Recent reports have reopened discussion of the prognostic value of elevated pre-treatment carcinoembryonic antigen (CEA) levels in colorectal cancer. Due to the discrepancies in the published results, we aimed to analyze the possible predictive value of CEA, both overall and in different tumoral stages in our environment.
Patients and methodsWe retrospectively studied 303 consecutive patients with colorectal cancer resected with curative intent by analysing tumour-related mortality. The frequency of patients with increased CEA levels (>5mg/L) was registered. Univariate and multivariate analyses of survival curves were performed, comparing patients with increased CEA levels and those with CEA levels within normal limits, both in the overall series and in the different pTNM tumoral stages.
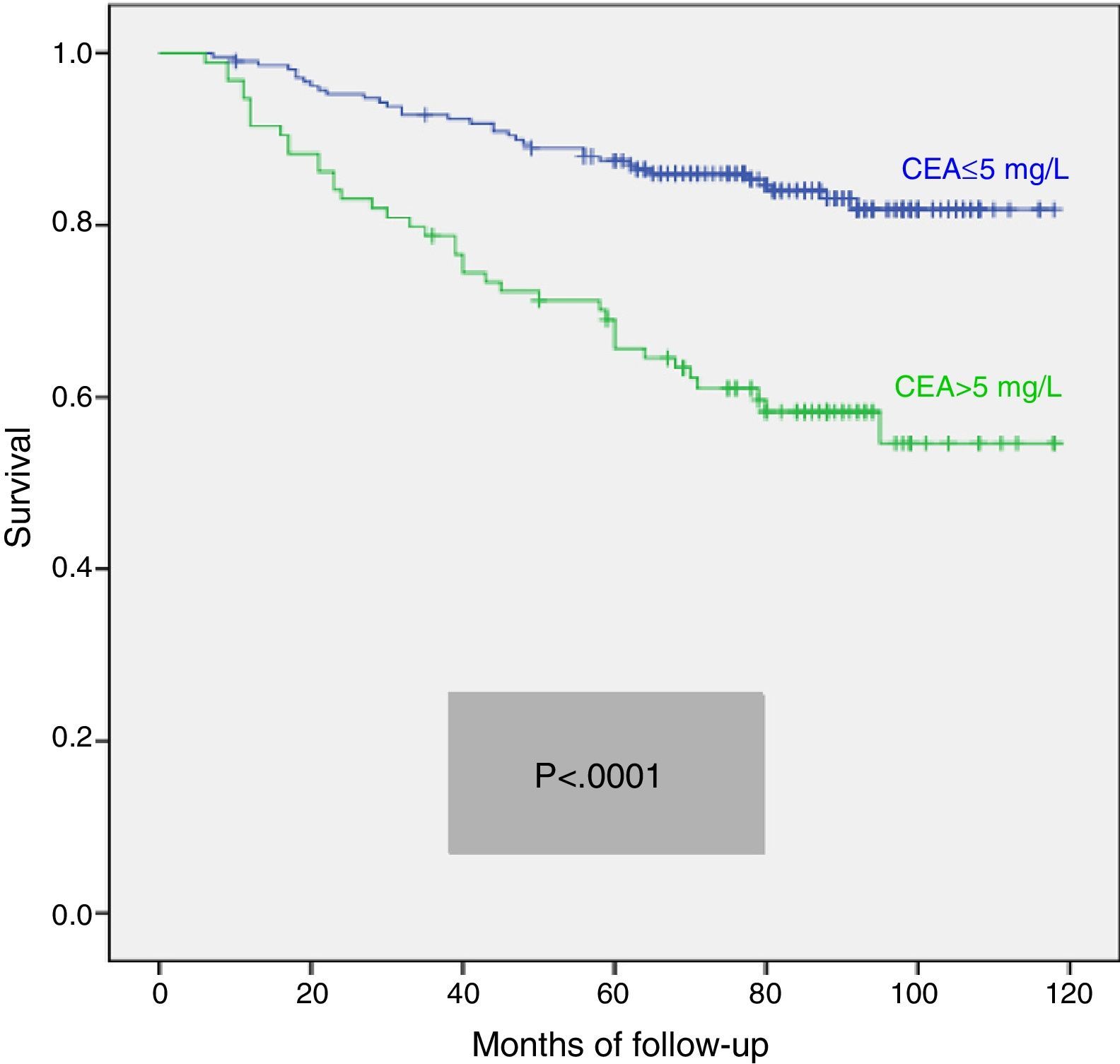
ResultsFrequency of patients with CEA>5mg/L was 31%. The median clinical follow-up was 83 months. A poor survival rate was registered in the multivariate analysis of the whole series in patients with high CEA levels: hazard ratio (HR)=1.81; 95% confidence interval (95% CI)=(1.15–3.10); p=0.012. This predictive value was only maintained in stage II in the survival analysis of the distinct tumoral stages (n=104): HR=3.02; 95% CI=(1.22–7.45); p=0.017.
ConclusionsBefore treatment, 31% of our patients with colorectal cancer resected with curative intent had pathological CEA values. In the overall series, a high pretreatment CEA level showed an independent prognostic value for poor survival. When pTNM tumoral stages were analyzed separately, CEA level had predictive value only in pTNM II tumours.
Publicaciones recientes han reactivado la discusión sobre el valour pronóstico de la elevación pretratamiento del antígeno carcinoembrionario (CEA) en el cáncer colorrectal. Debido a los resultados discordantes comunicados, pretendemos analizar en nuestro medio esta posible capacidad predictiva, globalmente y en los diferentes estadios tumorales.
Pacientes y métodosEstudiamos retrospectivamente 303 cánceres colorrectales resecados consecutivamente con intención curativa, analizando la mortalidad debida al tumour. Determinamos la frecuencia de casos con CEA pretratamiento patológico (>5mg/l). Comparamos mediante análisis univariante y multivariante las curvas de supervivencia entre los casos con CEA normal y patológico, tanto en el global de la serie como en los diferentes estadios pTNM.
ResultadosLa frecuencia de pacientes con CEA>5mg/l fue del 31%. La mediana de seguimiento clínico alcanzó los 83 meses. En el análisis multivariante de la serie global, la supervivencia fue desfavorable para los casos con CEA elevado: hazard ratio (HR)=1,89; intervalo de confianza al 95% (IC 95%)=(1,15–3,10); p=0,012. Al efectuar el análisis de supervivencia en los diversos estadios, únicamente se mantiene el valour predictivo en el estadio II (n=104): HR=3,02; IC 95%=(1,22–7,45); p=0,017.
ConclusionesAntes del inicio del tratamiento, un 31% de nuestros cánceres colorrectales resecados con intención curativa presentaron unos valores patológicos de CEA. Considerando la serie globalmente, la elevación del CEA pretratamiento presenta, de modo independiente, un valour pronóstico desfavorable sobre la supervivencia, pero al analizar su valour predictivo según los diferentes estadios, solo mantiene su significación en el estadio pTNM II.
Carcinoembryonic antigen (CEA) is an intracellular glycoprotein belonging to the immunoglobulin superfamily. It is present in low concentrations in embryonic and foetal intestine, and can also be found in insignificant amounts in the blood of healthy adults.1 As early as 1965, Gold et al.2 reported overexpression of CEA in 90% of colorectal cancer (CRC) tissue samples. Elevated serum CEA can also be found in other malignant processes, such as lung and breast cancer, or in benign conditions such as inflammatory bowel disease, diffuse liver disease and pancreatitis.3,4
CEA has been the most universally accepted and widely-used marker in CRC for decades.5 Following early publications describing high CEA levels in this disease,6 a Canadian study7 reported increased CEA in 35 of 36 CRCs, with no false positives. These results, which suggest a high diagnostic value, have not been confirmed, and the use of CEA for both screening and early diagnosis of CRC is not currently recommended due to its poor cost-effectiveness ratio.8 Most interest in the study of CEA has therefore centred on the determination of its possible prognostic utility in CRC.
In Spain, more cases of CRC are diagnosed annually than any other cancer.9 In addition to its high incidence, CRC is also associated with high mortality rates: it is the second leading cause of cancer-related death in Europe,10 and causes 12,000 deaths annually in Spain.9 Since risk for mortality varies greatly between individuals, it is very important to establish a prognosis as near to the time of tumour diagnosis as possible. Among the many predictive parameters proposed, postoperative tumour staging or pTNM is still considered the gold standard.11 However, major variations in mortality have been observed among patients classified in the same tumour stage.12,13 This limits the utility of the staging system, and raises the need for other prognostic variables not related to the pTNM classification.14
The predictive value of CEA has been analyzed in different circumstances. In some studies, levels of this marker measured at tumour diagnosis have been evaluated, both to assess the overall prognosis14,15 and to predict the response to neoadjuvant treatment,16–18 while others have examined persistently high CEA levels following surgery or administration of neoadjuvant treatment as a predictor of adverse prognosis.5 Finally, some authors have suggested periodically monitoring CEA levels during postoperative follow-up, since elevated values would lead to early suspicion of metastases or tumour recurrence, thereby increasing the chances that a new treatment might be curative.3,5,19
Despite numerous studies, the use of elevated pretreatment CEA as an independent predictor of prognosis in CRC1,15,20 remains uncertain, as recent studies have questioned its value.21,22 The last few years have seen a growing interest in the notion that the prognostic utility of pretreatment CEA differs in different TNM stages, which could help explain the discrepancy in results.14 Most studies in this context have been conducted in Asian patients, leading to the hypothesis that ethnicity could influence the evolution of CRC,23 and that this factor could have biased the study results. The few studies carried out in Spain have not found CEA to be an independent predictor of prognosis.15,24,25 Further national studies are therefore required, which prompted us to carry out this analysis of the overall and pTNM-specific predictive value of CEA (measured at the time of diagnosis) on the survival of patients who had undergone surgery for CRC.
Patients and methodsRetrospective observational study of a series of 303 patients with CRC, consecutively diagnosed and resected in our hospital, which had a catchment area of 230,000 inhabitants at the time of the analysis. The study was conducted between January 2004 and January 2007. The medical record review was approved by the hospital ethics committee. Each case was assessed by the centre's Digestive Tumours Committee, which prescribed individualized therapy: initial neoadjuvant treatment or direct surgery, according to hospital protocols. Elective resection with curative intent was performed by the same specialized surgical team. Patients undergoing palliative surgery or emergency procedures due to acute complications such as complete obstruction, perforation, bleeding or tumour abscess formation were excluded. Patients who died in the immediate postoperative period (30 days) were also excluded. Patients were followed up every 3 months for the first year, every 6 months for up to 5 years, and annually thereafter. The clinical follow-up time was calculated from the start of treatment until the patient died or the study end date was reached, with a minimum follow-up of over 5 years. The primary endpoint was survival, centred on tumour-related mortality, calculated using uni- and multivariate statistical techniques, including CEA as a possible factor.
CEA concentration, measured within 14 days of start of treatment (neoadjuvant or surgical), was assessed. The frequency of cases with elevated pretreatment CEA was determined, considering CEA values greater than 5mg/L as pathological.14
The following patient characteristics were analyzed: sex and age, anatomical location of the tumour, histological differentiation grade and tumour stage according to the American Joint Committee on Cancer classification system.11 The difference in frequency of these variables in the CEA ≤5mg/L and CEA >5mg/L groups was compared using chi-square and Fisher tests for categorical variables and the Student test for continuous variables. The CEA values were defined as mean and standard deviation, and the follow-up times as median and interquartile range.
In order to assess the possible relationship between elevated CEA and tumour stage, we compared the proportion of cases with pathological CEA in different pTNM stages. Similarly, we compared the mean CEA values measured in different tumour stages.
To study the prognostic value of pretreatment CEA, a univariate statistical analysis was first carried out, calculating Kaplan–Meier survival curves compared using the log rank test in the overall series and in each tumour stage. In order to assess the possible independent predictive significance of CEA, a multivariate study was then performed, both overall and in stage II tumours. The Cox stepwise regression model was used, including the following adjustment covariates: sex, age >65 years, rectal or colonic location of the tumour, grade of differentiation and pTNM tumour stage, calculating the hazard ratio (HR) as well as the 95% confidence intervals (95% CI). In the stage II neoplasms, classification of the lesion into categories T3 or T4 was added as a study variable.
Statistical calculations were carried out using SPSS 19 and R 2.9.2; p values <0.05 were considered significant.
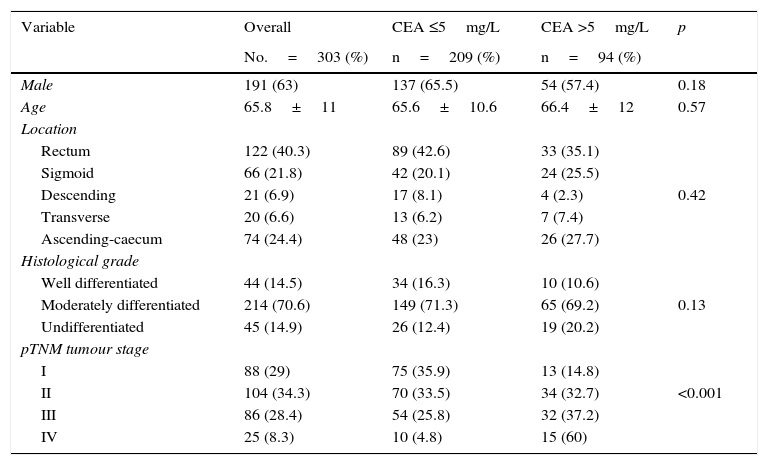
ResultsTable 1 summarizes the characteristics of our overall series. It also shows the differences found when the group of patients with elevated CEA was compared with the group with normal CEA. As can be seen, the only significant difference (p<0.0001) was the higher frequency of tumours in a more advanced stage in the group with CEA >5mg/L. The remaining parameters analyzed, both for the patient and the tumour, showed no significant differences between pathological and non-pathological CEA groups.
Characteristics of the overall series and proportion of normal (≤5mg/L) or pathological (>5mg/L) carcinoembryonic antigen (CEA) levels.
| Variable | Overall | CEA ≤5mg/L | CEA >5mg/L | p |
|---|---|---|---|---|
| No.=303 (%) | n=209 (%) | n=94 (%) | ||
| Male | 191 (63) | 137 (65.5) | 54 (57.4) | 0.18 |
| Age | 65.8±11 | 65.6±10.6 | 66.4±12 | 0.57 |
| Location | ||||
| Rectum | 122 (40.3) | 89 (42.6) | 33 (35.1) | |
| Sigmoid | 66 (21.8) | 42 (20.1) | 24 (25.5) | |
| Descending | 21 (6.9) | 17 (8.1) | 4 (2.3) | 0.42 |
| Transverse | 20 (6.6) | 13 (6.2) | 7 (7.4) | |
| Ascending-caecum | 74 (24.4) | 48 (23) | 26 (27.7) | |
| Histological grade | ||||
| Well differentiated | 44 (14.5) | 34 (16.3) | 10 (10.6) | |
| Moderately differentiated | 214 (70.6) | 149 (71.3) | 65 (69.2) | 0.13 |
| Undifferentiated | 45 (14.9) | 26 (12.4) | 19 (20.2) | |
| pTNM tumour stage | ||||
| I | 88 (29) | 75 (35.9) | 13 (14.8) | |
| II | 104 (34.3) | 70 (33.5) | 34 (32.7) | <0.001 |
| III | 86 (28.4) | 54 (25.8) | 32 (37.2) | |
| IV | 25 (8.3) | 10 (4.8) | 15 (60) | |
CEA: carcinoembryonic antigen; 95% CI: 95% confidence interval; HR: hazard ratio.
Pathological CEA levels (>5mg/L) were found in 94/303 patients (31%). The proportion of cases with pathological CEA in different pTNM stages was observed to gradually increase as the tumour stage increased: stage I: 13/88=14.8%; stage II: 34/104=32.7%; stage III: 32/86=37.2%; stage IV: 15/25=60%. These differences reached statistical significance: p<0.001.
In our overall series, mean CEA concentration was 11.2±22.5mg/L. Analysing the mean value measured in each pTNM stage also showed a gradual increase in each tumour stage: stage I: 4.6±9.4; stage II: 9.2±17.8; stage III: 12.6±20.5 and stage IV: 37.8±63.2 (p<0.001).
The median follow-up was 83 months (interquartile range: 64–93), with a 5-year tumour-related mortality of 21.4%, and 24.1% by the end of the study.
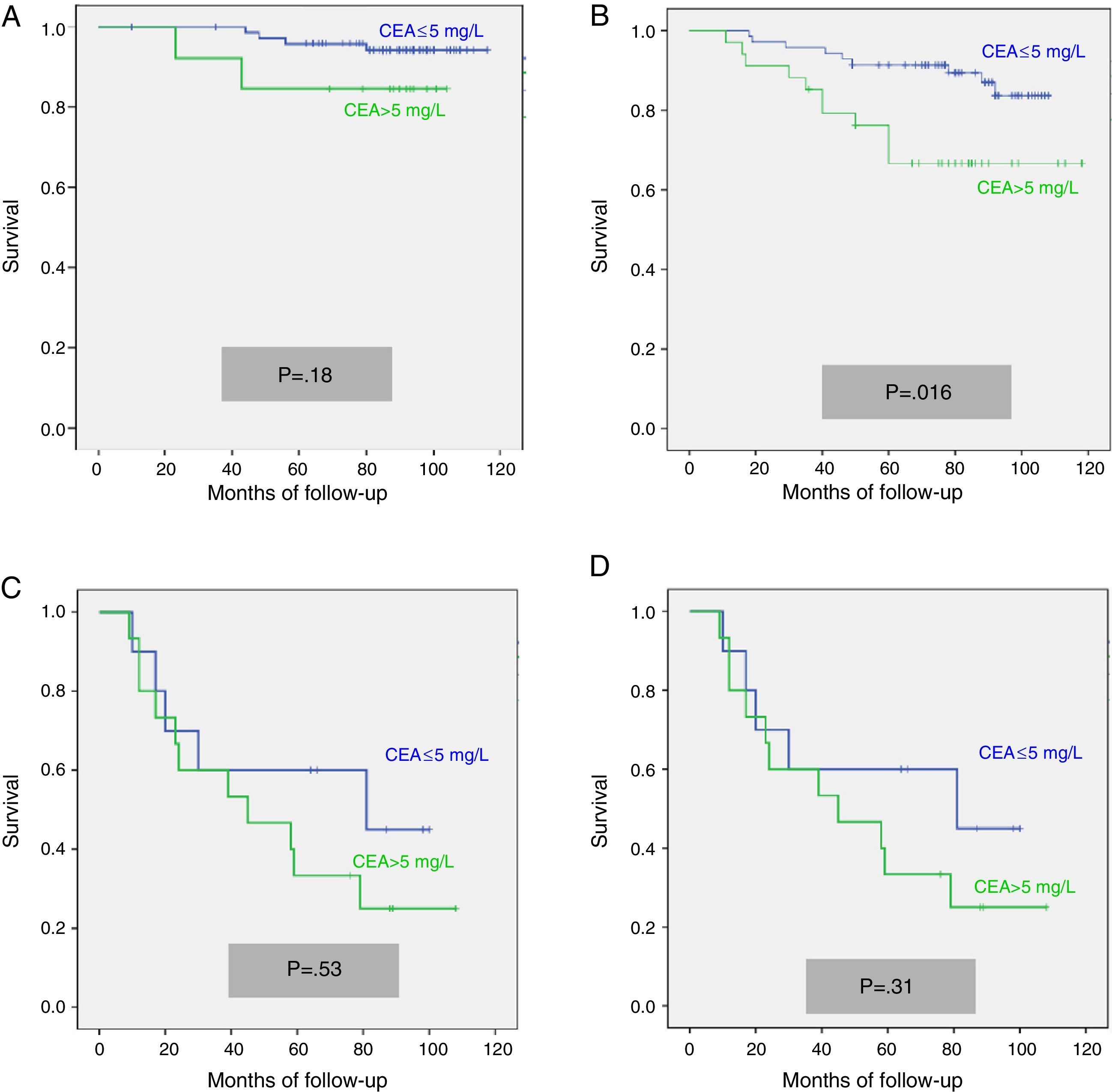
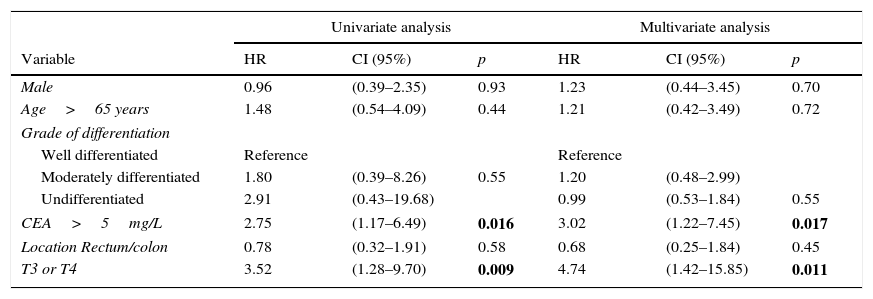
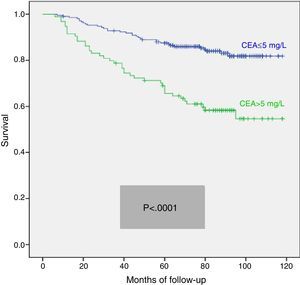
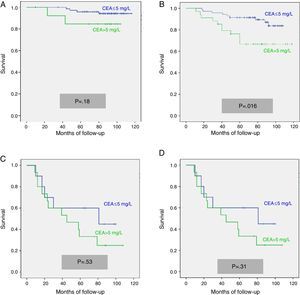
We calculated the Kaplan–Meier survival curves for cases with and without pathological CEA in both the overall series and the different pTNM stages. Overall, patients with pretreatment CEA ≤5mg/L showed significantly longer survival: (HR=3.01; 95% CI=[1.90–4.77]; p<0.0001). When patients were stratified according to tumour stage, the CEA variable lost statistical significance in stage I: (HR=3.03; 95% CI=[0.56–16.57]; p=0.18); stage III: (HR=1.26; 95% CI=[0.61–2.5]; p=0.53) and stage IV: (HR=1.71; 95% CI=[0.59–4.94]; p=0.31), maintaining its predictive value in stage II: (HR=2.75; 95% CI=[1.17–6.49]; p=0.016). Fig. 1 shows the survival curves for patients with normal and pathological CEA concentrations in our series as a whole. Fig. 2 shows the survival curves, according to the CEA, of patients with different pTNM stages.
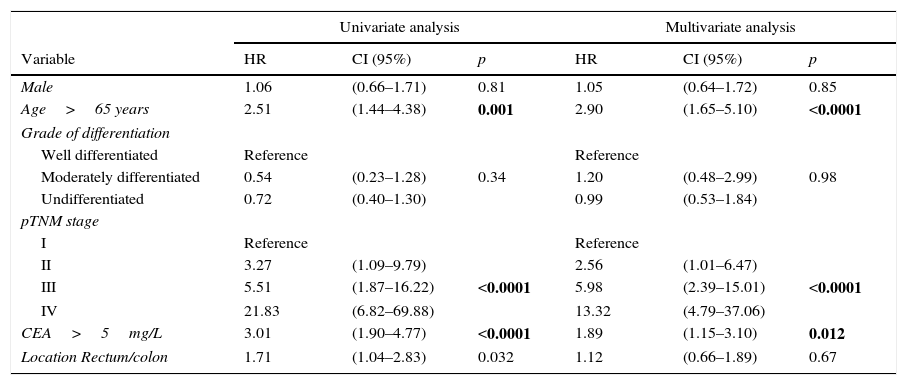
In view of the significant differences found in the overall series and the stage II sub-group, we performed multivariate analysis of survival according to CEA concentration. The adjustment variables used, as well as the results obtained in the overall series, are summarized in Table 2. We observed that only patient age over 65 years, pTNM tumour stage, and presence of a pathological CEA concentration were independent predictors of survival. Table 3 shows the survival curves of stage II cases (n=104; T3=92; T4=12). In this sub-group, only elevated CEA and category T4 were statistically significant predictors of poor survival in both the univariate and multivariate analysis.
Univariate and multivariate statistical analysis of survival curves in the overall series.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variable | HR | CI (95%) | p | HR | CI (95%) | p |
| Male | 1.06 | (0.66–1.71) | 0.81 | 1.05 | (0.64–1.72) | 0.85 |
| Age>65 years | 2.51 | (1.44–4.38) | 0.001 | 2.90 | (1.65–5.10) | <0.0001 |
| Grade of differentiation | ||||||
| Well differentiated | Reference | Reference | ||||
| Moderately differentiated | 0.54 | (0.23–1.28) | 0.34 | 1.20 | (0.48–2.99) | 0.98 |
| Undifferentiated | 0.72 | (0.40–1.30) | 0.99 | (0.53–1.84) | ||
| pTNM stage | ||||||
| I | Reference | Reference | ||||
| II | 3.27 | (1.09–9.79) | 2.56 | (1.01–6.47) | ||
| III | 5.51 | (1.87–16.22) | <0.0001 | 5.98 | (2.39–15.01) | <0.0001 |
| IV | 21.83 | (6.82–69.88) | 13.32 | (4.79–37.06) | ||
| CEA>5mg/L | 3.01 | (1.90–4.77) | <0.0001 | 1.89 | (1.15–3.10) | 0.012 |
| Location Rectum/colon | 1.71 | (1.04–2.83) | 0.032 | 1.12 | (0.66–1.89) | 0.67 |
CEA: carcinoembryonic antigen; 95% CI: 95% confidence interval; HR: hazard ratio.
Univariate and multivariate statistical analysis of survival curves in pTNM stage II tumours.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variable | HR | CI (95%) | p | HR | CI (95%) | p |
| Male | 0.96 | (0.39–2.35) | 0.93 | 1.23 | (0.44–3.45) | 0.70 |
| Age>65 years | 1.48 | (0.54–4.09) | 0.44 | 1.21 | (0.42–3.49) | 0.72 |
| Grade of differentiation | ||||||
| Well differentiated | Reference | Reference | ||||
| Moderately differentiated | 1.80 | (0.39–8.26) | 0.55 | 1.20 | (0.48–2.99) | |
| Undifferentiated | 2.91 | (0.43–19.68) | 0.99 | (0.53–1.84) | 0.55 | |
| CEA>5mg/L | 2.75 | (1.17–6.49) | 0.016 | 3.02 | (1.22–7.45) | 0.017 |
| Location Rectum/colon | 0.78 | (0.32–1.91) | 0.58 | 0.68 | (0.25–1.84) | 0.45 |
| T3 or T4 | 3.52 | (1.28–9.70) | 0.009 | 4.74 | (1.42–15.85) | 0.011 |
In CRC, CEA produced by tumour cells is released into the portal circulation, and may be metabolized by the liver in a first-pass effect. For this reason, and despite its increased production, serum CEA values can remain normal when measured in peripheral blood,6 where it only appears elevated in a small percentage of patients. In our series, the frequency of cases with pathological CEA (31%) was similar to that reported in other publications, ranging between 30% and 40%.16,26,27
Several studies have examined the possible factors behind the appearance of pathological CEA concentrations. Some suggest it could be due to certain variables such as the grade of tumour differentiation28 or its location.24 When we compared the characteristics of our case mix, both patients and tumours, we only found a significantly higher frequency of elevated CEA in the more advanced tumour stages. This was confirmed by the observation that mean CEA concentration and the proportion of cases with pathological CEA increased gradually and significantly with each increase in pTNM stage. These results are consistent with those reported in other studies,5,14,15 and can be explained by the correlation between a larger tumour cell mass in advanced stages and higher production of CEA.3
In CRC, CEA acts as an intercellular adhesion molecule and mediates cellular aggregation, facilitating tumour invasion and metastasis.5 For this reason, the potential adverse prognostic value of high pretreatment CEA levels in CRC was studied. Several studies have shown a significant relationship between the presence of initial pathological CEA values and poor outcome.1,14,29,30 At their consensus conference in 1999, the College of American Pathologists even went so far as to propose pretreatment CEA as a category I prognostic marker in CRC,31 giving it the same importance as TNM classification, lymph node or vascular invasion or the presence of residual cancer after surgery.32 This statement has been challenged on the grounds that, at the time of diagnosis, CEA levels can depend more on the tumour size than on the presence of possible occult metastases, which are really responsible for the poor prognosis,22 since it is generally agreed that tumour size in itself has no predictive value.33
The prognostic utility of pretreatment CEA in CRC, considered overall, has been widely debated in recent years, as some studies found it to have no independent predictive value.21,22 Discrepancies between studies may be due to various factors, such as sample size, duration of follow-up, and separate and combined analysis of rectal and colonic tumours.1 Another aspect to consider is the CEA cut-off point used, with values ranging between 2.4mg/L34 and 10mg/L29 being used. Like most studies,1,14,21,35,36 we defined CEA values greater than 5mg/L as pathological.
Although most studies refer to “preoperative” CEA values, we follow the lead of other authors16,17 and prefer to use the term “pretreatment” CEA, as our series also included patients who received neoadjuvant treatment before undergoing surgery. Considering the entire case mix, our results support the hypothesis that elevated pretreatment CEA in patients with CRC resected with curative intent predicts a threefold higher risk of tumour-related death. This prognostic value is independent of age, rectal or colonic location and tumour stage.
As mentioned above, pretreatment CEA levels could be a valid marker in CRC as a whole, but this validity can vary depending on the patient's tumour stage.14 This would also help to explain the different results obtained in global series, as these can include different proportions of pTNM stages. In the literature reviewed, pretreatment CEA has no prognostic value in pTNM stages I and IV,1,14,29 so the discussion centres on stages II and III. In pTNM stage II, the study findings are contradictory: most found elevated pretreatment CEA to have an independent prognostic value,1,14,37–39 while others found the opposite to be true.15,40,41 The results in pTNM stage III are also inconsistent: the significant predictive value of CEA in multivariate analysis reported by some authors15,28 has not been confirmed in more recent studies.1,14 A possible reason for this could involve the cut-off points for CEA, which were higher in studies that showed positive results than in those finding no independent prognostic value. In our study, when the pretreatment CEA was analyzed separately in each pTNM stage, we observed that it only maintained its prognostic value in stage II. In this stage, CRC is a relatively heterogeneous disease, both biologically and clinically, that includes tumours that reach but do not penetrate the serosa layer (T3), together with others that invade it (T4). Because of this, major differences have been found in the risk of recurrence after surgical resection, which can vary from 10% to 50%,12 with variations in 5-year-survival rates of up to 30%.11 This is why it is so important, particularly in colon cancer, to have prognostic factors that would allow clinicians to determine which cases would benefit most from adjuvant treatment, with the indications in this stage still under discussion. According to our results, elevated pretreatment CEA in stage II, irrespective of whether the tumour is T3 or T4, could help identify a sub-group of patients with a poor prognosis.
The main limitation of our study is its retrospective design. With few exceptions,38,39 most studies on the prognostic value of CEA share this characteristic, which limits the value of their findings. In addition, ours is a single-centre study. While this improves homogeneity and prevents bias arising from the involvement of several surgical teams, it limits the scope of the case mix and does not admit external evaluation. For this reason, our findings should be confirmed by other prospective multicentre studies, conducted preferably in Spain. If these confirm our findings in the series as a whole, elevated pretreatment CEA (>5mg/L) would be an easily obtained, low cost parameter that would help to predict lower post-resection survival from the moment of diagnosis, before tumour staging. Furthermore in pTNM stage II, both T3 and T4 tumours, it would help define a sub-group of patients with a theoretically poor prognosis, which could be taken into account when prescribing more aggressive treatment.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Borda A, Prieto C, Jiménez J, Vila J, Zozaya JM, Borda F. Valor pronóstico pretratamiento del antígeno carcinoembrionario en el cáncer colorrectal operado. ¿Es útil en todos los estadios del tumor? Gastroenterol Hepatol. 2016;39:191–198.