A total of 12 patients were recruited. HCV RNA was detected in the bile from ten patients.
ResultsThe mean viral load was 2.5log10IU/60mg bile salt. In the stool samples, HCV RNA was detected in ten patients (mean concentration 2.7log10IU/g of feces).
ConclusionsHCV RNA is readily detectable and is present at relatively high concentrations in the bile and stool samples of infected patients. This may be relevant as a source of infection in men who have sex with men. Biliary HCV secretion may perhaps play a role in the persistence of viral infection via an enterohepatic cycle of the virus or intrahepatic spread.
El ciclo de vida del virus de la hepatitis C (VHC) está estrechamente ligado al metabolismo lipídico. Recientemente, se ha descrito que el NPC1L1 (un transportador del colesterol) actúa como un receptor del VHC. Este receptor se expresa en la membrana canalicular de los hepatocitos y en el intestino, y actúa como uno de los principales transportadores durante la circulación enterohepática del colesterol.
ObjetivosPlanteamos la hipótesis de que el VHC podría tener un ciclo similar, por lo que nuestro objetivo fue estudiar la presencia del VHC en la bilis y en las heces de pacientes infectados.
Materiales y métodosSe obtuvieron muestras de sangre, heces y bilis duodenal de pacientes infectados por el VHC. La concentración vírica en la bilis se normalizó respecto a la concentración de sales biliares de cada muestra y también se evaluó la presencia de la proteína central del VHC. Se reclutaron un total de 12 pacientes. Se detectó el ARN del VHC en la bilis de 10 pacientes.
ResultadosLa media de la concentración vírica fue 2,5log10UI/60mg de sales biliares. En las muestras de heces, se detectó el ARN del VHC en 10 pacientes (media de la concentración 2,7log10UI/g de heces).
ConclusionesEl ARN del VHC es fácilmente detectable y está presente en concentraciones relativamente elevadas en las muestras de bilis y heces de pacientes infectados. Esto puede tener importancia como foco de infección en varones que mantienen relaciones sexuales con otros varones. Es posible que la secreción biliar del VHC pueda desempeñar un papel en la persistencia de la virosis a través de la circulación enterohepática del virus o la propagación intrahepática.
Chronic hepatitis C affects more than 170 million individuals who are at risk of developing cirrhosis and hepatocellular carcinoma.1 The impact of this disease is evident, and the mortality due to hepatitis C virus (HCV) infection has superseded the mortality from HIV infections in the United States.2 New, oral direct-acting antivirals (DAAs) are showing remarkable results. Nevertheless, DAAs have some shortcomings, such as high cost, risk of developing resistance-associated variants and drug-drug interactions.3–5 A better understanding of the viral cycle may facilitate the development of alternative treatment strategies or strategies that complement DAAs.
HCV circulates in human serum associated with several lipoproteins forming lipo-viral particles (LVPs).6–8 LVP formation occurs during lipoprotein synthesis in hepatocytes, which is a process that may shield the virus from neutralizing antibodies.9 LVPs are characterized by their low density, presence of apolipoprotein B (apoB) and enrichment of triglycerides.10 HCV is not evenly distributed in the different plasmatic lipid fractions and is especially abundant in the very-low-density fractions, which comprise triglyceride-rich lipoproteins (TRL).11
TRL particles have a density less than 1.006g/mL and have a core made of triglycerides and cholesterol esters surrounded by a phospholipid monolayer of free cholesterol, apoB and other lipoproteins.12 There are two types of TRLs: very-low-density lipoproteins (VLDLs) and chylomicrons. VLDLs are assembled with one apoB100 molecule per particle and are secreted by hepatocytes, whereas chylomicrons contain one molecule of apoB48, which is a truncated form of apoB and is enterocyte specific.13 In humans, apoB100 is synthesized primarily by the liver, whereas apoB48 is synthesized only in the intestines. Intriguingly, apoB100 and apoB48 are equally represented in LVPs purified from HCV-infected patients.14 This observation led to the suggestion that the intestine could be a reservoir of HCV and that enterocytes could contribute to an estimated 18% of the plasmatic viral load.14 An intestinal contribution to the overall HCV load in infected patients could be explained either by viral extrahepatic replication in the enterocyte or by the intestinal absorption of HCV.
Based on evidence suggesting that enterocytes can be a reservoir for HCV,14,15 it is plausible that HCV has an enterohepatic cycle similar to that of cholesterol: secretion into the bile, reabsorption in the enterocyte and secretion bound to chylomicrons. This possibility would imply that some HCV particles could escape reabsorption by enterocyte, shedding to the feces of infected patients. However, detection of HCV particles in bile and stools of infected patients remains controversial. Thus, we designed a study to evaluate the presence of HCV in the bile and feces of Chilean patients chronically infected with the virus. We also assessed whether the viral load correlated with different compartments and plasmatic cholesterol and triglyceride levels.
Materials and methodsStudy populationPatients chronically infected with HCV who were followed up at the Viral Hepatitis Unit of the Clinical Hospital, Pontificia Universidad Católica de Chile (PUC) were recruited and invited to participate in this study. The protocols and consent forms used in this study were approved by the Ethical Review Board of the Faculty of Medicine, PUC (protocol number 12-199) and were in accordance with local regulations and the ethical principles originated in the Declaration of Helsinki and with ICH/Good Clinical Practice. Patients were informed about the study and completed a written consent form before donating blood, bile and stool. Patient inclusion criteria were the following: 1) chronic hepatitis C defined as detectable HCV RNA in the plasma for more than 6 months; 2) age greater than 18 years; 3) compensated liver disease (bilirubin<3mg/dL unless having Gilbert's syndrome, albumin>3g/dL, INR<2, no hepatic encephalopathy, no ascites or recent (1 month) history of variceal bleeding); 4) no history of cholecystectomy or known gallstones; 5) no current HCV antiviral treatment; 6) no medications for dyslipidaemia within the preceding 2 months; 7) no abdominal surgery that could alter the biliary or intestinal anatomy; 8) HCV RNA levels>10,000IU/mL; 9) no evidence of sitosterolemia; 10) negative pregnancy test in urine (for females); and 11) signed informed consent document. Subjects who had HIV or hepatitis B virus (HBV) coinfection, who were undergoing anticancer chemotherapy, or who had received previous antiviral treatment with DAAs were excluded from the study.
Liver fibrosis was assessed via a liver biopsy or Fibroscan and was categorized according to the METAVIR system.16
Peripheral blood, bile and feces were collected for biochemical and virological assessments. Standard clinical tests, including a lipid profile (total cholesterol, triglycerides, HDL cholesterol, LDL cholesterol), a liver profile (ALT, AST, GGTP, alkaline phosphatase, total and conjugated bilirubin, prothrombin time/INR) and HCV RNA levels were determined from plasma samples.
Sample collection and biochemical analysisPeripheral blood was collected into EDTA-Vacutainer™ tubes (Becton Dickinson, Franklin Lakes, NJ, USA), and plasma samples recovered from the donors were stored at 80°C until processed. Bile samples were obtained using the technique of biliary drainage (duodenal aspirate). This technique is routinely performed in our clinical service for both clinical and research purposes.17 Briefly, after 8h fasting, patients underwent an upper digestive endoscopy under conscious sedation with midazolam. An oro-duodenal small diameter tube was positioned with the endoscope. After removing the endoscope, the gallbladder was stimulated with amino acids and bile was aspirated from the duodenum.18 Bile samples were stored at −80°C until processing. Serum lipids and liver function tests were determined by standard clinical chemical assays. Biliary cholesterol and bile acids were measured by enzymatic methods19,20 and phospholipids were assessed by a colorimetric method.21 Stool samples were preserved in 5mL of RNALater™ Stabilization Solution (Ambion, Thermo Fisher Scientific, Waltham, MA, USA) from the time of collection and were frozen at −80°C for later analysis.22 All measurements in bile and feces were corrected according to the input volume.
Viral load determination and HCV genotypingHCV RNA levels in plasma, bile and feces were determined using the COBAS TaqMan HCV Test™ v2.0 (Roche Molecular Systems, Inc, Rotkreuz, Switzerland) with a lower limit of quantification (LLOQ) of 25IU/mL and lower limit of detection (LLOD) of 9.3IU/mL.23 HCV genotyping of the virus present in the plasma was performed using the reverse hybridization line probe assay INNO-LiPA HCV II kit™ (Innogenetics, Ghent, Belgium) according to the manufacturer's instructions.24,25
RNA extraction and RT-PCR in stool samplesA technique for extracting HCV RNA from stool has been described elsewhere.26 For convenience and more reliable results, we used the PowerMicrobiome™ RNA Isolation Kit (MoBio Laboratories, Carlsbad, CA, USA), which is optimized for removing PCR inhibitors from the stool and gut material. To rule out the possibility that fecal HCV RNA or core protein is a result of contamination from occult blood loss in the gut, an immunochemical assay to detect human hemoglobin (Hemosure™) was performed on the stool samples. This assay is highly specific and sensitive (50ng human hemoglobin/mL), requires no specific diet and is readily available at our institution.27
The HCV RNA extracted by the PowerMicrobiome™ RNA Isolation Kit from the feces was eluted in 60μL of nuclease-free water. Thirty microliters diluted in 470μL of 1× PBS were used to quantify the HCV RNA using the COBAS TaqMan HCV Test™ v2.0. In each case, the results (in IU) were corrected by the input in milligrams of stool and then by the Power PowerMicrobiome™ elution volume. In parallel, the HCV RNA was detected using a one-step reverse transcription (RT) polymerase chain reaction (PCR) using the SuperScriptTM III one-step RT-PCR system with Platinum-Taq DNA Polymerase™ (Invitrogen, Life Technologies, Carlsbad, CA, USA) kit with a lower limit of detection of 1×103 HCV genomic copies per mL following a protocol previously described.25
Determination of core protein levelsThe HCV core protein levels are correlated with HCV RNA levels and may be used as an alternative marker of HCV infection. The level of core protein in the plasma, bile and feces was quantified using the ARCHITECT HCV Ag assay™ (Abbott Laboratories, Abbott Park, IL, USA) following the manufacturer's instructions. The dynamic range of this assay is 3–20,000fmol/L. For this purpose, samples frozen at −80°C (thawed once) were used.
Data analysisThe HCV RNA viral load, given its exponential distribution, was logarithmically transformed using base 10, and geometrical means were used. Values below the limit of quantification (25IU/mL) were computed as 12IU/mL, and undetectable values were computed as 4IU/mL for statistical analyses. Similarly, undetectable HCV core protein determinations were considered to be 2fmol/L for the statistical analyses. As previously mentioned, the viral load determinations in the bile were normalized to the bile acid concentration for a meaningful comparison, and the viral loads were expressed as IU/60mg bile salts, which is the normal concentration of bile salts per mL of bile.28,29 Viral load determinations in feces were expressed as IU/g of stool. To determine if the viral load was correlated with the amounts of core protein and between compartments, a Pearson correlation analysis was performed using Graph Pad V6 and SPSS v20. A p value <0.05 was considered significant.
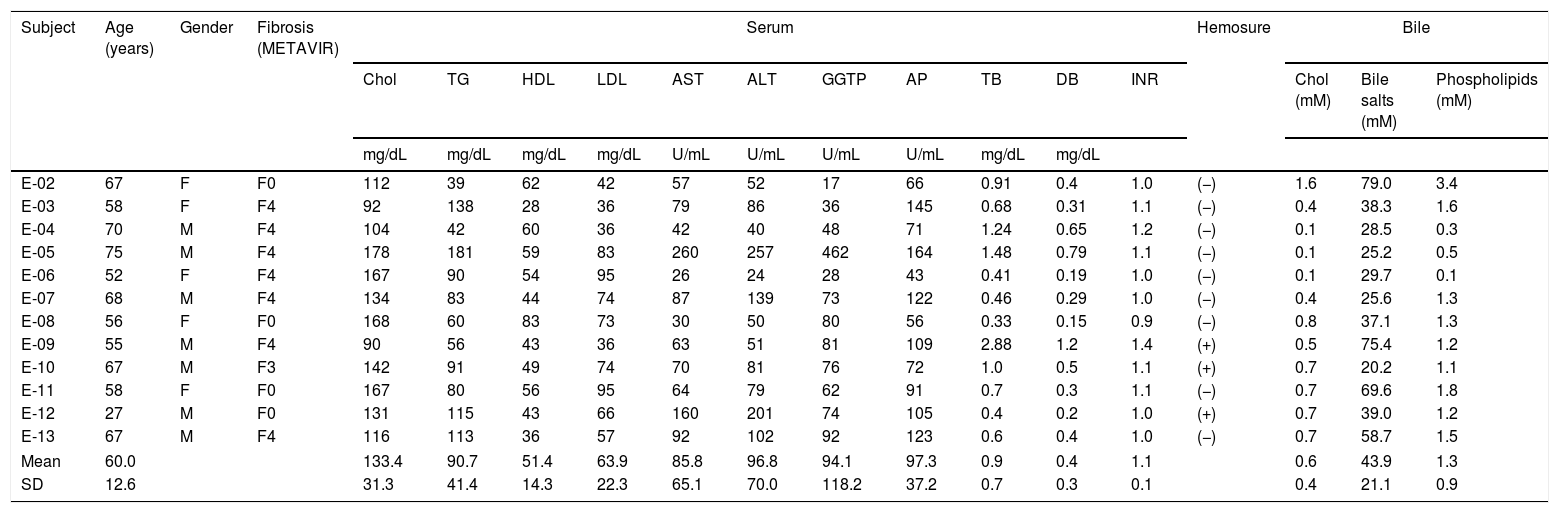
ResultsPatient characteristicsThe clinical features and the serum and bile compositions at the time of sample collection for the twelve patients included in this study are shown in Table 1. The average age of patients was 60 years (range 27–75 years), with roughly equal gender distribution (male 58%). An HCV genotypic analysis confirmed the prevalence of genotype 1b in the majority of patients (n=9), which is consistent with the epidemiology in our country.30 Seven of the twelve patients were cirrhotic at the time of sampling. All patients with cirrhosis were well compensated. Two patients had failed to a previous course of treatment with peginterferon alfa 2b plus ribavirin; the remaining patients were treatment naïve.
Patients’ clinical features and serum and bile compositions.
| Subject | Age (years) | Gender | Fibrosis (METAVIR) | Serum | Hemosure | Bile | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chol | TG | HDL | LDL | AST | ALT | GGTP | AP | TB | DB | INR | Chol (mM) | Bile salts (mM) | Phospholipids (mM) | |||||
| mg/dL | mg/dL | mg/dL | mg/dL | U/mL | U/mL | U/mL | U/mL | mg/dL | mg/dL | |||||||||
| E-02 | 67 | F | F0 | 112 | 39 | 62 | 42 | 57 | 52 | 17 | 66 | 0.91 | 0.4 | 1.0 | (−) | 1.6 | 79.0 | 3.4 |
| E-03 | 58 | F | F4 | 92 | 138 | 28 | 36 | 79 | 86 | 36 | 145 | 0.68 | 0.31 | 1.1 | (−) | 0.4 | 38.3 | 1.6 |
| E-04 | 70 | M | F4 | 104 | 42 | 60 | 36 | 42 | 40 | 48 | 71 | 1.24 | 0.65 | 1.2 | (−) | 0.1 | 28.5 | 0.3 |
| E-05 | 75 | M | F4 | 178 | 181 | 59 | 83 | 260 | 257 | 462 | 164 | 1.48 | 0.79 | 1.1 | (−) | 0.1 | 25.2 | 0.5 |
| E-06 | 52 | F | F4 | 167 | 90 | 54 | 95 | 26 | 24 | 28 | 43 | 0.41 | 0.19 | 1.0 | (−) | 0.1 | 29.7 | 0.1 |
| E-07 | 68 | M | F4 | 134 | 83 | 44 | 74 | 87 | 139 | 73 | 122 | 0.46 | 0.29 | 1.0 | (−) | 0.4 | 25.6 | 1.3 |
| E-08 | 56 | F | F0 | 168 | 60 | 83 | 73 | 30 | 50 | 80 | 56 | 0.33 | 0.15 | 0.9 | (−) | 0.8 | 37.1 | 1.3 |
| E-09 | 55 | M | F4 | 90 | 56 | 43 | 36 | 63 | 51 | 81 | 109 | 2.88 | 1.2 | 1.4 | (+) | 0.5 | 75.4 | 1.2 |
| E-10 | 67 | M | F3 | 142 | 91 | 49 | 74 | 70 | 81 | 76 | 72 | 1.0 | 0.5 | 1.1 | (+) | 0.7 | 20.2 | 1.1 |
| E-11 | 58 | F | F0 | 167 | 80 | 56 | 95 | 64 | 79 | 62 | 91 | 0.7 | 0.3 | 1.1 | (−) | 0.7 | 69.6 | 1.8 |
| E-12 | 27 | M | F0 | 131 | 115 | 43 | 66 | 160 | 201 | 74 | 105 | 0.4 | 0.2 | 1.0 | (+) | 0.7 | 39.0 | 1.2 |
| E-13 | 67 | M | F4 | 116 | 113 | 36 | 57 | 92 | 102 | 92 | 123 | 0.6 | 0.4 | 1.0 | (−) | 0.7 | 58.7 | 1.5 |
| Mean | 60.0 | 133.4 | 90.7 | 51.4 | 63.9 | 85.8 | 96.8 | 94.1 | 97.3 | 0.9 | 0.4 | 1.1 | 0.6 | 43.9 | 1.3 | |||
| SD | 12.6 | 31.3 | 41.4 | 14.3 | 22.3 | 65.1 | 70.0 | 118.2 | 37.2 | 0.7 | 0.3 | 0.1 | 0.4 | 21.1 | 0.9 | |||
Fibrosis: liver fibrosis by METAVIR scale (F0 to F4). Chol: total cholesterol. TG: triglycerides. HDL: high-density lipoprotein. LDL: low-density lipoprotein. AST: aspartate aminotransferase. ALT: alanine aminotransferase. GGTP: gamma-glutamyl transpeptidase. AP: alkaline phosphatase. TB: total bilirubin. DB: direct bilirubin. SD: standard deviation.
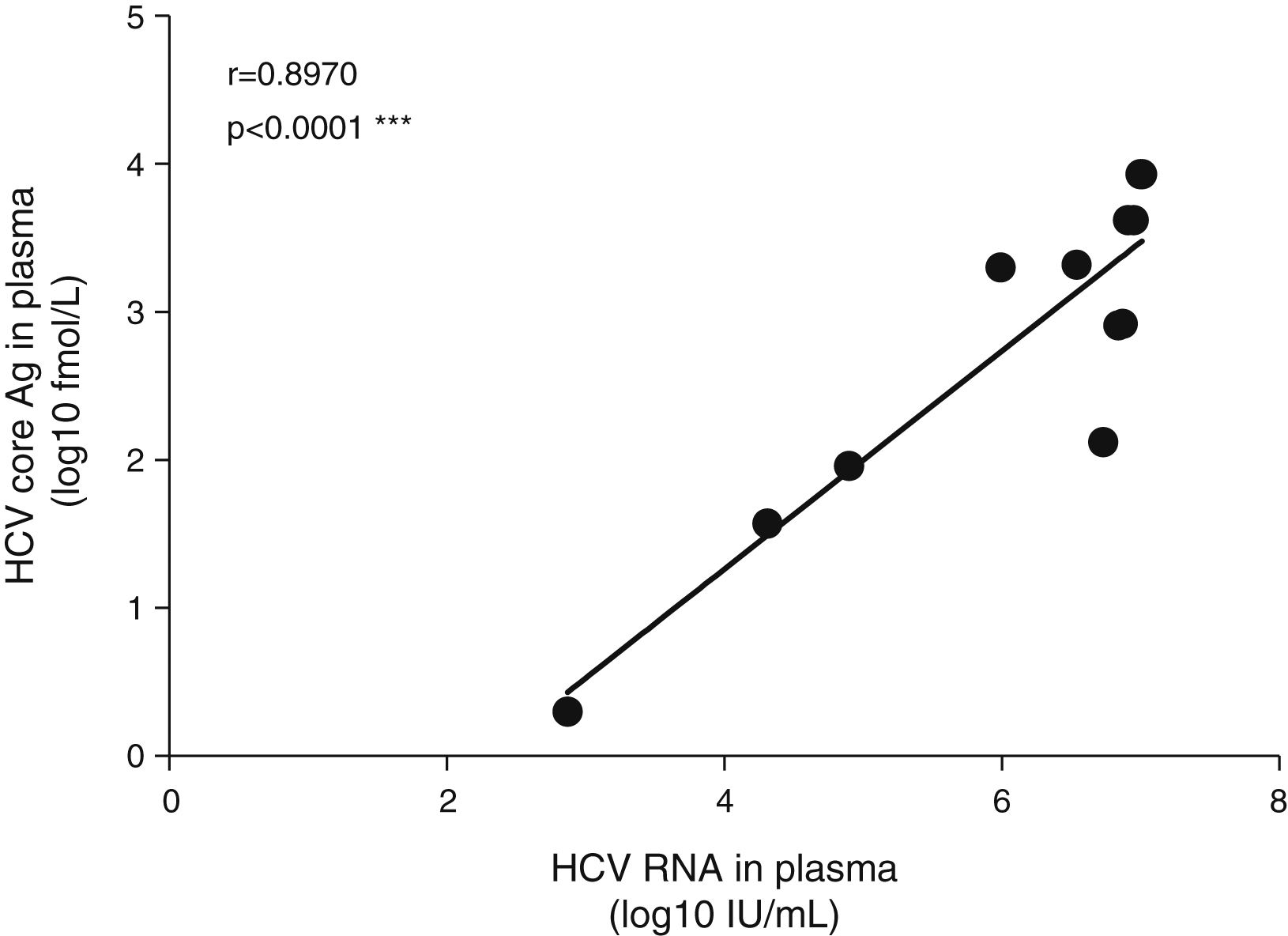
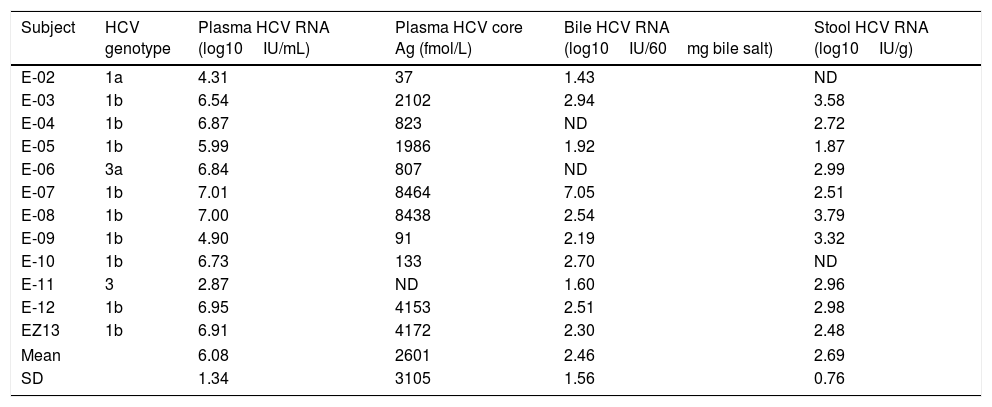
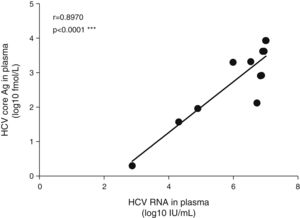
The mean HCV viral load in the plasma was 6.08±1.34log10IU/mL. As expected, the HCV core Ag in plasma showed a high correlation with the plasmatic HCV viral load (Fig. 1). We detected HCV RNA in the bile of 10 patients (83%), and the mean viral load was 2.5log10IU/60mg bile salts (range: undetectable–7.0log10IU/60mg bile salt), which is (on average) 3.6log10 less than the average plasma viral load (Table 2). In feces, HCV RNA was alsodetected in 10 patients (83%). The average viral load was 2.7log10IU/g (range: undetectable–7log10IU/g). The internal control RNA was detected in all samples indicating the lack of RT-PCR inhibitors. Patients with a positive immunochemical fecal occult blood test did not exhibit greater levels of biliary or fecal HCV viral load. The presence of the HCV core protein was confirmed in the bile of only one patient and was not detected in any of the evaluated stool samples.
HCV genotype, viral load and HCV core protein quantification in plasma, bile and stool samples from patients chronically infected with hepatitis C virus.
| Subject | HCV genotype | Plasma HCV RNA (log10IU/mL) | Plasma HCV core Ag (fmol/L) | Bile HCV RNA (log10IU/60mg bile salt) | Stool HCV RNA (log10IU/g) |
|---|---|---|---|---|---|
| E-02 | 1a | 4.31 | 37 | 1.43 | ND |
| E-03 | 1b | 6.54 | 2102 | 2.94 | 3.58 |
| E-04 | 1b | 6.87 | 823 | ND | 2.72 |
| E-05 | 1b | 5.99 | 1986 | 1.92 | 1.87 |
| E-06 | 3a | 6.84 | 807 | ND | 2.99 |
| E-07 | 1b | 7.01 | 8464 | 7.05 | 2.51 |
| E-08 | 1b | 7.00 | 8438 | 2.54 | 3.79 |
| E-09 | 1b | 4.90 | 91 | 2.19 | 3.32 |
| E-10 | 1b | 6.73 | 133 | 2.70 | ND |
| E-11 | 3 | 2.87 | ND | 1.60 | 2.96 |
| E-12 | 1b | 6.95 | 4153 | 2.51 | 2.98 |
| EZ13 | 1b | 6.91 | 4172 | 2.30 | 2.48 |
| Mean | 6.08 | 2601 | 2.46 | 2.69 | |
| SD | 1.34 | 3105 | 1.56 | 0.76 | |
ND: not detectable. SD: standard deviation.
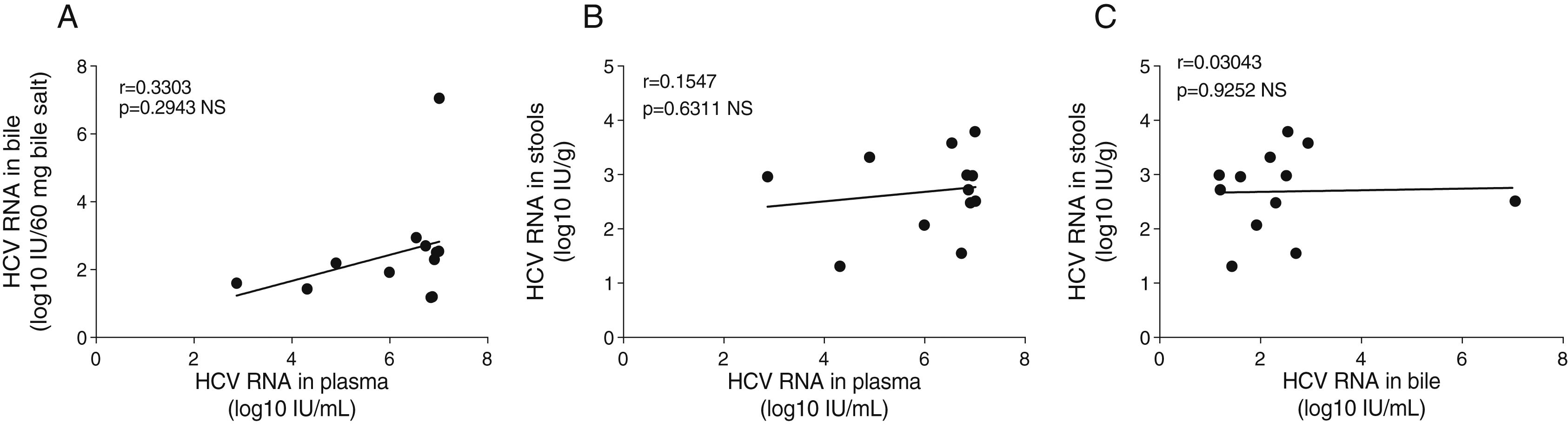
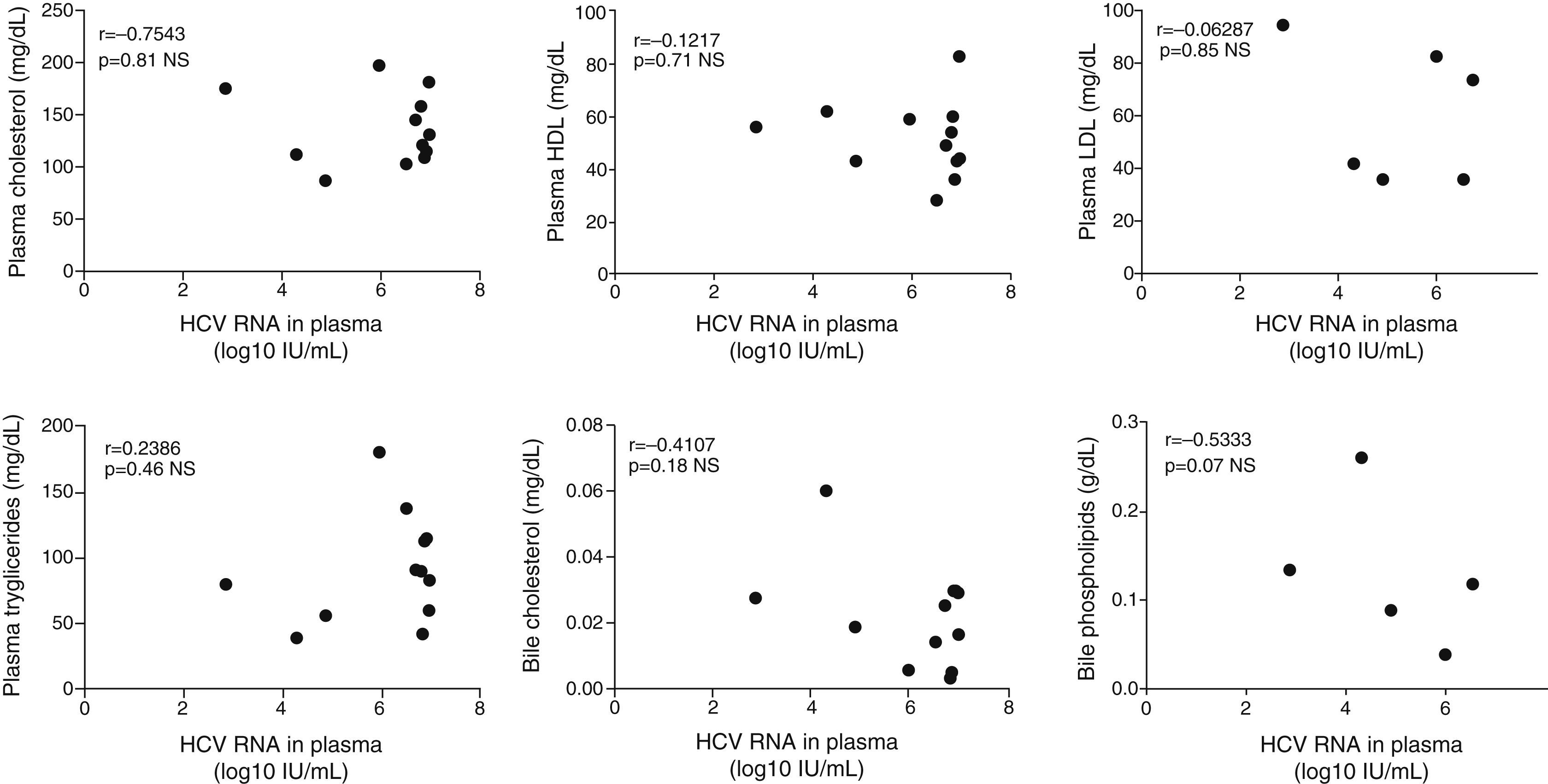
The plasmatic HCV viral load was not significantly associated with the biliary HCV RNA titers or with the fecal viral load (Fig. 2A and B). Similarly, biliary and stool viral loads were not correlated (Fig. 2C). The standardized biliary viral load was weakly correlated with the presence of plasmatic HCV core protein (r=0.603, p=0.038). The fecal viral load was not significantly associated with the lipidic components of plasma or bile (Fig. 3). The plasma viral load did not show a significant association with the concentration of the plasmatic or biliary lipidic components (Fig. 3).
The existence of extrahepatic reservoirs for HCV replication has long been suspected but remains highly controversial. Previous studies, including several from our laboratory,24,25,31,32 have observed HCV RNA to be associated with peripheral blood mononuclear cells (PBMCs). Additionally, Deforges et al. reported that the small intestine can be infected by HCV and strongly suggested that this organ may be a reservoir for HCV and a source of LVPs.15 The main evidence of HCV replication in this extrahepatic compartment was the detection of HCV non-structural proteins NS3 and NS5A in intestinal biopsies. HCV protein expression was observed in enterocytes, which are cells that synthesize and secrete chylomicrons. Although this evidence strongly suggests viral replication, herein, we put forward an alternative and yet unexplored possibility. HCV may also have an enterohepatic cycle similar to the cycle described for cholesterol. If true, HCV would constantly be secreted into the bile and would be reabsorbed by the enterocyte and secreted back into the system bound to chylomicrons. Furthermore, the presence of HCV would be expected in the bile (secreted) and stool (not reabsorbed) of chronically infected individuals. In this model, HCV replication in the enterocyte would not be required to explain the apoB48 and high triglyceride content of LVPs. In this study, to indirectly evaluate this alternative model, by establishing whether HCV is present in the bile and stool of chronically infected HCV patients. In these studies we not only detected HCV RNA but we also quantified its levels in each samples. When comparing the HCV viral load in the bile and plasma of the same individual, the viral load was an average of 3.6log10 less in the bile compared to the plasma. No correlation was found between the viral titers in the different compartments and the concentration of the lipidic components of the plasma or bile. Nonetheless, the results of this study suggest that HCV can be shed into the bile and can later be found in the stool.
There has been an alarming increase in incidence of acute HCV infection in HIV-infected men who have sex with men (MSM),33 which has been speculated to be related to permucosal transmission.34 The presence of HCV RNA in measurable quantities in stools of infected patients may explain in part these outbreaks.
Three earlier studies reported the amplification of HCV RNA from the bile of chronically infected patients.35–37 These studies detected viral RNA in 19/34 patients (56%), whereas we were able to detect HCV RNA in 10/12 patients (83%). One important difference between our study and the previous report is that we quantified the HCV RNA in the bile using the Roche COBAS TaqMan assay. We also quantified the HCV RNA in the bile components and used this value to normalize the HCV RNA concentration. In our opinion, this normalization provided a better comparison between samples obtained from different patients.
HCV core antigen was determined using a commercial kit, but we could detect this protein in the bile of just one patient and none in the stool samples. Heidrich et al.38 in a recent study tested the stools of 98 chronically infected patients and were able to detect HCV RNA in 69% of the samples, but HCV core protein with the same assay (ARCHITECT) in only 24% of samples. Unfortunately the core protein level was not provided nor was the HCV RNA level determined.
Aside from the already commented study of Heidrich, the presence of HCV RNA in stool has been tested in other two studies. In the earliest paper,39 attempts to identify HCV RNA in secretions, including feces, were unsuccessful. A second study was able to detect and measure the HCV viral load in relatively high amounts (1–3 logs lower than plasma) in feces in 4 out of 6 chronically infected patients.40 However, in that report, the authors did not rule out blood contamination as the possible source of viral RNA. To overcome this limitation, in our study, a highly sensitive immunochemical method was used to detect blood in the stool, which was negative in 9 of the 12 patients. The three remaining patients did not exhibit a greater viral load in the bile or stool, which suggests a limited role of blood contamination as a source of virus in the tested samples.
One of our objectives was to determine whether viral particles were shed into the bile and stool of chronically infected HCV patients. We also sought to detect the presence of an HCV structural protein. Unfortunately, we were unable to detect the HCV core protein in these secretions using the Abbott ARCHITECHT assay. This failure could be explained by either the technical limitations of this assay, which is designed to detect viral protein in plasma samples or by the lower sensitivity of the assay compared to the COBAS TaqMan test considering that the bile and stool samples exhibited lower viral loads when compared to plasma. Despite the lack of core protein detection, the presence of viral RNA suggests that the virus, even though at lower quantities, can be found both in the bile and stool. This observation supports a model in which the virus is shed into the bile where it can later be excreted or reabsorbed by the enterocyte.
The proposed model raises a number of additional questions. How can the virus be reabsorbed by the enterocytes or any other intestinal cell if these cells are known to lack the CD81 virus receptor? The discovery of new viral entry factors has revealed that several of these factors are related to lipid metabolism, including the LDL receptor,41 scavenger receptor class B type I (SR-BI),42 and NPC1L1.43 Interestingly, SR-B1 is found on the apical and basal poles of enterocytes and may provide an alternative entry pathway for the virus.44,45 Additionally, NPC1L1 is also expressed on the apical membrane of enterocytes.43,46 Previous studies have proposed NPC1L1 as a target for HCV therapeutic interventions.43 In this context, our findings are very clinically relevant and suggest that HCV reabsorption by enterocytes could be targeted for clinical intervention using an NPC1L1 antagonist, such as ezetimibe, which is a drug that disrupts the enterohepatic cycle of endogenous cholesterol.47 Ezetimibe binds to the extracellular loop C at a different domain than cholesterol and the HCV virion and blocks the internalization of the NPC1L1/cholesterol complex.48 Therefore, antagonizing the NPC1L1 cholesterol receptor with ezetimibe may have an inhibitory effect in hepatic and/or intestinal uptake.
The presence of HCV RNA in the bile and stool, together with the description that HCV circulates in the plasma equally bound to hepatic-derived apoB100 and intestinally derived apoB48,14 leads to the striking possibility that the blockage of intestinal NPC1L1 can disrupt the possible enterohepatic mode of HCV circulation. While compelling, this hypothesis remains highly speculative and must be specifically evaluated in clinical studies.
Conflict of interestsA.S. has received fees as a speaker for MSD, Roche, BMS, he has participated in Advisory Board Meetings for MSD, Abbvie, Gilead, Vertex, Roche, and Janssen and he has stock ownership of Gilead and Achillion. The others authors declare that they have no conflict of interest.
This study was funded by grants FONDECYT #1130357 from the Comisión Nacional de Investigación Científica y Tecnológica (CONICYT) to AS and MLL, FONDECYT #1130303 to JFM and P09/016-F of Iniciativa Científica Milenio del Ministerio de Economía, Fomento y Turismo to MLL.