New parameters complementary to clinical TNM classification are needed, to orient preoperative on the possibility of a R0 gastric cancer resection. We analysed the possible predictive value of blood neutrophil/lymphocytic ratio (N/L) in relation to resectability.
MethodsTwo hundred and fifty-seven gastric cancers consecutively diagnosed and without neoadjuvant treatment were retrospectively studied. Univariate and multivariate analysis of the frequency of R0 cases was performed between groups with a normal N/L ratio (<5) and pathological N/L ratio (≥5). Furthermore, we studied the subgroup of operated patients (n=156) analysing the frequency of R0 resection according to N/L ratio <5 or ≥5.
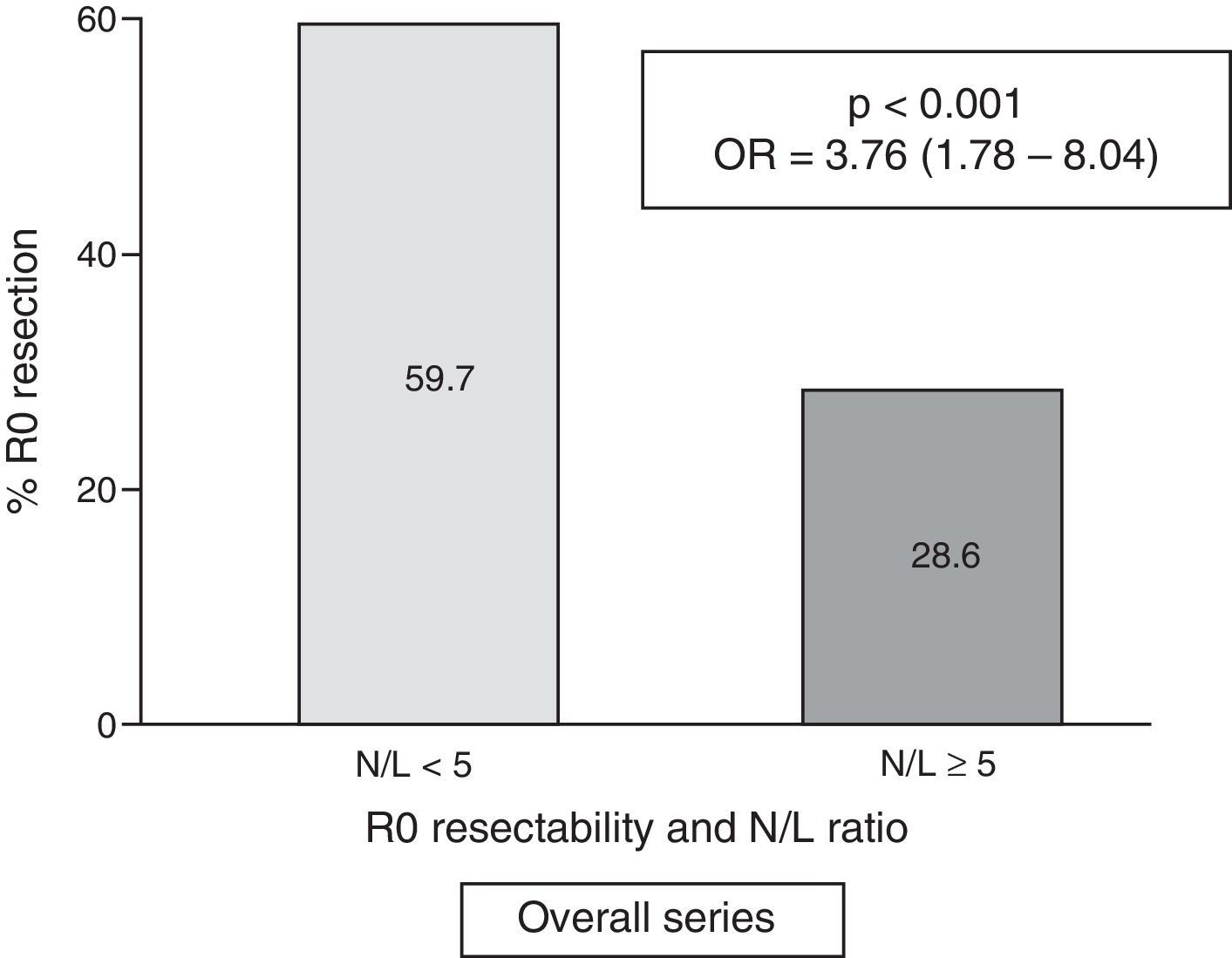
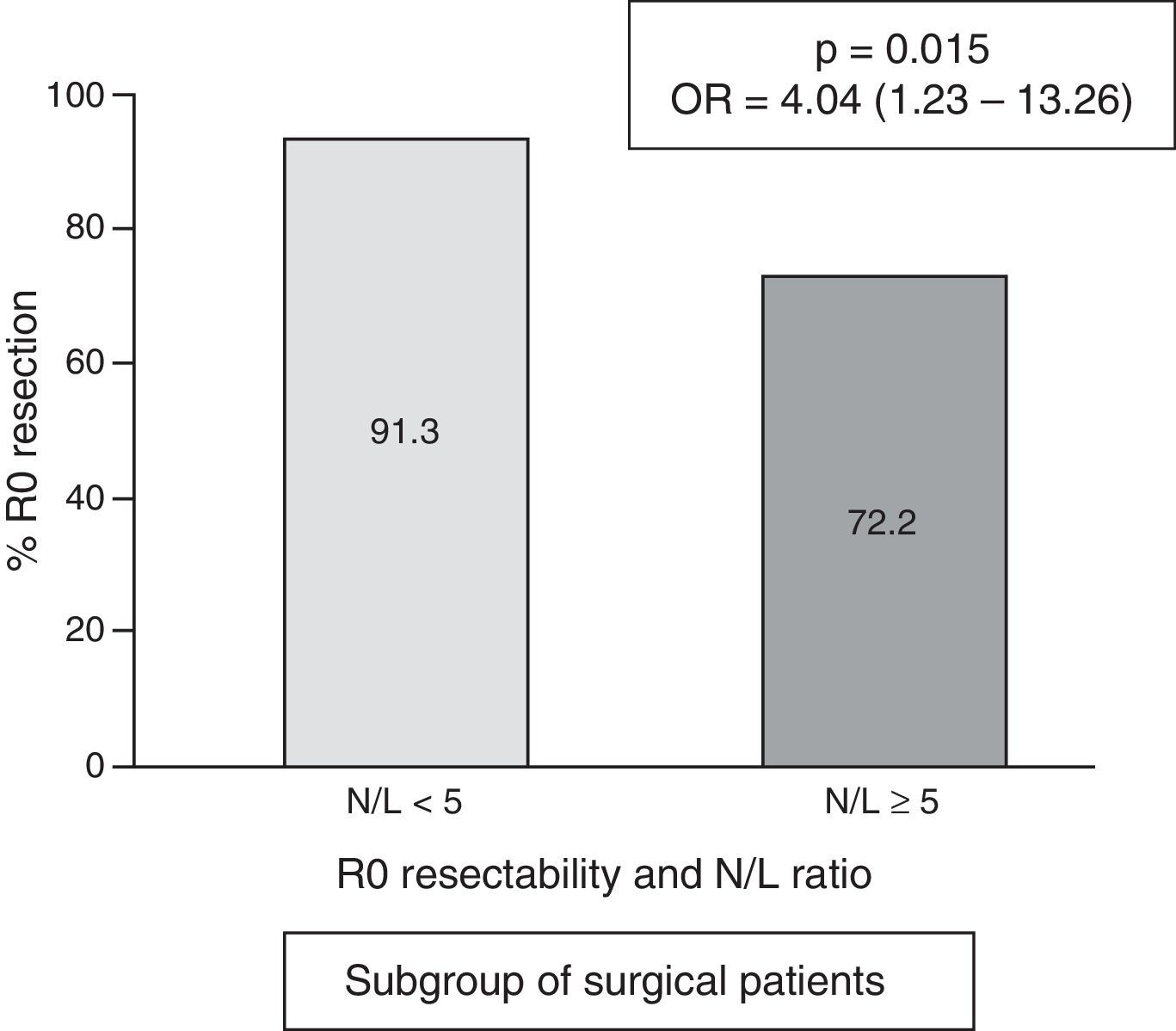
ResultsOne hundred and fifty-six patients underwent surgical intervention, of which 139 had R0 resections. A high N/L ratio was registered in 46 cases (17.9%). Globally, resectability was higher in patients with a N/L ratio <5:59.7% vs. N/L ratio ≥5:28.6% (p<.001; OR=3.76; 95% CI=1.78–8.04). The relation between N/L ratio<5 and R0 resection was confirmed in the multivariate (p=.006; OR=3.86; 95% CI=1.46–10.22). In the operated subgroup, the higher frequency of R0 resection achievement is maintained in cases with N/L ratio <5:91.3% vs. 72.2% (p=.015; OR=4.04; 95% CI=1.23–13.26).
ConclusionsThe presence of a N/L ratio<5 at the diagnosis of a gastric carcinoma is related in a significant and independent way with a higher frequency of R0 tumoral resection, globally. This higher proportion of R0 resection cases in patients with a N/L<5 ratio is confirmed in the subgroup of operated patients.
Se necesitan nuevos parámetros, complementarios al TNM clínico, para orientar preoperatoriamente acerca de la resecabilidad R0 del cáncer gástrico. Analizaremos el posible valor predictivo del cociente neutrófilos/linfocitos (N/L) circulantes sobre dicha resecabilidad.
MétodosEstudiamos retrospectivamente 257 carcinomas gástricos, diagnosticados consecutivamente y sin tratamiento neoadyuvante. Realizamos un análisis univariante y multivariante de la frecuencia de casos con resección R0 entre los grupos con cociente N/L «normal» (<5) y «patológico» (≥5). Adicionalmente, estudiamos el subgrupo de pacientes operados (n=156), analizando la frecuencia de resección R0, según su cociente N/L<5 o≥5.
ResultadosFueron operados 156 casos, con 139 resecciones R0. Registramos un cociente N/L elevado en 46 casos (17,9%). Globalmente, la resecabilidad R0 fue superior en los pacientes con cociente N/L<5: 59,7% frente al cociente≥5: 28,6% (p<0,001; OR=3,76; IC 95%=1,78-8,04). En el análisis multivariante se confirma la relación entre cociente N/L<5 y resección R0 (p=0,006; OR=3,86; IC 95%=1,46-10,22). En el subgrupo de pacientes operados se mantiene la mayor frecuencia de resección R0 en los casos con cociente<5: 91,3% frente a 72,2% (p=0,015; OR=4,04; IC 95%=1,23-13,26).
ConclusionesDe modo global, un cociente N/L<5 en el momento del diagnóstico del cáncer gástrico se relaciona de modo significativo e independiente con una mayor frecuencia de resección tumoral R0. En el subgrupo de pacientes operados se confirma esta mayor proporción de resección R0 en los casos con cociente N/L<5.
Gastric cancer is a heterogeneous disease with several different clinical, epidemiological and molecular features.1 Incidence of this type of cancer has fallen over the last few decades, particularly in Western countries.2 Nevertheless, it is still associated with a high mortality rate that is only second to lung cancer,5 and accounts for an estimated 740,000 deaths worldwide per year,3 10% of all cancer exitus4 According to statistics, 5-year survival in gastric carcinoma is as low as 25%,2 because tumour diagnosis is frequently made at an advanced stage.6 Survivability depends on radical resection (R0) of the tumour,3,7 although the feasibility of this measure cannot always be predicted, and many patients are not, in fact, candidates for resection.3
The resectability of the lesion is guided by tumour staging, clinical tumour node metastasis (TNM) or pre-treatment.8 Despite improvements in diagnostic studies, between 5% and 20% of patients undergoing scheduled surgery with curative intent present greater locoregional progression than expected, particularly peritoneal or visceral metastasis not previously detected during staging. This rules out resection9,10 and limits the predictive validity of clinical TNM.11,12 It is very important that gastric cancer patients be correctly classified, as this will not only indicate the possible need for neoadjuvant therapy in patients with advanced disease,13 but also prevent unnecessary, costly laparotomies that are associated with significant morbidity. Before starting treatment, therefore, it would be of great benefit to have access to other parameters applicable to all diagnosed cases of gastric cancer that do not rely on TNM and are potentially predictive of R0 resectability.14 For this reason, we analysed the possible predictive value of a series of blood variables that could complement tumour staging. These parameters include tumour markers, such as carcinoembryonic antigen and carbohydrate antigen 19-9, that are shown to have a sensitivity of less than 40% for predicting metastases.15 Some recent studies have suggested that the circulating neutrophil/lymphocyte (N/L) ratio could be related to the frequency of metastatic spread in gastric cancer3,16 that renders the tumour unresectable. The N/L ratio is part of the systemic inflammatory response against the tumour.17 Three meta-analyses published in 201518–20 confirmed poor prognosis in cases of gastric tumours resected in patients with a high pre-surgical N/L ratio, suggesting an association between this ratio and the progression of gastric cancer in these patients. Nevertheless, no studies have hitherto analysed the prognostic value of pre-treatment determination (surgical and non-surgical patients) of the N/L ratio to predict the feasibility of R0 resection, regardless of the patient's clinical TNM stage. The objective of our study, therefore, was to analyse the extent to which an elevated peripheral blood N/L ratio can predict the feasibility of radical resection in all patients diagnosed with gastric cancer, and to determine whether or not this ratio correlates with preoperative TNM staging. Additionally, in the subgroup of surgical patients who underwent surgery, we aimed to determine the ability of the N/L ratio to predict the frequency of R0 resection in gastric cancer patients.
Patients and methodsWe retrospectively analysed a series of patients with gastric carcinoma registered from January 2005 to December 2009 in our hospital, which had a catchment population of 230,000 at the time of the study. Authorisation to review the computerised medical records was obtained from the hospital's ethics committee. The 257 cases included were consecutively diagnosed and confirmed by gastroscopy with biopsy samples. Patients had no haematologic disease or infectious or inflammatory processes that could alter the results of the N/L ratio calculation. All patients receiving neoadjuvant treatment were excluded in order to rule out the effect this could have on tumour resectability.
Computed tomography and endoscopy was performed to determine tumour extension and establish pre-treatment TNM staging. Four patients underwent diagnostic laparoscopy. Therapeutic decisions were made by the Interdisciplinary Digestive Tumour Committee, composed of specialists in gastroenterology, digestive surgery, radiology, pathology and medical oncology, and followed the protocols used in our hospital. Indications for treatment were grouped as follows: (A) not operable due primarily to the patient's psychological and physical status (the latter was determined on the basis of the pre-treatment American Society of Anesthesiologists [ASA] score: 1: healthy person; 2: mild, controlled systemic disease; 3: severe systemic disease that is not disabling, and 4: severe, disabling disease that is a constant threat to life) and their age; (B) not operable due to the apparent unresectability of the tumour due to metastasis shown on the extension study, according to the 7th edition of the American Joint Committee on Cancer, cancer staging manual,21 and (C) operable, and subsequently undergoing surgery with curative intent. Patients underwent operations by the same specialised surgical team. Radical resection, or R0, was confirmed by absence of microscopic residual tumour in the pathology report.
We tested for differences in age (Student's t test) and ASA score (Chi2 test) between inoperable cases due to patient characteristics and the rest of the series.
The peripheral blood N/L ratio was determined from the sample taken immediately before the start of treatment, once cancer had been diagnosed. This period did not exceed 7 calendar days. After testing for normal or non-normal distribution using the Kolmogorov–Smirnov test, we determined the median and interquartile range of N/L ratio values in the series as a whole. We used the Mann–Whitney test to compare the possible differences in N/L ratio values between groups with and without R0 tumour resection.
Using the ROC curve, we established an optimal N/L ratio cut-off value of 5 or more to indicate pathology. We performed a univariate analysis of the frequency of R0 resectability using Chi2 and Fisher's exact tests, determining the odds ratio (OR) and the 95% confidence interval (95% CI). The following parameters were evaluated: sex; age over 70 years (the optimal cut-off age determined by the ROC curve); anatomical location of the tumour: proximal, medial or distal segment; Lauren classification: intestinal or diffuse, and degree of differentiation in endoscopic biopsies (well, moderately and poorly differentiated and undifferentiated); TNM tumour diagnosis (i-iv); and finally, N/L ratio <5 or ≥5.
Variables with a p value<0.05 were included in a multivariate binary logistic regression analysis in which R0 resectability was the dependent variable, and the OR and 95% CI were calculated.
Finally, we performed a separate study of the subgroup of patients who were considered operable and underwent surgery with curative intent. In this subgroup, we quantified cases with N/L ratio ≥ 5 and compared the frequency of R0 resections achieved between patients with and without pathological N/L ratio.
Statistical calculations were performed on SPSS® 19 for Windows (SPSS Inc., Chicago, IL, USA). Statistical significance was set at p<0.05.
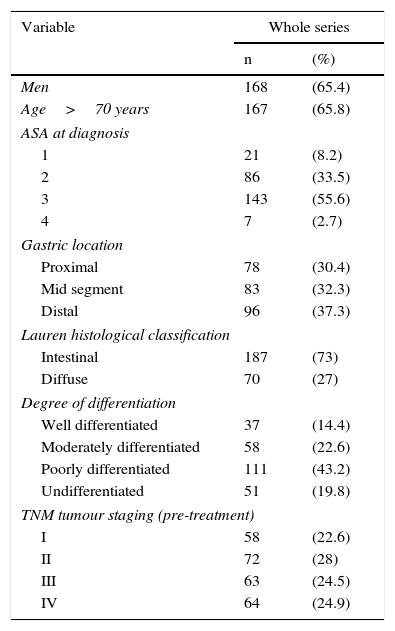
ResultsThe main patient (n=257) and tumour characteristics are shown in Table 1.
Patient characteristics (n=257).
| Variable | Whole series | |
|---|---|---|
| n | (%) | |
| Men | 168 | (65.4) |
| Age>70 years | 167 | (65.8) |
| ASA at diagnosis | ||
| 1 | 21 | (8.2) |
| 2 | 86 | (33.5) |
| 3 | 143 | (55.6) |
| 4 | 7 | (2.7) |
| Gastric location | ||
| Proximal | 78 | (30.4) |
| Mid segment | 83 | (32.3) |
| Distal | 96 | (37.3) |
| Lauren histological classification | ||
| Intestinal | 187 | (73) |
| Diffuse | 70 | (27) |
| Degree of differentiation | ||
| Well differentiated | 37 | (14.4) |
| Moderately differentiated | 58 | (22.6) |
| Poorly differentiated | 111 | (43.2) |
| Undifferentiated | 51 | (19.8) |
| TNM tumour staging (pre-treatment) | ||
| I | 58 | (22.6) |
| II | 72 | (28) |
| III | 63 | (24.5) |
| IV | 64 | (24.9) |
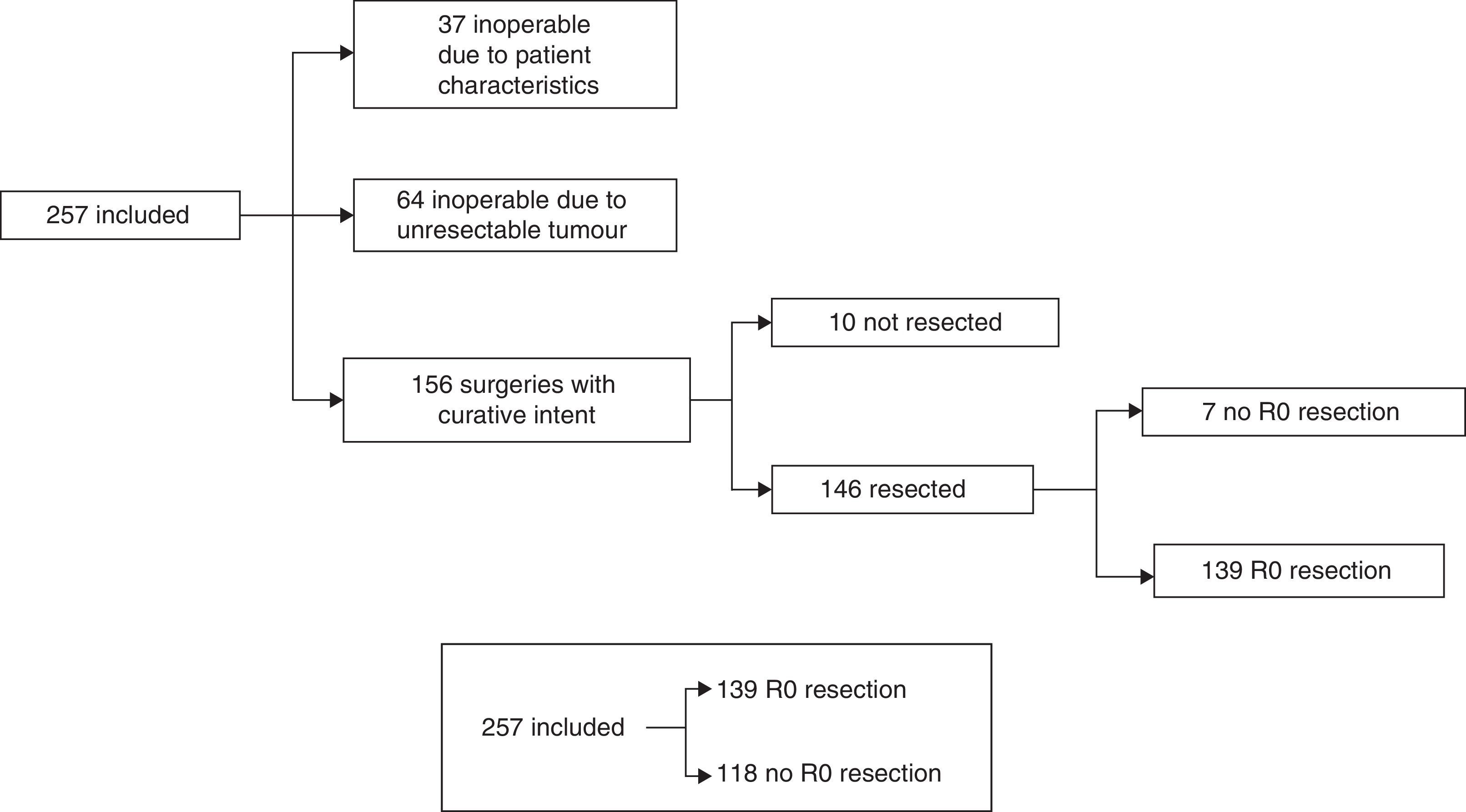
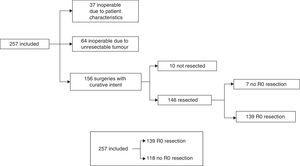
The treatment decision process is shown as a flow chart in Fig. 1, and includes: (A) inoperable due to patient characteristics: 37/257 (14.4%); (B) inoperable due to unresectable tumour: 64/257 (24.9%), and (C) operable with curative intent: 156/257 (60.7%). In group C, patients initially considered operable, 6/156 cases (3.8%) only underwent exploratory laparotomy, 4/156 (2.6%) only underwent palliative bypass surgery, and in 7/156 (4.5%), only resection with microscopic residual tumour (R1) was achieved. In the remaining 139 cases (89.1%), R0 tumour resection was achieved. Our global series was finally divided into cases with R0 resection: 139/257 (54.1%) and rest of patients: 118/257 (45.9%) (Fig. 1).
The mean age of group A, considered inoperable due to patient characteristics, was 83.8±9.9 years, significantly higher than the other cases: 70.8±11.1 (p<0.001). Likewise, the ASA score in group A was higher than in the rest of the series: group A ASA 1=0/37 (0%); ASA 2=3/37 (8.1%); ASA 3=30/37 (81.1%) and ASA 4=4/37 (10.8%). Other cases: ASA 1=21/221 (9.5%); ASA 2=83/221 (37.6%); ASA 3=113/221 (51.1%) and ASA 4=3/221 (1.4%), p<0.001.
The cut-off points for the continuous variables used to evaluate R0 resectability (ROC curve) were: age>70 years, area under the curve=0.659, and N/L ratio ≥ 5, area under the curve=0.652.
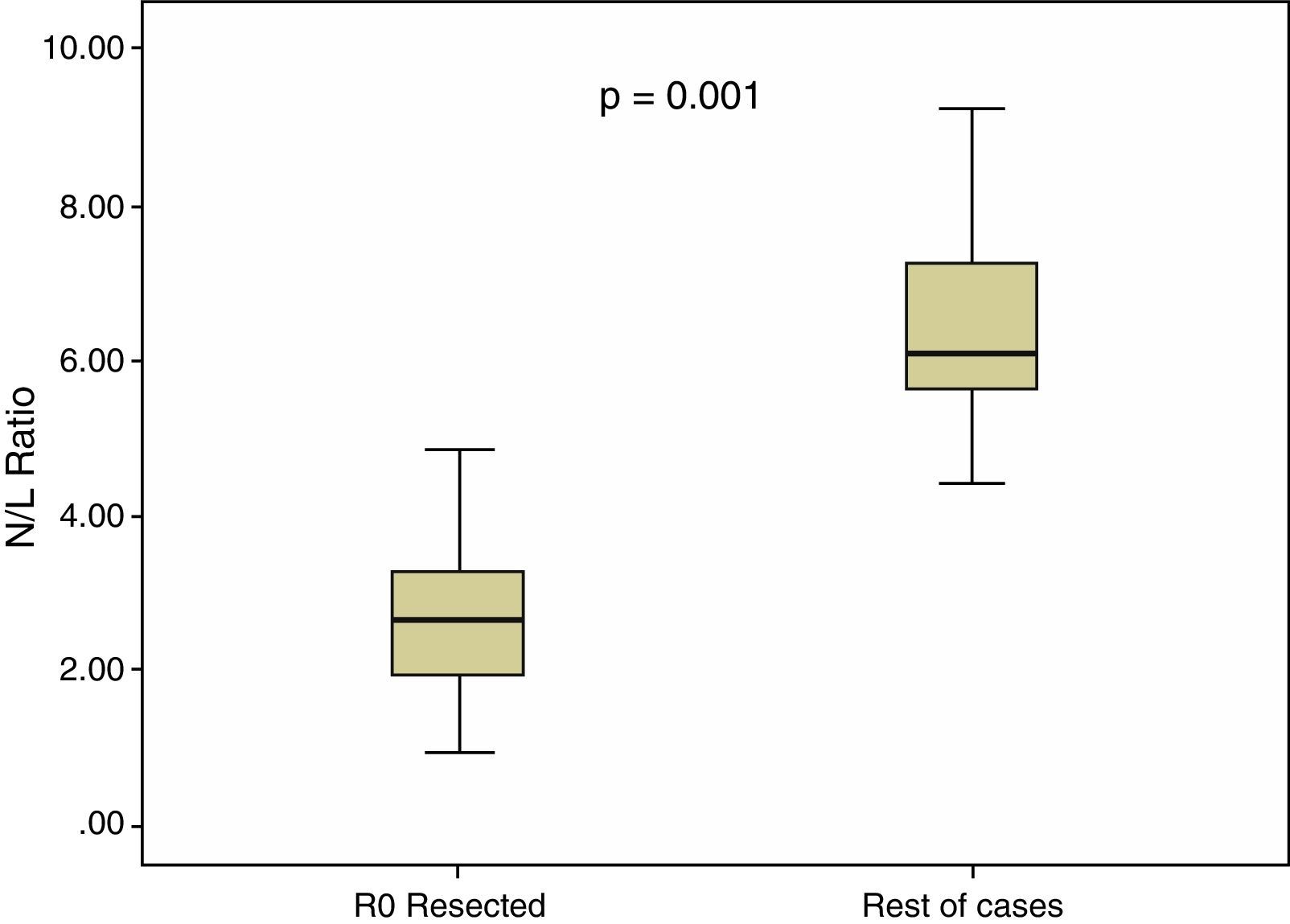
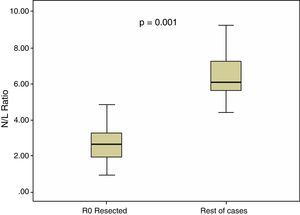
In all our cases, the median N/L ratio value was 2.70, with an interquartile range of 1.80–3.90. In patients with R0 resection, median N/L ratio was 2.30, with an interquartile range of 1.70–3.40, which was significantly higher (p=0.001) than the non-R0 resection group: median=3.05, interquartile range 2.20–5.60. These differences are shown in Fig. 2.
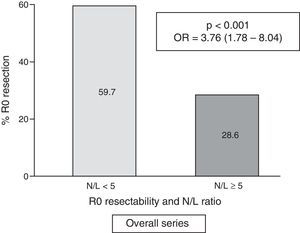
In the overall series, we observed a high N/L ratio value (≥5) in 46 cases (17.9%).
We found no significant differences in patient characteristics between subgroups with normal N/L ratio and N/L ratio ≥ 5: age=71.7±11.8 vs. 76.3±10.8 years, (p=0.21); male sex: 66.2 vs. 61.4% (p=0.54), and pre-treatment ASA: 1=8.9%; 2=36.1%; 3=52.6% and 4=2.3% vs. 1=4.5%; 2=20.5%; 3=72.1% and 4=4.5%, respectively, (p=0.10).
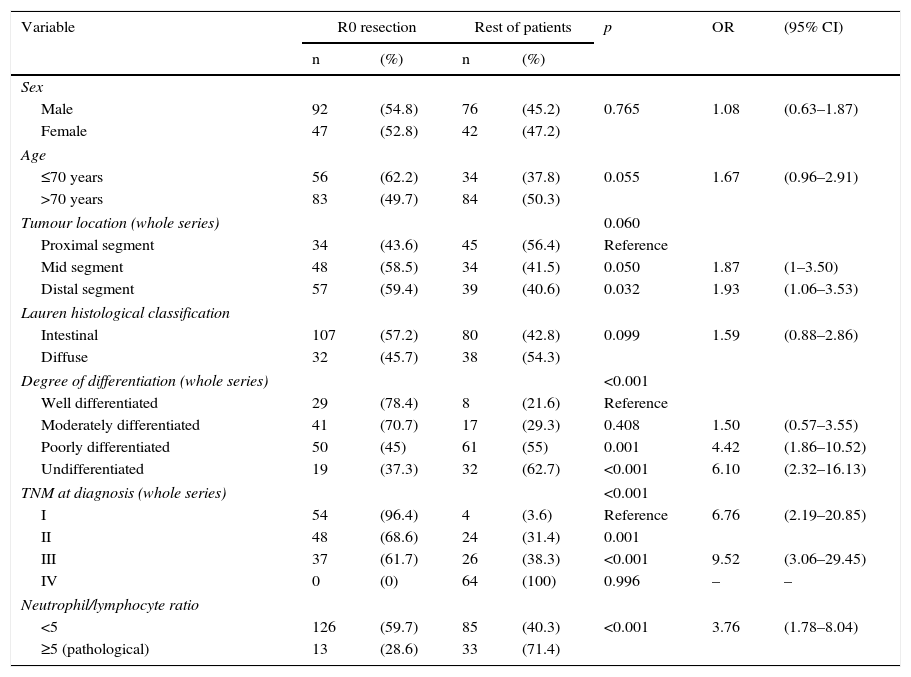
Table 2 shows the results of the univariate analysis of the frequency of R0 resectability using the different patient and tumour parameters analysed. Fig. 3 shows the frequency of R0 resections according to the normal or pathological N/L ratio.
Univariate analysis of the frequency of R0 resectability.
| Variable | R0 resection | Rest of patients | p | OR | (95% CI) | ||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | ||||
| Sex | |||||||
| Male | 92 | (54.8) | 76 | (45.2) | 0.765 | 1.08 | (0.63–1.87) |
| Female | 47 | (52.8) | 42 | (47.2) | |||
| Age | |||||||
| ≤70 years | 56 | (62.2) | 34 | (37.8) | 0.055 | 1.67 | (0.96–2.91) |
| >70 years | 83 | (49.7) | 84 | (50.3) | |||
| Tumour location (whole series) | 0.060 | ||||||
| Proximal segment | 34 | (43.6) | 45 | (56.4) | Reference | ||
| Mid segment | 48 | (58.5) | 34 | (41.5) | 0.050 | 1.87 | (1–3.50) |
| Distal segment | 57 | (59.4) | 39 | (40.6) | 0.032 | 1.93 | (1.06–3.53) |
| Lauren histological classification | |||||||
| Intestinal | 107 | (57.2) | 80 | (42.8) | 0.099 | 1.59 | (0.88–2.86) |
| Diffuse | 32 | (45.7) | 38 | (54.3) | |||
| Degree of differentiation (whole series) | <0.001 | ||||||
| Well differentiated | 29 | (78.4) | 8 | (21.6) | Reference | ||
| Moderately differentiated | 41 | (70.7) | 17 | (29.3) | 0.408 | 1.50 | (0.57–3.55) |
| Poorly differentiated | 50 | (45) | 61 | (55) | 0.001 | 4.42 | (1.86–10.52) |
| Undifferentiated | 19 | (37.3) | 32 | (62.7) | <0.001 | 6.10 | (2.32–16.13) |
| TNM at diagnosis (whole series) | <0.001 | ||||||
| I | 54 | (96.4) | 4 | (3.6) | Reference | 6.76 | (2.19–20.85) |
| II | 48 | (68.6) | 24 | (31.4) | 0.001 | ||
| III | 37 | (61.7) | 26 | (38.3) | <0.001 | 9.52 | (3.06–29.45) |
| IV | 0 | (0) | 64 | (100) | 0.996 | – | – |
| Neutrophil/lymphocyte ratio | |||||||
| <5 | 126 | (59.7) | 85 | (40.3) | <0.001 | 3.76 | (1.78–8.04) |
| ≥5 (pathological) | 13 | (28.6) | 33 | (71.4) | |||
CI: 95% confidence interval; OR: odds ratio.
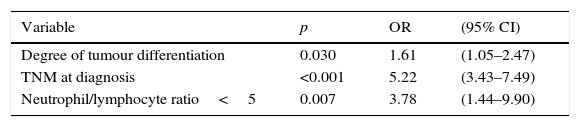
Statistically significant (p<0.05) parameters in the univariate resectability analysis, i.e., degree of tumour differentiation, clinical TNM staging and N/L ratio ≥ 5, were included in a multivariate model. The results are shown in Table 3.
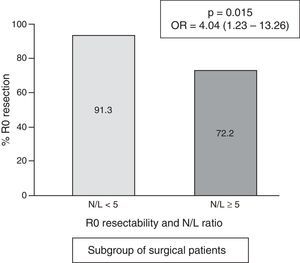
In the subgroup of patients undergoing surgery, the frequency of cases with an N/L ratio ≥ 5 was 18/156=11.5%. The median N/L ratio value was 2.3, with an interquartile range of 1.70–3.40. In surgical patients with a “normal” N/L ratio (<5), the frequency of achieving R0 resection was 91.3% vs. 72.2% in cases with pathological N/L ratio (p=0.015; OR=4.04; 95% CI=1.23–13.26) (Fig. 4).
DiscussionResective surgery is the cornerstone of treatment following a gastric carcinoma diagnosis.7 In gastric carcinoma, surgery is only indicated when it is performed with curative intent, since purely palliative surgery increases morbimortality and does not improve survival. The only exception to this rule is situations such as obstruction, refractory pain or persistent bleeding.22
When evaluating treatment, the first consideration is whether the patient meets criteria for surgery, in other words, if they are psychologically and physically able to withstand resective surgery. This decision may be based on subjective criteria, particularly when evaluating the patient's psychological status. In our case, all therapeutic decisions throughout the study were taken by an interdisciplinary committee formed by a team of specialists. The N/L ratio was not taken into consideration when deciding the therapeutic strategy. In this way, we avoided any potential bias arising from classifying patients as inoperable. As expected, a retrospective comparison of inoperable patients (due to their characteristics) vs. operable cases showed that the former were older, with higher ASA scores.
The resectability of the tumour was assessed after the patient had been classified as operable. This therapeutic criterion was established before starting treatment, taking into account tumour extension as shown on tumour staging studies, and accepting that surgery with curative intent must be ruled out in the presence of peritoneal or distant metastases.23,24 Following these criteria, surgery was ruled out in 64 patients due to an unresectable tumour.
Staging is based on radiological studies, particularly computed tomography11 and less frequently MRI or positron emission tomography.25 These techniques have a limited capacity to determine locoregional tumour stratification and detect peritoneal metastases or small, superficial liver lesions.11 Endoscopic ultrasound can improve the assessment of locoregional tumour spread, perigastric fluid collection,26 and peritoneal carcinomatosis,11,26 but is of little use in identifying distant metastases,9 and the usefulness of other studies, such as staging laparoscopy, is less well documented. Although staging laparoscopy with or without ultrasound is more specific for the diagnosis of metastases, it is also more invasive and costly,9,22 and should be limited to T3–T4 tumours with no obvious adenopathic involvement or metastasis.22
The radical resection criterion can only be established following surgery and histological study of the resected tumour, which should confirm microscopically margin-negative resection, together with adequate lymphadenectomy. Unresectability criteria include tumour involvement of the liver, pancreas, contents of the hepatoduodenal ligament, aorta, coeliac trunk, splenic artery, common hepatic artery, superior mesenteric artery and vein, inferior vena cava and abdominal wall.9
In our series, patients receiving neoadjuvant treatment were excluded due to the risk of this therapy interfering with the radical resection rate.27 R0 resection was achieved in 89.1% of tumours initially considered resectable. This is among the highest R0 resection rates reported in published studies, which have shown that a large number of patients considered candidates for surgery with curative intent on the basis of pre-treatment tumour extension studies were later found on laparotomy to have non-resectable tumours.10,11,28
Given the limitations of preoperative TNM tumour staging,11 therapeutic strategies in gastric cancer would be greatly improved if other resectability predictor variables that do not rely on TNM could be identified.29 In addition to avoiding unnecessary laparotomies, these markers would facilitate early detection of patients with unresectable tumours that could benefit from neoadjuvant therapy followed possibly by rescue surgery at a later date.27
As mentioned above, the peripheral blood N/L ratio is an indication of the patient's systemic inflammatory response to various malignancies,30–32 including gastric cancer.33 The tumour microenvironment includes immune cells. Neutrophils and lymphocytes form part of the tumour stroma and correlate closely with circulating neutrophils and lymphocytes in peripheral blood,29 and the N/L ratio forms part of the host's systemic inflammatory response against the tumour.17 Neutrophils facilitate tumour progression by inducing mutation of tumour suppressor genes, secreting cytokines and enzymes that promote tumour proliferation and metastasis, and releasing elastase and matrix metallopeptidase 9, which degrades the extracellular matrix. Neutrophils also inhibit apoptosis and stimulate angiogenesis by secreting vascular endothelial growth factor.29,34,35 Meanwhile, decreased lymphocyte levels impair cytolysis and the activity of natural killer cells and activated T cells.36 Elevated N/L ratios have been associated with worse survival following apparently curative gastric carcinoma resection.18–20,37,38 Some authors explain this worse prognosis by associating elevated N/L ratios with tumour growth, expressed as a higher rate of T4 lesions37 and metastatic tumours.3,16 This, and the lack of evidence in the literature, prompted us to investigate whether elevated N/L ratios before treatment could predict R0 resectability in all gastric cancer patients, and we therefore determined this factor in both unresected patients and in those in whom radical resection was achieved.
Several different cut off levels have been used7,20,36,38,39 to determine the effect of the N/L ratio in gastric cancer, but the ideal level has yet to be determined. We followed the criteria of other authors,7,40 and set our cut-off level at ≥5. This level was detected in 1 of every 6 patients. In our study, as in other similar papers,33,41 patients with a pathological N/L ratio were well-matched with the normal N/L ratio subgroup in terms of age, sex, or ASA score. The N/L ratio was significantly lower in patients in whom R0 resection was achieved than in the remaining cases. We evaluated the possible correlation between N/L ratio and resectability, comparing the pathological and normal N/L ratio subgroups in terms of the frequency of cases with R0 resection. Our results show that an elevated N/L ratio at the time of cancer diagnosis was significantly associated with a lower likelihood of R0 resectability, with an OR of close to 4.
The significance of the N/L ratio persisted in the multivariate analysis, thus confirming the effectiveness of this factor in predicting radical tumour resectability. The N/L ratio was not related to pre-treatment TNM or any other variable associated with greater tumour aggressiveness, such as poor differentiation.33
We observed both a lower frequency of cases with pathological N/L ratio and a lower N/L ratio value in the subgroup of 156 patients undergoing surgery with curative intent. Since operable cases mostly involve less advanced tumours, our results support the proposed correlation between the N/L ratio and the degree of tumour progression.3,16,37
The analysis of the subgroup of surgical patients also showed a significantly higher frequency of R0 resection in patients with normal N/L ratio, confirming that these patients are 4 times more likely to undergo R0 than patients with a pathological N/L ratio. Nevertheless, the validity of our results is limited by the small number of patients in whom no R0 resection was possible.
Determining the peripheral blood N/L ratio is a simple, non-invasive, low-cost procedure. An elevated N/L ratio determined at the time of cancer diagnosis could help identify patients with less likelihood of being candidates for radical tumour resection. In these cases, other options should be considered before indicating surgery with curative intent, due to the increased risk of this resulting in a costly non-therapeutic laparotomy and its associated morbidity. Our findings could also be used as an indication for staging laparoscopy in patients with pathological N/L ratios, particularly those that have already been considered candidates for surgery with curative intent. This, however, is merely a suggestion, and further studies are needed to confirm this hypothesis. Laparoscopy is a highly accurate method of confirming the presence of peritoneal and visceral metastases that would rule out surgery and indicate the need for neoadjuvant therapy.9 However, it is a costly, invasive procedure, and the criteria for indicating this strategy are still under discussion.
Our study, in line with other papers consulted, is limited by its retrospective design, and as such our findings should be viewed with caution. The single-centre study design ensures that all patients are treated by the same specialised surgical team, thus eliminating any potential surgeon bias. The disadvantage of this design, however, lies in the absence of external validity and a smaller caseload, the limitations of which are highlighted by the frequency of R0 resection in the subgroup of patients undergoing surgery with curative intent. All the foregoing, coupled with the lack of evidence from previous studies, particularly in Spain, suggests the need to confirm the value of the pre-treatment N/L ratio as a predictor of R0 resection in gastric carcinoma patients in further, preferably prospective, multicentre studies.
Conflicts of interestThe authors declare they have no conflicts of interest.
The authors would like to thank Berta Ibáñez (NavarraBiomed, Fundación Miguel Servet; REDISSEC) for her contribution to the statistical analysis.
Please cite this article as: Borda A, Vila J, Fernández-Urién I, Zozaya JM, Guerra A, Borda F. Valor predictivo pretratamiento del cociente neutrófilos/linfocitos circulantes sobre la posibilidad de resección R0 en el cáncer gástrico. Gastroenterol Hepatol. 2017;40:1–9.