Diagnostic discrimination between inflammatory bowel disease (IBD) and functional gastrointestinal disorders is complex, as they cause similar signs and symptoms. Faecal calprotectin (FC) is a useful marker in this context, and can be used to select patients who will most benefit from colonoscopy. The aim of this study was to evaluate the utility of FC in discriminating between organic disease and functional disorders.
Materials and methodsThe study included 264 patients presenting with gastrointestinal complaints consistent with an organic pathology. FC levels were determined and diagnostic accuracy was assessed using the area under the curve obtained from the final diagnosis.
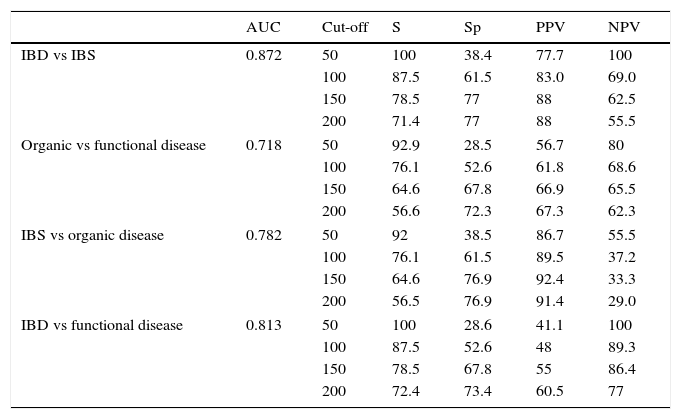
ResultsCalprotectin levels in organic bowel disease patients were significantly higher (median 254μg/g; 95% confidence interval [CI], interquartile range 105–588.5) than in functional disease patients (95μg/g; 95% CI, 47.25–243.92) (p<.0001). Similarly, in patients with IBD, the values obtained were higher (270.85μg/g; 95% CI, 96.85–674.00) than in those with irritable bowel syndrome (79.70μg/g; 95% CI, 36.50–117.25) (p<.0001). For a cut-off of 150μg/g, FC had an area under the ROC curve to discriminate between organic and functional disease of 0.718, and 0.872 to discriminate between irritable bowel syndrome and IBD.
ConclusionOur study supports the importance of FC as a marker in the evaluation of patients with IBD. The best diagnostic accuracy is obtained at a cut-off value of 150μg/g.
La discriminación entre enfermedades inflamatorias intestinales (EII) y trastornos funcionales gastrointestinales es compleja debido a que pueden presentar cuadros clínicos similares. La calprotectina fecal (CPF) es un marcador inflamatorio útil para este fin y permite seleccionar a los pacientes que más se pueden beneficiar de someterse a una colonoscopia. El objetivo fundamental de este estudio fue valorar la utilidad de la CPF para diferenciar entre enfermedades orgánicas y funcionales.
Material y métodosSe determinó la concentración de CPF de 264 pacientes que presentaban signos o síntomas gastrointestinales indicativos de enfermedad orgánica, y se calculó su precisión diagnóstica mediante el área bajo la curva a partir del diagnóstico final.
ResultadosLos pacientes con enfermedad orgánica presentaron valores de CPF mayores (mediana 254μg/g; intervalo de confianza [IC] 95%, rango intercuartil 105-588,5) que el grupo con enfermedad funcional (95μg/g; IC 95%, 47,25-243,92; p<0,0001), así como el grupo con EII (270,85μg/g; IC 95%, 96,85-674,00) obtuvo valores más elevados que el grupo con síndrome del intestino irritable (79,70; IC 95%, 36,50-117,25; p<0,0001). Para un valor de corte de 150μg/g se obtuvo un valor del área bajo la curva de 0,718 para discriminar entre enfermedad orgánica y funcional, y de 0,872 para discriminar entre síndrome del intestino irritable y EII.
ConclusiónEn este estudio se corrobora el alto valor de la CPF en la evaluación de pacientes con sospecha de EII. La mejor eficacia diagnóstica se consigue con un cut-off de 150μg/g para la discriminación entre EII y síndrome del intestino irritable.
Abdominal pain or discomfort, accompanied by diarrhoea or constipation, is a common cause of primary care and gastroenterology consultations.1 Many clinical symptoms that manifest in abdominal pain of functional origin are also common with other organic disorders,2 particularly inflammatory bowel disease (IBD), so it is important to identify these patients early in order to avoid unnecessary studies.
Irritable bowel syndrome (IBS) is the most common gastrointestinal functional disorder, with a prevalence in Europe of around 10-15%. On the other hand, IBD is less common, with a prevalence of 0.1–0.2% for ulcerative colitis (UC) and 0.05–0.1% for Crohn's Disease (CD).3 IBS has a benign course but is unpleasant, painful and greatly reduces the patient's quality of life.4 The most common symptoms are abdominal pain, bloating and/or impaired defecation. The pathophysiological basis has not been fully established, but several factors have been proposed: alterations in intestinal motility, visceral hypersensitivity, psychological disorders and inflammatory and postinfectious mechanisms.5 About 3% of primary care consultations and 16–25% of gastroenterology consultations are due to this condition.6 In contrast, IBD is a chronic inflammatory disorder of the gastrointestinal tract comprising 2 major entities: UC and CD, whose progression alternates bouts of inflammatory activity with periods of remission and can lead to severe complications, requiring in some cases hospital admissions. UC is characterised by continuous inflammation of the mucosa of the colon, whereas CD presents a discontinuous and transmural involvement that may involve any part of the digestive tract, although it most commonly affects the terminal ileum and in the colon. As for its aetiology, there are no new relevant data on IBD,7 but its cause is believed to be multifactorial, resulting from a complex interaction between genetic, environmental and individual factors of the patients’ immune system.
The clinical suspicion of IBD generally results in the practice of a blood test, a stool analysis, an endoscopy (sigmoidoscopy or colonoscopy), a biopsy and imaging studies, which help to exclude other causes and confirm the diagnosis.
Endoscopy remains the gold standard, as it allows the intestinal mucosa to be directly viewed and biopsied. However, it is an examination that has some risks and limitations because it is an invasive, operator-dependent procedure that is both unpleasant and requires preparation and anaesthesia for paediatric patients, in addition to being relatively expensive. It is estimated that more than 60% of the colonoscopies performed in young patients reveal no abnormalities and are therefore potentially avoidable.8
Given the high demand for gastroenterology consultations and endoscopic examinations, a test that allows physicians to confidently rule out IBD will benefit patients and gastroenterology departments, since it would avoid non-critical invasive tests. The ideal biomarker should make the use of colonoscopy much more efficient thanks to a high sensitivity so as not to cause delays in diagnosis, and specificity to avoid unnecessary examinations.9 Several non-invasive markers have been recommended for this purpose, including the typical biochemical parameters of inflammation, erythrocyte sedimentation rate and C-reactive protein, but they are not sensitive enough or specific enough for these conditions.10
The determination of calprotectin in faeces has becoming established in recent years as a useful new marker of IBD. Several studies have demonstrated that there is an association between the levels of calprotectin and the degree of inflammation; therefore, it can be used to monitor response to treatment and to predict the risk of recurrence.11,12
Calprotectin is an abundant and widely distributed protein13 which is mainly found in neutrophil polymorphonuclear leukocytes, monocytes and reactive macrophages,14 representing 60% of the total protein content of their cytoplasm.15 Its biological function is not precisely understood, but its protective activity is apparent in inflammatory, infectious and proliferative processes, in which its plasma levels increase 8–40 fold.16 Its faecal concentration is about 6 times greater than its plasma concentration. Its resistance to heat and metabolic degradation by bacterial enzymes and by the intestinal proteases facilitate its elimination intact in the faeces.17 Its elevation is due to an increase in the permeability of the mucosa that induces the migration of polymorphonuclear neutrophilic leukocytes and monocytes to the intestinal lumen, where after they complete their activity they die, releasing a large amount of calprotectin.
Studies have shown that elevated levels are not affected by extraintestinal inflammation and are only elevated in certain inflammatory diseases with intestinal involvement2 (e.g., cystic fibrosis, rheumatoid arthritis, CD, UC and bacterial infections) and neoplastic diseases (e.g., colorectal cancer).
Faecal calprotectin (FCP) allows patients to be managed and controlled properly and avoids unnecessary testing of those patients with a functional disorder.
The main objective of this study was to evaluate the utility of FCP to distinguish between intestinal diseases of an organic and functional nature and between IBD and IBS, estimating the diagnostic performance of this test by assessing the ROC curve and assessing its sensitivity (S), specificity (Sp), positive predictive value (PPV) and negative predictive value (NPV) for different cut-off points.
Materials and methodsProspective observational study from April to August 2015 of patients referred to the Gastroenterology and Paediatric Gastroenterology Unit of the Hospital Clínico Universitario Virgen de la Arrixaca (Murcia), whose FCP was determined upon presentation of symptoms and signs indicative of organic disease (intense and disabling abdominal pain, chronic diarrhoea, major changes in bowel habits, haematochezia and weight stagnation in children). Patients with a history of cancer or intestinal polyps were excluded.
A general physical examination was performed and a complete medical history was obtained by interviewing all patients or appropriate relatives about their intestinal and extraintestinal symptoms, their duration, other illnesses, travel history, medications (antibiotics and nonsteroidal anti-inflammatory drugs), family history of intestinal diseases (IBD, coeliac disease or colorectal cancer) and substance abuse. All patients were submitted to the necessary tests until a definitive diagnosis was obtained, including: blood tests, stool cultures and tests (searching for infection, leukocytes, faecal occult blood and FCP) and endoscopic studies (colonoscopy and endoscopic capsule), as appropriate. Endoscopic patients were those without known IBD and with: haematochezia, intense and disabling lower abdominal pain of more than 2 months of duration, changes in bowel habits (predominantly constipation) of at least 2 months of duration, uncomplicated diarrhoea (excluding infectious origin or malabsorption), as well as patients in follow-up for UC or CD requiring a new endoscopic evaluation. Patients with incomplete colonoscopy or inadequate bowel cleansing were excluded. The procedures used in the patients were performed after obtaining informed consent.
Patients were classified into 2 groups according to their diagnosis:
- •
Organic disease, including: IBD (CD and UC), allergies, coeliac disease, cystic fibrosis, acute infectious diarrhoea and gastritis caused by H. pylori.
- •
Functional disease: IBS, food intolerances, non-erosive gastroesophageal reflux disease, functional dyspepsia, bacterial overgrowth, functional abdominal pain and functional abdominal pain secondary to the consumption of non-steroidal anti-inflammatory drugs.
Stool samples were collected in sterile vials and sent to the laboratory in less than 48h, where they were frozen at −20°C until their analysis, which took place in less than a month. FCP is stable in faeces at room temperature for at least 72h and up to 4 months frozen at a temperature below −20°C. The FCP concentration was determined using the ELISA kit from Bühlmann (Basel, Switzerland) using monoclonal antibody against calprotectin. Samples were prepared and analysed according to the manufacturer's instructions.
Statistical analysisData analysis was performed with the SPSS® v. 11 statistical package. The Kolmogorov–Smirnov test showed that FCP concentration values did not follow a normal distribution. Consequently, the results are expressed in median and interquartile range.
No differences were found in the distribution by age or gender (p=0.103 and p=0.239, respectively), and therefore the data were processed together.
The values between the different groups were compared with the Kruskal–Wallis statistical test. We assumed a statistically significant difference when p<0.05. The diagnostic performance, the S, the Sp, the PPV and the NPV were evaluated.
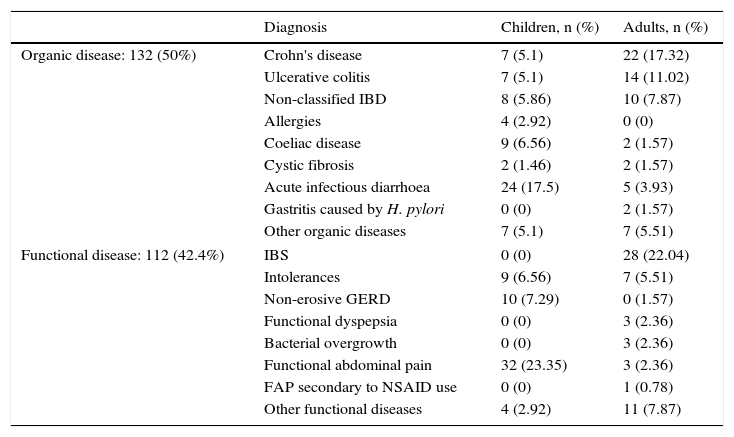
ResultsIn the study conducted from April to August 2015, 127 adults (80 women and 47 men) between 17 and 82 years and with a mean age of 42.7 years, and 137 children (63 girls and 74 boys) between 2 and 16 years and with a mean age of 6.7 years, who visited the Gastroenterology Unit because they presented some symptom of gastrointestinal disease indicative of organicity, were consecutively enrolled. 28 patients were excluded from the study because they did not deliver the stool sample for the determination of FCP or because they had an incomplete colonoscopy or inadequate colonic cleansing at the time of the colonoscopy. The diagnosis by age groups is shown in Table 1. No differences were found in FCP values between children and adults, either grouped by disease or all together (p>0.05).
Classification of patients according to the final diagnosis and age group.
| Diagnosis | Children, n (%) | Adults, n (%) | |
|---|---|---|---|
| Organic disease: 132 (50%) | Crohn's disease | 7 (5.1) | 22 (17.32) |
| Ulcerative colitis | 7 (5.1) | 14 (11.02) | |
| Non-classified IBD | 8 (5.86) | 10 (7.87) | |
| Allergies | 4 (2.92) | 0 (0) | |
| Coeliac disease | 9 (6.56) | 2 (1.57) | |
| Cystic fibrosis | 2 (1.46) | 2 (1.57) | |
| Acute infectious diarrhoea | 24 (17.5) | 5 (3.93) | |
| Gastritis caused by H. pylori | 0 (0) | 2 (1.57) | |
| Other organic diseases | 7 (5.1) | 7 (5.51) | |
| Functional disease: 112 (42.4%) | IBS | 0 (0) | 28 (22.04) |
| Intolerances | 9 (6.56) | 7 (5.51) | |
| Non-erosive GERD | 10 (7.29) | 0 (1.57) | |
| Functional dyspepsia | 0 (0) | 3 (2.36) | |
| Bacterial overgrowth | 0 (0) | 3 (2.36) | |
| Functional abdominal pain | 32 (23.35) | 3 (2.36) | |
| FAP secondary to NSAID use | 0 (0) | 1 (0.78) | |
| Other functional diseases | 4 (2.92) | 11 (7.87) | |
FAP, functional abdominal pain; GERD, gastroesophageal reflux disease; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome.
Of all patients studied, 132 (50%) were classified as having organic intestinal disease and 112 (42.4%) as having gastrointestinal functional disease. The rest (20) were patients without a definitive diagnosis at the time. Of those with organic disease, 68 had IBD, of which 29 presented CD, 21 UC and in the remaining 18, the type of IBD was not differentiated. Of the group with functional disease, 28 were classified as IBS. The rest of the patients are shown in Table 1.
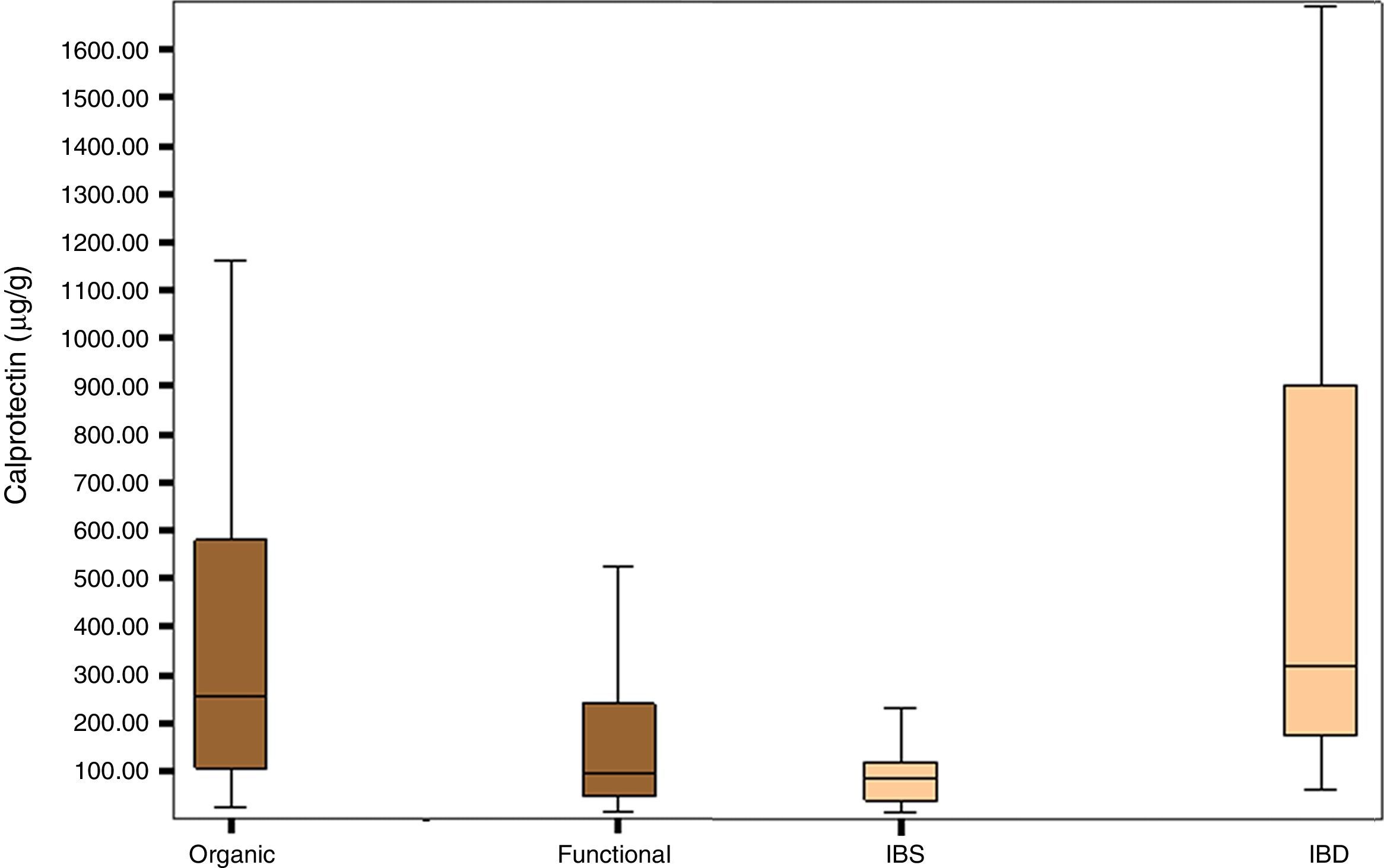
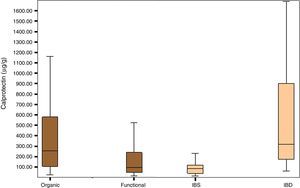
Fig. 1 shows the distribution of FCP values in the organic, functional, IBS and IBD disease groups.
The group of patients with organic disease presented FCP values (median; confidence interval percentage, interquartile range: 254μg/g; 95% CI, 105–588.5) greater than the group with functional disease (95μg/g; 95% CI, 47.25–243.92; p<0.0001). Furthermore, the group of patients with IBD had significantly higher FCP values (270.85μg/g; 95% CI, 96.85–674.00) than the group of patients with IBS (79.70μg/g; 95% CI, 36.50–117.25); p<0.0001) (Table 2).
Median and interquartile range of faecal calprotectin (μg/g) in patients with organic and functional gastrointestinal disease.
| Organic | Functional | IBS | IBD | |
|---|---|---|---|---|
| n | 132 | 112 | 28 | 68 |
| Median | 254.3 | 95.00 | 79.70 | 270.85 |
| Interquartile range | 105–588.5 | 47.25–243.92 | 36.50–117.25 | 96.85–674.00 |
| p | p<0.0001 | p<0.0001 | ||
IBD, inflammatory bowel disease; IBS, irritable bowel syndrome.
The concentrations were significantly higher in the group of patients with another type of non-IBD organic disease (153μg/g; 95% CI, 82.5–340.5) compared to the group with functional disease (95μg/g; 95% CI, 47.25–243.92; p<0.0001).
The concentrations were also significantly higher in the group of patients with non-IBD organic disease (153μg/g; 95% CI, 82.5–340.5; p=0.034) compared to patients with another type of non-IBD functional disease (101μg/g; 95% CI, 49–249.95).
No statistically significant differences were found between FCP values in patients with CD compared to patients with UC (p=0.443). There were also no differences in the group of patients with another type of non-IBS functional disease compared to the group with IBS (p=0.123).
No patient diagnosed with IBD had FCP values below 50μg/g; the lowest value was 60 in an adult and 73 in a child. Of the patients with IBS, 16 (57%) obtained a value >50μg/g. Of these, in 9 cases (33%), the value was between 50 and 100μg/g and in 7 cases (25%), it was greater than 100μg/g, with the highest value being 396μg/g.
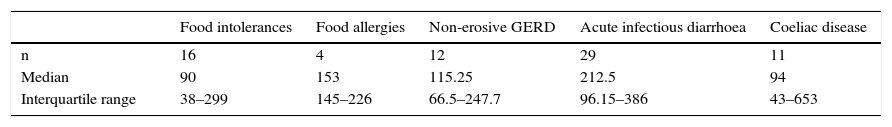
The FCP values in patients with food intolerances, food allergies, non-erosive gastroesophageal reflux disease, acute infectious diarrhoea and coeliac disease are shown in Table 3.
Median values and interquartile range of faecal calprotectin (μg/g) in patients with functional diseases other than irritable bowel syndrome and organic diseases other than inflammatory bowel disease.
| Food intolerances | Food allergies | Non-erosive GERD | Acute infectious diarrhoea | Coeliac disease | |
|---|---|---|---|---|---|
| n | 16 | 4 | 12 | 29 | 11 |
| Median | 90 | 153 | 115.25 | 212.5 | 94 |
| Interquartile range | 38–299 | 145–226 | 66.5–247.7 | 96.15–386 | 43–653 |
GERD, gastroesophageal reflux disease.
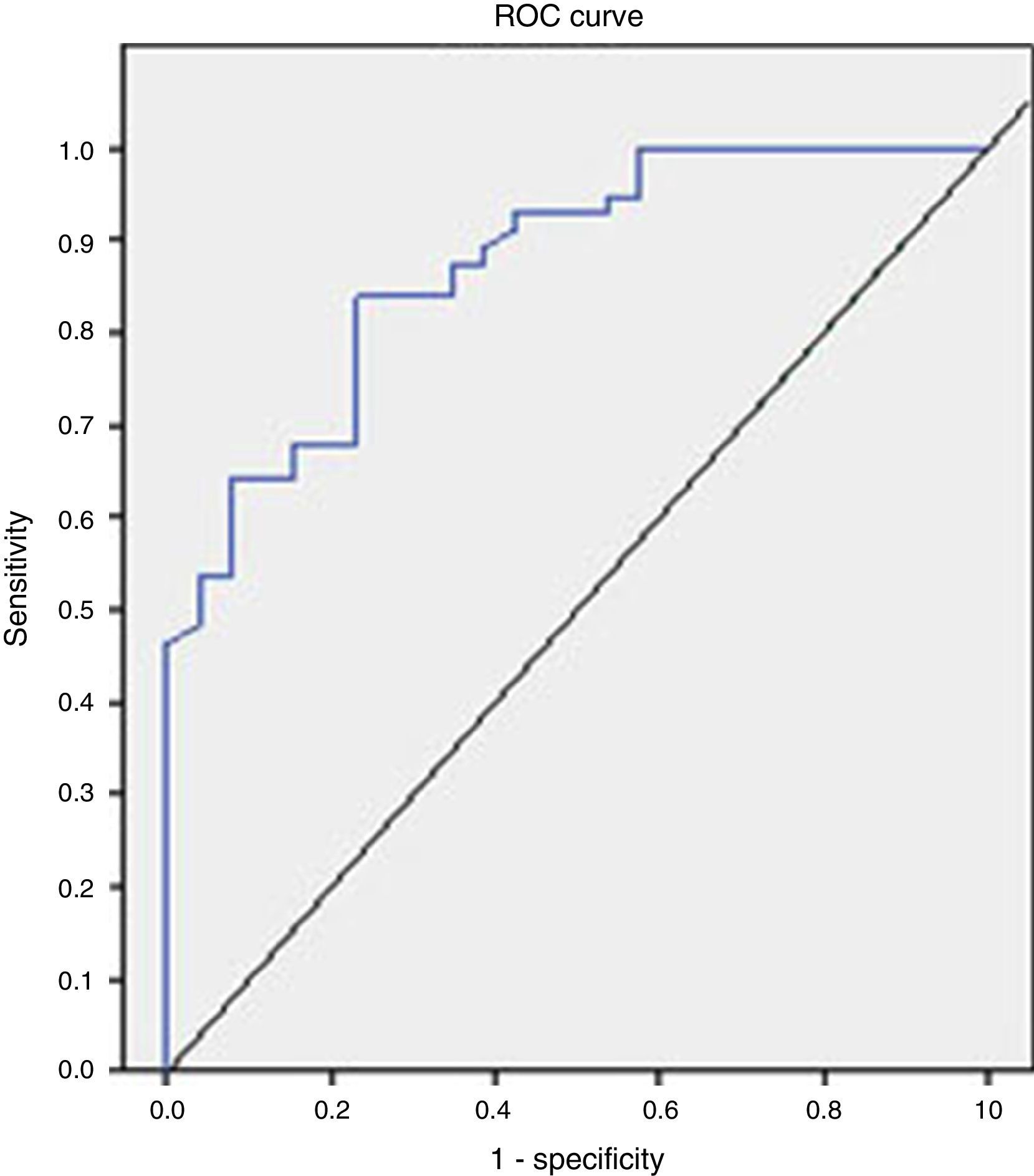
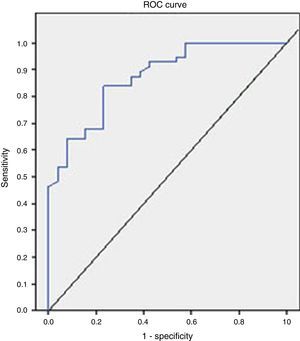
The analysis of the ROC curve that is represented in Fig. 2 showed the good diagnostic performance of FCP in distinguishing IBD from IBS, with an AUC=0.872 (95% CI=0.795–0.949, S=100%, E=38.4%, PPV=77.7%, NPV=100%), good for IBD compared to all other functional diseases (AUC=0.776), and moderate in the differentiation of organic versus functional disease (AUC=0.718).
The values of S, Sp, PPV and NPV with different cut-offs are shown in Table 4.
Diagnostic accuracy for faecal calprotectin with different cut-offs depending on the final diagnosis.
| AUC | Cut-off | S | Sp | PPV | NPV | |
|---|---|---|---|---|---|---|
| IBD vs IBS | 0.872 | 50 | 100 | 38.4 | 77.7 | 100 |
| 100 | 87.5 | 61.5 | 83.0 | 69.0 | ||
| 150 | 78.5 | 77 | 88 | 62.5 | ||
| 200 | 71.4 | 77 | 88 | 55.5 | ||
| Organic vs functional disease | 0.718 | 50 | 92.9 | 28.5 | 56.7 | 80 |
| 100 | 76.1 | 52.6 | 61.8 | 68.6 | ||
| 150 | 64.6 | 67.8 | 66.9 | 65.5 | ||
| 200 | 56.6 | 72.3 | 67.3 | 62.3 | ||
| IBS vs organic disease | 0.782 | 50 | 92 | 38.5 | 86.7 | 55.5 |
| 100 | 76.1 | 61.5 | 89.5 | 37.2 | ||
| 150 | 64.6 | 76.9 | 92.4 | 33.3 | ||
| 200 | 56.5 | 76.9 | 91.4 | 29.0 | ||
| IBD vs functional disease | 0.813 | 50 | 100 | 28.6 | 41.1 | 100 |
| 100 | 87.5 | 52.6 | 48 | 89.3 | ||
| 150 | 78.5 | 67.8 | 55 | 86.4 | ||
| 200 | 72.4 | 73.4 | 60.5 | 77 | ||
AUC, area under the curve; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; NPV, negative predictive value; PPV, positive predictive value; Sp, specificity; S, sensitivity.
No significant correlation was found between the FCP values and the white blood cell count (p=0.650), neutrophil count (p=0.067) and lymphocyte count (p=0.679) through a univariate analysis. A correlation, although weak, was seen between the FCP value and faecal occult blood (ρ=0.392, p<0.05). Likewise, a correlation was found with plasma levels of albumin (ρ=−0.413; p<0.05).
DiscussionThe diagnosis of IBD has traditionally been based on the assessment of digestive symptoms. Digestive symptoms are very common and nonspecific, which means that when they are used as the only filter for the indication of a colonoscopy, the results are inefficient.17 For this reason, there is a need in medical practice for a simple test to help select those patients with symptoms indicative of inflammatory disease requiring more complex diagnostic tests.
Although the study was conducted with a small number of patients, the findings are in line with previous studies in the paediatric and adult population, showing how a single FCP test can help gastroenterologists in the differential diagnosis between gastrointestinal diseases of an organic and functional nature.18 It should be noted that the adult population studied is young (the median age is 42 years), and mostly female, with 80 women (63%) versus 47 men (37%). We also note that the prevalence of different gastrointestinal diseases is consistent with the literature. In fact, all patients diagnosed with IBS were adults, of whom 22 (78.6%) were women with a mean age of 37.6 years versus 6 men (11.4%), which is consistent with the available epidemiological data that show that IBS affects women more frequently, especially adults under 45 years of age.19 We also note the high number of children (24) with acute infectious diarrhoea who underwent the FCP test.
It should be remembered that in addition to IBD, conditions of very different aetiology, such as infections, food allergies, cystic fibrosis, gastritis, rectal bleeding, poorly controlled coeliac disease or therapy with non-steroidal anti-inflammatory drugs can give high calprotectin results.20 In our patients, FCP concentrations were significantly higher in the IBD group compared to the group with another type of organic disease. However, our results also do not allow us to establish FCP values that distinguish between both disease groups.
The cut-off value in children aged 1–17 years and in healthy adults has been established below 50μg/g, in accordance with the limit indicated in the trial.21 In children under one year, different reference values have been proposed, since in newborns intestinal permeability is higher.22 Therefore, a test result with values within the normal range (FCP<50μg/g faeces) would support the diagnosis of functional disease as a non-inflammatory cause of the symptoms.23 Referral to a gastroenterologist is usually advised with a FCP value greater than 50, but also when there is a high suspicion of IBD, even with a normal FCP, for example, if there is a strong family history. Taking this reference value, our data give an S of 100% and an Sp of 38.4% to distinguish between IBD and IBS. According to our results, in order to optimise the diagnostic efficacy of the test, it would be advisable to establish the upper limit of FCP concentrations higher than 150μg/g faeces, by which we would obtain an acceptable S (78.5%) and significantly increase the Sp (77%). We have established FCP values between 50 and 150μg/g faeces to be a grey area, in which case we recommend repeating the test after 4–6 weeks or in the event of a new outbreak of symptoms.
Taking into account the results obtained in the analysis of the ROC curve, the FCP shows a high value in the differential diagnosis of IBD versus IBS and moderate for the differentiation of organic versus functional disease.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Lozoya Angulo ME, de las Heras Gómez I, Martinez Villanueva M, Noguera Velasco JA, Avilés Plaza F. Calprotectina fecal, marcador eficaz en la diferenciación de enfermedades inflamatorias intestinales y trastornos funcionales gastrointestinales. Gastroenterol Hepatol. 2017;40:125–131.