Eosinophilic oesophagitis (EoE) is a disease caused by an immune response to food antigens in contact with the oesophageal mucosa. Its diagnosis is defined by the combination of oesophageal dysfunction symptoms and inflammation of the oesophageal mucosa predominantly by eosinophils. Its chronic course and frequent progression to subepithelial fibrosis and oesophageal strictures indicate the need for treatment. The information provided by recent clinical trials and systematic reviews has led to the development of new clinical guidelines, endorsed by several European scientific societies. This review includes the most relevant aspects of the new guidelines, updates the EoE concept and reports its epidemiology and risk factors, associated conditions and its natural history in children and adults. Diagnostic criteria are provided, and tests for EoE diagnosis and monitoring and therapeutic options are analysed based on the best scientific evidence and consensus opinion of experts.
La esofagitis eosinofílica (EoE) es una enfermedad causada por una respuesta inmune frente a antígenos alimentarios en contacto con la mucosa esofágica. Su diagnóstico se define por la combinación de síntomas de disfunción esofágica e inflamación de la mucosa esofágica, predominantemente eosinofílica. Su curso crónico y la frecuente progresión hacia fibrosis subepitelial y estenosis esofágicas indican la necesidad de tratamiento. La información proporcionada por ensayos clínicos y revisiones sistemáticas recientes ha permitido desarrollar una nueva guía clínica, avalada por varias sociedades científicas europeas. Esta revisión recoge los aspectos más relevantes de la nueva guía, actualiza el concepto de EoE, informa de su epidemiología y factores de riesgo, condiciones asociadas y su historia natural en niños y adultos. Se proporcionan los criterios diagnósticos, se analizan las pruebas para diagnóstico y monitorización de la EoE, y las opciones terapéuticas en base a la mejor evidencia científica y la opinión consensuada de expertos.
Eosinophilic oesophagitis (EoO) is a chronic inflammatory disease of the oesophagus. It is characterised clinically by symptoms related to oesophageal dysfunction and histologically by eosinophil-predominant inflammation of the oesophagus. Since the initial characterisation of EoO as a separate clinical pathological syndrome from eosinophilic gastroenteritis in the early 1990s by two independent groups of researchers,1,2 its prevalence has increased dramatically, affecting at least one in every 2000 inhabitants in Europe and North America.3 As a result, EoO is currently the second most common cause of chronic oesophagitis after gastro-oesophageal reflux disease (GORD) and the main cause of dysphagia and food impaction in children and young adults. Although it is not associated with mortality or risks of malignancy, its chronic nature and progressive behaviour adversely impact patients’ quality of life.4
Over the two decades of the disease's history, the volume of scientific evidence available on the different epidemiological, pathophysiological, clinical and therapeutic aspects of EoO has grown exponentially and has been summarised in four consensus documents and clinical practice guidelines developed by expert groups.5–8 However, major advances over recent years, including several randomised, placebo-controlled clinical trials (RCTs) and meta-analyses with systematic reviews, which are not included in earlier guidelines, mean that these are currently obsolete. Furthermore, the overall quality of all earlier guidelines (evaluated using the AGREEII tool) is limited since no specific methods were used to establish the quality of evidence or the weight of the statements and recommendations they provided.9
New clinical guidelines developed by an international group of experts using the GRADE system to classify evidence and the strength of recommendations have just been published under the auspices of United European Gastroenterology.10 The recommendations were based on the best available evidence, and when such evidence was not available or was considered to be inconsistent, consensus was sought among expert authors and the best clinical practice. After two rounds of voting, the recommendations were decided upon by consensus at a final face-to-face meeting. The new guidelines10 (the main recommendations are presented in this review) aim to provide doctors who treat children and adults with EoO (including gastroenterologists, allergists, paediatricians, ear, nose and throat specialists, pathologists, dieticians, general practitioners and emergency doctors) with a structured framework for the diagnosis and treatment of the disease.
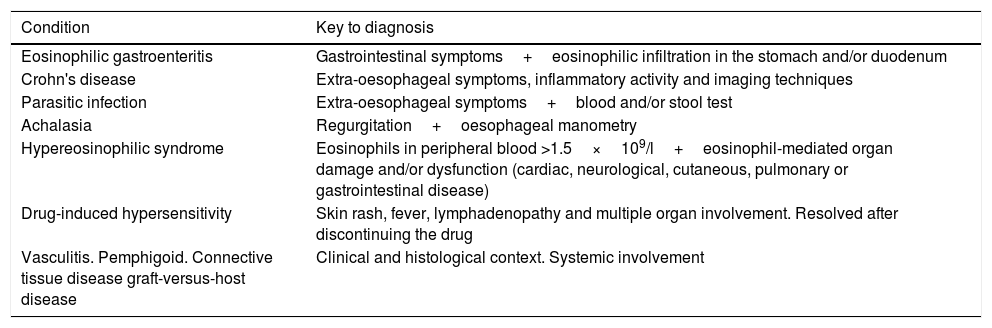
Definition of eosinophilic oesophagitis: evolution and current conceptAccording to the latest definition, EoO is a chronic, immune-mediated oesophageal disease characterised clinically by symptoms related to oesophageal dysfunction and histologically by eosinophil-predominant inflammation. Clinical manifestations and histological findings should not be interpreted in isolation, and other local and systemic causes of oesophageal eosinophilia should be excluded (Table 1).
Local and systemic causes of oesophageal eosinophilia to be ruled out before diagnosing eosinophilic oesophagitis.
| Condition | Key to diagnosis |
|---|---|
| Eosinophilic gastroenteritis | Gastrointestinal symptoms+eosinophilic infiltration in the stomach and/or duodenum |
| Crohn's disease | Extra-oesophageal symptoms, inflammatory activity and imaging techniques |
| Parasitic infection | Extra-oesophageal symptoms+blood and/or stool test |
| Achalasia | Regurgitation+oesophageal manometry |
| Hypereosinophilic syndrome | Eosinophils in peripheral blood >1.5×109/l+eosinophil-mediated organ damage and/or dysfunction (cardiac, neurological, cutaneous, pulmonary or gastrointestinal disease) |
| Drug-induced hypersensitivity | Skin rash, fever, lymphadenopathy and multiple organ involvement. Resolved after discontinuing the drug |
| Vasculitis. Pemphigoid. Connective tissue disease graft-versus-host disease | Clinical and histological context. Systemic involvement |
The first guidelines for EoO, published in 2007, considered the presence of symptoms of oesophageal dysfunction, eosinophilic infiltration of the oesophagus (defined by epithelial biopsies with >15 eosinophils per high-power field), together with lack of response to proton pump inhibitors (PPI) or, alternatively, normal oesophageal acid exposure determined by pH-metry, as diagnostic criteria.5 A dichotomous diagnostic criterion was established that assumed that GORD and EoO were mutually exclusive disorders, with GORD being the only oesophageal disease capable of responding to PPI therapy. However, this assumption was counterintuitive since the likelihood of both diseases coexisting in the same patient was high, usually affecting young males.11 In 2011, the first prospective series to systematically evaluate PPI therapy in patients with oesophageal eosinophilia and symptoms suggestive of EoO showed that up to 50% responded to PPIs.12 Furthermore, there was no significant difference in clinical, endoscopic and histological findings between PPI responders and non-responders and as a result, there was an extensive overlap between GORD (determined by monitoring oesophageal pH) and EoO. After this study, subsequent guidelines excluded oesophageal pH monitoring as a criterion for EoO diagnosis,6 but continued to consider response to PPI as sufficient motive to rule out EoO. The definition of a novel potential phenotype of the disease in 2011, called “PPI-responsive oesophageal eosinophilia”, to classify patients with features of EoO who achieved complete remission with PPI therapy, replaced GORD as the main differential diagnosis of EoO. Since 2011, numerous studies have clearly demonstrated that PPI-responsive patients not only share clinical, endoscopic and histological features but also molecular features.11 No other inflammatory gastrointestinal disease, except for “PPI-responsive oesophageal eosinophilia”, is defined by its response to a type of medication rather than by its genotypic and phenotypic traits. Therefore, the main novelty of this updated clinical guideline is the retraction of the previous term, no longer viewing PPI therapy as a diagnostic criterion for EoO but rather as a therapeutic agent.13 As a result, clinical and histological remission after PPI therapy is currently part of the EoO spectrum and should not be considered a separate entity, although additional data are required in children. Likewise, GORD is not a differential diagnosis of EoO and both entities can coexist, either independently or as an interaction.
Epidemiology and risk factors of eosinophilic oesophagitisSeveral studies have provided information on the frequency of EoO based on registries of cases, series of endoscopies and oesophageal biopsies, and more recently population-based studies. The results of these population-based studies have recently been summarised in a meta-analysis.3 The prevalence of EoO has rapidly increased in several countries in Europe and North America, with a global incidence of 1–20 new cases per 100,000 inhabitants per year and a prevalence of 13–49 cases per 100,000 inhabitants, and these figures are significantly higher in studies published after 2008.
In the particular case of Spain, the results of a population-based study analysing the study period between 2005 and 2011 estimated a mean annual incidence for adults of 6.4 cases per 100,000 inhabitants and a prevalence of 44.6 cases per 100,000 inhabitants.14 These figures have recently been updated for 2007–2016 and show a marked increase, with a mean incidence of 13.7 cases/100,000 inhabitants/year and a prevalence of 81.7 patients/100,000 inhabitants.15 EoO accounts for 7% of diagnoses in adults referred for endoscopy due to oesophageal symptoms.16 However, this frequency increases to 23% and 50% if only those patients with dysphagia or food impaction are considered, respectively.17,18 These data should be corroborated in paediatric studies since the disease seems to be clearly underdiagnosed in children.19,20
EoO can occur at any age. In children, most studies coincide in showing incidence peaks that increase with age21; among adults, the highest frequency of cases has been described between the ages of 30 and 50.22 Nearly all studies have shown that the frequency of EoO is higher in males than in females, both in children and in adults, with an odds ratio (OR) of 2.01 (95%CI: 1.63–2.48) in a meta-analysis of population-based studies.3 Although familial clustering has been described, twin and family studies have shown that concurrent cases of EoO within a family are much more strongly associated with environmental components than with genetic causes.23 Likewise, an association between a single nucleotide polymorphism (SNP) in the thymic stromal lymphopoietin (TSLP) gene and another SNP in its receptor has been described. The latter is encoded in the pseudoautosomal region of sex chromosomes.
Along with male gender, atopy has been considered another major risk factor for EoO: rhinitis, bronchial asthma and eczema are definitely significantly more common among patients with EoO compared with the general population.24 IgE-mediated food allergy has also been related to EoO, and even the treatment of food-induced anaphylaxis using oral immunotherapy could be a risk factor for the development of de novo EoO.25 However, to date, it has not been possible to demonstrate that atopy alone predisposes patients to EoO. In fact, EoO as a particular form of food allergy appears to be independent of IgE action26 and instead appears to be associated with IgG4.27 Moreover, besides atopy, EoO has been associated with other disorders. The oldest of these was its association with coeliac disease, the result of an evident publication bias, which has since been ruled out by several solid studies.28–30 Other described associations include hypereosinophilic syndrome, inflammatory bowel disease, oesophageal atresia and some connective tissue disorders, such as Marfan or Ehlers–Danlos syndromes,31 but it has not been possible to demonstrate a causal or temporal association between any of these and EoO.
Diagnosis of eosinophilic oesophagitisThe most common clinical manifestations of EoO in older children and adults are solid-food dysphagia and food impaction and non-swallowing associated chest pain.32,33 In younger children, the most common symptoms overlap to a great extent with those of gastro-oesophageal reflux, and include vomiting, abdominal pain, food refusal and failure to thrive.21 These symptoms should help confirm the suspected diagnosis and the need for an endoscopy, always accompanied by biopsies, since endoscopic findings alone cannot reliably establish a diagnosis of EoO.34 Because inflammatory changes in EoO are frequently patchy and may not be present in all biopsies,35,36 at least 6 biopsies should be obtained from two different sites in the oesophagus, typically in the proximal and distal halves of the oesophagus. Diagnostic sensitivity increases with the number of biopsies and reaches 100% after taking at least 6 biopsies, which should be targeted at areas of endoscopic abnormality, mainly whitish exudates and longitudinal furrows since these show peak eosinophilic infiltration.36–38 Biopsies should be taken despite a normal endoscopic appearance of the oesophagus since this has been reported in 10% and 32% of adult and paediatric patients, respectively.39,40 At the time of initial diagnosis, gastric and duodenal mucosal biopsies should also be obtained in order to rule out eosinophilic gastroenteritis, especially in children or in the presence of other concomitant gastrointestinal symptoms. These biopsies are not accurate in subsequent patient assessments.41
Since the initial description of EoO, the histological threshold for eosinophil density necessary for the diagnosis of this disease has varied,42 starting in 2011 at 15 eosinophils per high-power field (HPF). In addition to this criterion providing uniformity for all patients, it also makes it possible to distinguish between the two diseases associated with eosinophilic infiltration of the oesophageal mucosa, EoO and GORD, since GORD is associated with low eosinophil counts, generally less than 5 eosinophils per HPF. It is important to once again highlight that GORD and EoO are not mutually exclusive disorders and can coexist in the same patient. The cut-off point of 15 eosinophils per HPF has recently shown a sensitivity of 100% and a specificity of 96% in the diagnosis of EoO.43 However, this threshold is somewhat arbitrary since the size of an HPF varies between microscope manufacturers42 and should always be evaluated within the clinical context, especially in those cases with counts compatible with EoO obtained from asymptomatic patient samples. Reporting eosinophil density per square millimetre and communication with the pathologist upon questionable findings are useful in clinical practice, as is evaluating additional histological markers that characterise EoO, such as the presence of eosinophil microabscesses, dilated intercellular spaces, epithelial surface layering of eosinophils, elongation of conjunctival papillae and lamina propria fibrosis. Haematoxylin-eosin staining is sufficient for the assessment of histological features of EoO in clinical practice. The usefulness of a new EoO-specific histopathological scoring system (EoEHSS) has not yet been evaluated.44
Once the initial diagnosis of EoO has been established, monitoring of disease activity, which includes both the presence or absence of symptoms and eosinophilic inflammation in the oesophageal mucosa in response to therapeutic interventions, is a shared need with other chronic diseases. The ability of symptoms (or their absence) to predict inflammatory activity in EoO has been repeatedly reported to be very limited. Development of the EoO-specific activity index (EEsAI),45 which quantifies potential difficulties anticipated by patients when consuming foods with different consistencies, as well as dietary or behavioural adaptations to overcome such difficulties, has a very modest predictive capacity for estimating the inflammatory activity of the disease, as has been shown in a recent prospective, multicentre study.46
EoO-associated endoscopic findings have been standardised by the EREFS (acronym for Exudates, Rings, Oedema, Furrows and Strictures) classification, which was validated in adult patients, with good inter-observer agreement.47 However, the ability of this classification to diagnose and monitor EoO activity has so far been very limited.48,49 Therefore, although this practice is widespread,50 doctors should not make assumptions about the biological activity of EoO based exclusively on symptoms or endoscopic findings, but should also base their decisions on biopsy results.
Natural history of eosinophilic oesophagitisThe first data on the history of the disease in the absence of treatment were provided by a Swiss series of 30 adult patients with a mean follow-up of 7.2 years, which documented the persistence of dysphagia and oesophageal eosinophilic infiltration over time.51 For paediatric EoO, two series of 89 and 330 children with up to 8 years of follow-up21,52 confirmed the chronic and relapsing nature of EoO after discontinuing treatment. Adults diagnosed with EoO during childhood continue to have symptoms and require treatment. The duration of the untreated disease constitutes the main risk factor for oesophageal fibrotic remodelling and stricture formation,53 the prevalence of which increases from 47% to 88% when diagnostic delay (and the absence of treatment) increases from 2 to more than 20 years,54 and doubles with each 10-year increase in patient age at diagnosis.55 The frequency of functional abnormalities detected by high-resolution oesophageal manometry also increases with disease duration.56 Some preliminary data show the ability of both topical steroids and dietary treatment to reverse oesophageal remodelling in children.57,58 There is no evidence that EoO is a pre-malignant condition.
Because of its chronic and progressive nature, EoO has an impact on the quality of life and psychosocial adjustment of affected children and their families, producing anxiety and depression, sleeping difficulties and school problems. Among adults, EoO affects psychosocial functioning, but not physical well-being or mental functioning. Anxiety derived from concerns related to the long-term consequences of the disease, long-term medications or highly restrictive diets and the risk of food impaction hinder social interactions. Quality of life is worse at all ages in cases with more pronounced oesophageal symptoms and active histological disease.4
Treatment of eosinophilic oesophagitisEoO treatment includes the use of drugs or dietary modifications capable of inducing and maintaining remission of symptoms and oesophageal eosinophilic infiltration and endoscopic dilation to resolve strictures or reduced calibre of the oesophagus, as a consequence of the fibrotic remodelling process of the oesophagus.
Treatment with proton pump inhibitorsOf the different drugs with anti-inflammatory capacity that are useful in EoO, several clinical trials and prospective studies in adults and children have shown that PPI therapy is capable of inducing histological remission of the disease (defined as a reduction of eosinophilic infiltration to below 15 eosinophils per HPF) in 50–57% of patients.59–61 The first prospective study conducted in paediatric patients also showed a histological remission rate equivalent to 47%.61 A recent systematic review with meta-analysis, including 33 studies with 619 patients with EoO, showed that PPIs induced histological remission (defined by <15 eosinophils per HPF) in 50.5% (95%CI: 42.2–58.7%) and symptomatic improvement in 60.8% (95%CI: 48.38–72.2%) of treated patients. No significant differences were noted in patients’ age, study design or type of PPI assessed. However, a trend towards increased efficacy was observed when the total dose was divided into two daily doses, and among patients with pathological gastro-oesophageal reflux in pH-metry.62 Recommended PPI doses in adults are 20–40mg omeprazole twice daily or equivalent; in children, 1–2mg/kg omeprazole or equivalent.
When pharmacological treatment is discontinued, symptoms and oesophageal eosinophilia typically recur over a 3–6 month period.12 Therefore, the long-term therapeutic strategy for pharmacological treatments is to use the lowest effective dose to keep the disease in remission. A recent prospective series in children showed that 78% remained in remission at one year with half the dose used for induction.61 In adults, PPI therapy at half the initial dose maintains clinical and histological remission in at least 75% of patients after at least one year of follow-up.63,64 Among relapsing patients, the majority regain remission after dose escalation. No data have been published on PPI safety problems in patients with EoO.
Topical corticosteroidsAlthough no specific formulation has yet been approved and marketed to treat EoO, to date 13 randomised trials have been conducted in children and adults with topical corticosteroids, which are summarised in several systematic reviews and meta-analyses.65–69 These studies have confirmed the high efficacy of these drugs to induce histological remission in EoO. However, comparative interpretation of the results is complex due to variations in inclusion criteria, drugs used (fluticasone or budesonide), daily doses, duration of treatment (from 2 to 12 weeks), drug delivery system (inhalers with dry powder, suspensions of varying viscosity or effervescent tablets) and criteria used to define histological remission (from <1 to <20 eosinophils per HPF).
A system to deliver the drug along the oesophagus is essential to achieve an adequate and maintained coverage of its mucosa, avoiding corticoid deposits in other tissues. A clinical trial comparing the efficacy of 1mg budesonide administered twice daily as a viscous solution versus an aerosol showed a significantly higher rate of complete histological remission in the first group (64% vs 27%). Drug mucosal contact time, measured by scintigraphy, was significantly longer in patients treated with viscous budesonide, especially in the distal oesophagus.69 The topical corticosteroid administration methods therefore determine drug mucosal contact time and the highest histological remission rate. A new investigational budesonide formula, administered as effervescent tablets specifically designed to release the drug in the oesophagus, has demonstrated the capacity to induce universal histological remission along the entire length of the oesophagus after only 2 weeks of treatment.70
Unlike histological remission, data on the efficacy of topical corticosteroids in symptom resolution are less clear, with several trials unable to demonstrate significant advantages over placebo. Use of non-validated symptom scoring scales, inclusion of patients with more severe disease or not considering adaptations in the eating behaviour of patients to minimise dysphagia are possible explanations that are being tackled in more recent studies.71
Although available data are limited, treatment with topical corticosteroids is also effective in maintaining long-term disease remission in steroid-responsive patients.72–74
Swallowed topical corticosteroids for the treatment of EoO seem to show a favourable safety profile, with no serious adverse effects. This is similar to the situation documented in patients treated with placebo according to data obtained from the clinical trials conducted to date. Oesophageal candidiasis is the most common adverse effect (5–10%), with most cases being asymptomatic and found incidentally during post-treatment endoscopies. Viscous, orodispersible and aerosol formulations have shown no differences in the frequency of associated candidiasis. Small recent series in paediatric patients treated with long-term topical corticosteroids have generated some uncertainty regarding potential suppression of the pituitary–adrenal axis,75 which has not been confirmed in other series76 and clinical trials versus placebo.70,71 Moreover, decreased plasma cortisol levels have been the only documented effect, with no clinical signs of adrenal insufficiency or growth impairment.74 Until solid data are available from long-term clinical trials, monitoring of serum cortisol in children with EoO who are receiving high doses of swallowed topical corticosteroids for long periods of times or concomitant use of inhaled/nasal corticosteroids for other associated allergies may be advisable.10
Dietary treatmentThe appeal of dietary treatment for EoO stems from the absence of adverse effects if adequate nutrition is guaranteed, its high potential efficacy comparable to or superior to that of some pharmacological options, with a lower cost for healthcare systems. During the two decades of EoO history, several dietary interventions have been evaluated, including exclusive feeding with an elemental formula, food allergy testing-based elimination diets, and empirical elimination of those food most likely to cause EoO.
The elemental diet, which is devoid of antigenic capacity, as an exclusive diet for children with EoO, provided the first evidence on the efficacy of a dietary intervention for inducing remission of EoO,77 and is the most effective dietary intervention,78 capable of inducing histological remission in 90.8% (95%CI: 84.7–95.5%) of EoO patients of all ages. However, several disadvantages limit its use in clinical practice, including its bad taste (which requires frequent use of nasogastric tubes), lack of adherence, psychological and social effects when avoiding all types of conventional food and high cost. The use of the exclusive elemental diet should only be considered after failure of a pharmacological treatment and other elimination diets, especially in young children, and for a limited time.10
Food elimination based on the results of skin prick or epicutaneous patch tests was evaluated for the first time in 2002. After excluding an average of five foods, histological remission was achieved in 49% of treated paediatric patients,79 documented by endoscopy. EoO-specific causative foods among responsive patients were exclusively attributed by symptom recurrence after reintroduction of food, reported by the parents, without performing oesophageal biopsies. A decade later, the same research group updated its results to an overall efficiency of 53%.80 However, several studies have further reported worse results with this strategy. A meta-analysis revealed that the global efficacy of the food allergy testing-based elimination diet to achieve histological remission was 45.5% (95%CI: 35.4–55.7%), with wide heterogeneity (I2: 75%) indicating low reproducibility.78 The efficacy rates were significantly lower in adults than in children (32.2% vs 47.9%). Food elimination guided by blood IgE microarrays achieved EoO remission in only 7% of adult patients, leading to the early discontinuation of a study.81 The predictive capacity of a combination of multiple skin prick and blood test results, measuring immediate or delayed hypersensitivity reactions, to identify foods responsible for EoO (defined by sequential reintroduction in responders to an empirical 6-food diet) was totally unsatisfactory.82 As a result, the European Academy of Allergy and Clinical Immunology (EAACI) has recommended not performing these tests to try and identify foods responsible for EoO26 because their diagnostic accuracy is insufficient to design effective diets.
Due to the unfeasibility of the elemental diet in clinical practice and the low sensitivity and specificity of skin prick tests to identify EoO food triggers, the efficacy of an empirical six-food elimination diet (consisting in eliminating the six foods most commonly associated with food allergy among Chicago's paediatric population from the diet: cow's milk protein, wheat, egg, soy, nuts, and fish and shellfish) was tested in 2006,83 achieving histological remission of the disease in 74% of treated children. Similar results have been achieved in patients of all ages and are summarised in a meta-analysis of seven observational studies which provided a homogeneous efficacy (I2=0%) of 72% (95%CI: 66–78%) in children and adults.78 Sequential reintroduction of each of the excluded foods under endoscopic and histological control makes it possible to accurately identify the foods responsible for EoO in each patient.84 However, the high degree of dietary restriction and the large number of endoscopies required to complete this process, together with the fact that most responsive patients will have only one or two EoO food triggers, has resulted in simpler strategies, such as the four-food elimination diet (milk, wheat, egg and legumes/soy), with an overall efficiency for achieving EoO remission of 54% in adults85 and 64% in children.86 More recently, a prospective multicentre study conducted in 120 patients of all ages has evaluated the effectiveness of a two-food elimination diet (milk and gluten-containing cereals) followed by a four-food elimination diet, in cases of no histological resolution, reserving the six-food diet as a final rescue option. Clinical and histological remission rates of 43%, 60% and 79%, respectively, were achieved and the number of endoscopies and diagnostic process time were reduced by 20% compared with starting with a six-food diet.87
Once the foods responsible for EoO have been identified in each individual patient (90% of those responding to an empirical two- or four-food elimination diet will have only one or two food triggers), long-term avoidance maintains disease remission. Two studies conducted in adults showed that all adults who avoided the responsible food(s) showed clinical and histological remission of EoO up to 3 years later,88,89 without the need for drugs. In children, maintenance of remission has been reported after 4 years of follow-up.90
Endoscopic dilation in eosinophilic oesophagitisDilation with balloons, bougie dilators or rigid dilators is the only endoscopic treatment available for EoO. Its effectiveness has been reviewed in a meta-analysis of 27 observational studies involving a total of 845 patients and 1820 oesophageal dilations. A symptomatic improvement was documented in 95% of patients (95%CI: 90–98%), with a highly variable duration of symptomatic relief. Complications were uncommon, including perforation (0.38% of cases), haemorrhage (0.05%) and hospitalisation (0.67%), and no deaths.91 Oesophageal dilation does not control the chronic inflammation that contributes to oesophageal remodelling and should therefore not be used in isolation without an effective, concomitant anti-inflammatory treatment (PPI, topical corticosteroids or diet).
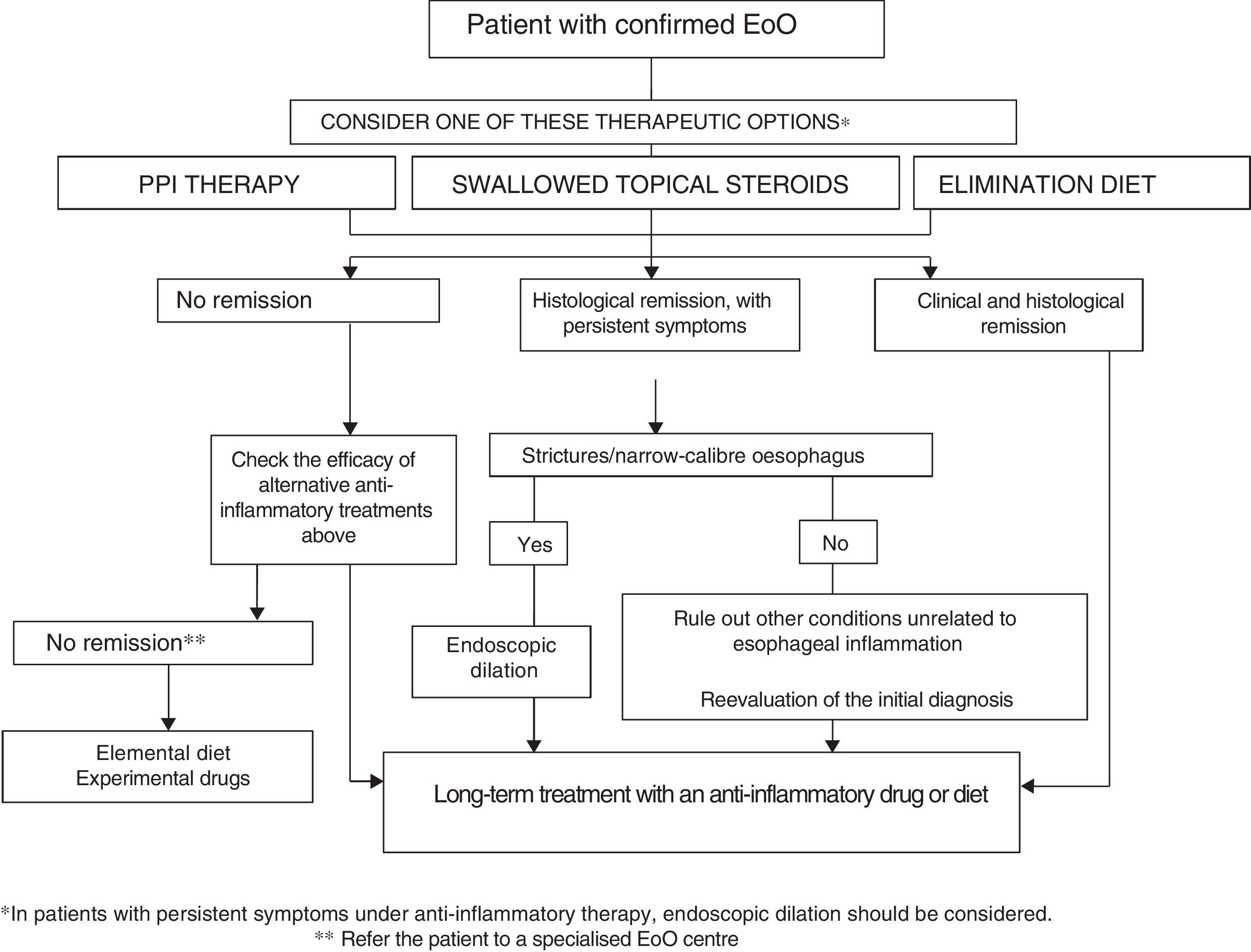
How to choose treatment for a patient with eosinophilic oesophagitis?EoO is a chronic disease in which oesophageal inflammation progresses over time to fibrotic remodelling, leading to narrow-calibre oesophagus and oesophageal strictures. Inflammatory activity and fibrotic remodelling may be reversed with anti-inflammatory PPI therapy, topical steroids or diets, while strictures may be resolved by endoscopic dilation. Making a therapeutic analogy with inflammatory bowel disease,92 a treatment aimed at curing oesophageal inflammation (mucosal healing) plus endoscopic dilation should be considered in all patients with EoO in the event of fibrostenotic findings. Figure 1 shows the proposed therapeutic algorithm for EoO.
Both diet and topical steroids or PPIs could be considered as a first-line option with anti-inflammatory capacity. The choice of therapy should be discussed individually with the patient, but because of their low cost, safety, convenience and moderate effectiveness, PPIs may be a reasonable initial option. After a period of 6–12 weeks, the effectiveness of the initial treatment should be assessed by endoscopy. In there is no response to PPI therapy, the choice between swallowed topical corticosteroids or diet should again be discussed individually with the patient and his/her family, and may depend on age (adolescents and young adults usually show poor adherence to diet), severity of the disease (severe symptoms should be treated preferably with topical corticosteroids), patient lifestyle, preferences and ability to understand food labels. The therapeutic option may change over time due to adverse effects, the patient's unwillingness to continue, negative impact on quality of life or family resources. Two recent series have reported that patients with EoO who respond to diet or topical steroids may also achieve remission with PPIs and vice versa.93,94
Endoscopic dilatation should be offered to all patients with fibrostenotic abnormalities (including oesophagus with calibre <13mm) that cause dysphagia or food impaction, despite the use of dietary or pharmacological treatment. Endoscopic dilation should not be the only intervention used since it has no effect on oesophageal inflammation. In cases of severe and symptomatic oesophageal stricture, dilation together with topical corticosteroids may quickly achieve clinical, endoscopic and histological remission of EoO.
Other treatments for eosinophilic oesophagitisThe effectiveness of azathioprine or 6-mercaptopurine in EoO has been evaluated in only a small series of cases, showing that these drugs induced and maintained steroid-free disease remission for a period of up to 5 years, with relapse after discontinuation of therapy.95
Sodium cromoglicate and antihistamines have no effect on symptoms or oesophageal eosinophilia39; however, the leukotriene receptor antagonist, montelukast, has shown no capacity to induce or maintain topical corticosteroid-induced remission.96,97 A selective CRTH2 antagonist evaluated in a randomised placebo-controlled trial induced modest yet significant improvements in symptoms and oesophageal inflammation without achieving normalisation.98
Several biological agents used in studies for severe asthma have also been evaluated in EoO. The effectiveness of the anti-interleukin (IL)-5 monoclonal antibodies mepolizumab and reslizumab has been evaluated in children, adolescents and adults with EoO in three double-blind randomised trials.99–101 Compared to placebo, symptoms showed no improvement in two of the three studies, although a significant reduction in eosinophilic infiltration was documented in all cases, without achieving histological remission. An anti-IL-13 antibody (QAX576) recently evaluated in a clinical trial versus placebo showed a 60% reduction in mean eosinophil count and downregulated expression of genes involved in EoO pathophysiology in oesophageal biopsies, with no improvement in symptoms.102 Other anti-IL-13 drugs are currently being evaluated. Omalizumab, an anti-IgE antibody, showed no relevant effects on oesophageal symptoms or oesophageal eosinophilia compared with placebo in another clinical trial.27 Finally, two doses of infliximab, an anti-tumour necrosis factor-alpha agent, did not lead to clinical or histological improvement in a series of 3 adults with EoO.103
Conflict of interestThe authors declare that there is no conflict of interest related to the content of this article.
Please cite this article as: Lucendo AJ, Molina-Infante J. Esofagitis eosinofílica: diagnóstico y tratamiento actual basado en la evidencia. Gastroenterol Hepatol. 2018;41:281–291.