Cholangiocarcinomas are heterogeneous biliary tract tumors that cause devastating disease. Perihilar cholangiocarcinoma (PHC) is the most common type of biliary tract cancer and are associated with a high mortality. Diagnoses of PHC depend on the results of its clinical presentation, serum biomarkers and imaging techniques. Pre-operative managements including pre-operative biliary drainage (PBD) and portal vein embolization (PVE) could reduce mortality. The best chance of long-term survival and potential cure is surgical resection with negative surgical margin. Lymph node metastasis over N2 nodes precludes long-term survival. The benefit of concomitant vascular resection remains uncertain. Liver transplantation combined with neoadjuvant chemotherapy with radiotherapy is a promising option in highly selected patients with unresectable tumors. Herein, an overview is provided of developments in diagnosis, peri-operative management and surgical treatment among patients with PHCs.
Los colangiocarcinomas son tumores heterogéneos de las vías biliares, que provocan enfermedades graves. El colangiocarcinoma perihiliar (CPH) es el tipo más frecuente de cáncer de las vías biliares y se asocia con elevada mortalidad. El tipo de diagnóstico del CPH depende de los efectos de la presentación clínica, los biomarcadores séricos y las técnicas de diagnóstico por la imagen. Los tratamientos preoperatorios, entre los cuales se encuentran el drenaje biliar preoperatorio (DBP) y la embolización de la vena porta (EVP), podrían reducir la mortalidad. La mejor opción para lograr una supervivencia a largo plazo y la posible curación es la resección quirúrgica con borde quirúrgico negativo. La metástasis ganglionar N2 impide la supervivencia a largo plazo. El beneficio de la resección vascular concomitante continúa siendo incierto. El trasplante de hígado combinado con quimiorradioterapia prequirúrgica es una opción prometedora en pacientes cuidadosamente seleccionados con tumores inoperables. En este documento ofrecemos una visión general del desarrollo que se ha producido en el diagnóstico, el tratamiento perioperatorio y el tratamiento quirúrgico de pacientes con CPH.
Cholangiocarcinomas (CCAs) are the most common primary tumors of the biliary tract, comprising 3% of all gastrointestinal malignancies. In 1965, Dr. Gerald Klatskin, who described the distinctive clinical and pathological features of adenocarcinomas of the hepatic duct at its bifurcation within the porta hepatis, named these hilar tumors as Klatskin tumors, also known as Perihilar cholangiocarcinoma (PHC). Globally, the highest prevalence of PHC is in Southeast Asia, such as Thailand, showing a peak incidence of 84.6 per 100,000 in men, and 36.8 per 100,000 in women.1 The etiology of PHC is unclear, but several risk factors such as Opisthorchis viverrini, Clonorchis sinensis, hepatolithiasis, primary sclerosing cholangitis (PSC), chronic viral hepatitis B and C, cirrhosis, diabetes, obesity and Caroli's disease are associated with PHC.1 The best chance of long-term survival and potential cure is negative margin (R0) resection. However, due to the silent clinical nature of PHC, many patients are unresectable at the time of diagnosis. In this review, we mainly discuss the preoperative diagnosis, management and current surgical treatment in PHC.
Histology and tumor characteristicsHistopathologically, over 90% of PHCs are mucin-producing adenocarcinomas, which can be demonstrated by intracellular mucin.2 Other histologic types are rare, such as squamous cell carcinoma, papillomatosis, small cell carcinoma, undifferentiated carcinoma, embryonal rhabdomyosarcoma, papillary carcinoma, leiomyosarcoma, and cystadenocarcinoma. Causing annular thickening of the bile duct or extending longitudinally along the bile ducts in the submucosa are the specific growth patterns of PHCs. Based on the morphological growth appearance, PHC can be classified as mass-forming exophytic and intraductal subtypes. Intraductal PHC can be further subclassified as nodular and sclerosing subtype. Sclerosing tumors, which comprising 70% of all PHCs, tend to invade periductal neural tissue and vessels, resulting in marked fibrosis and inflammation of periductal tissues.2,3 The nodular subtype is characterized by irregular nodules of tumor that project into the lumen of the duct. In some cases, features of both sclerosing and nodular type may coexist, which is described as “nodular-sclerosing”. Less commonly, the most recently described subtype is an intraductal tubulopapillary neoplasm, which presents with a papillary form and has a more favorable prognosis.4
Diagnosis and workupEarly diagnosis of PHC is difficult because most patients are asymptomatic until the disease progresses and spread to outside the biliary tree. It is reported that 90% patients clinically present with biliary problems such as painless jaundice, and 10% patients present with cholangitis when tumor has grown large enough to obstruct the biliary tree.5
Serum biomarkersTumor biomarkers such as carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA) can be elevated in both benign and malignant gastrointestinal tumors. Despite none of those has reached adequate both sensitivity and specificity for PHC, CA19-9 and CEA serum levels are associated with the tumor stage. The higher the preoperative CA19-9 and CEA serum levels, the less resectability rate and the worse survival of patients.6
ImagingImaging plays a decisive role in the diagnosis, staging and assessment of resectability.
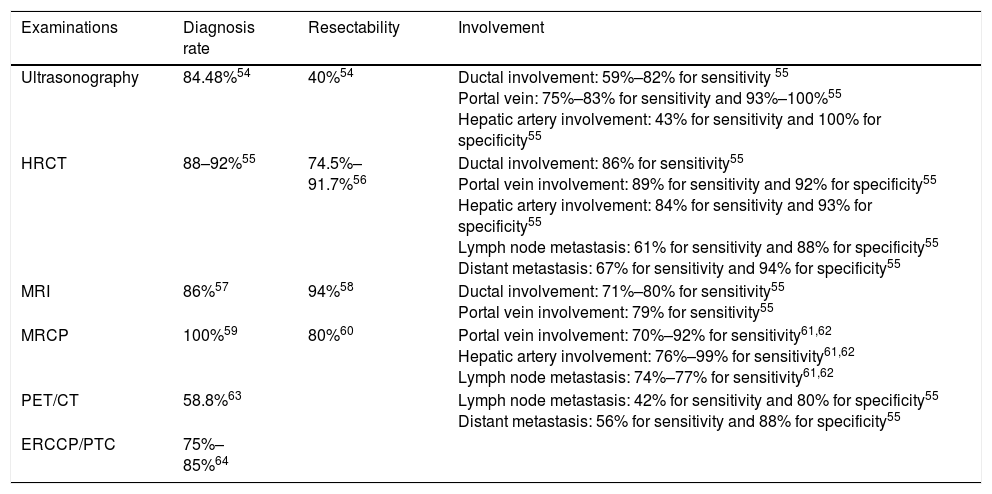
Ultrasonography is usually the initial test to evaluate patients with suspected bile duct obstruction. However, apart from the difficulty of finding small infiltrating CCAs, ultrasound has limited value in the diagnosis of extrahepatic metastasis.
High-resolution computed tomography (HRCT), which could accurately depict the thickening of the bile duct wall and the spread of tumors to the liver parenchyma or hilar vessels, is the most commonly used imaging modality for assessing resectability of PHCs. Despite the high accuracy of HRCT for evaluation of portal vein and hepatic artery involvement, determining nodal or peritoneal metastases is significantly lower.
Magnetic resonance imaging (MRI) in conjunction with magnetic resonance cholangiopancreatography (MRCP) have been increasingly used for PHCs as it allows for a clearer delineation of the intrahepatic extension of the tumor within the bile ducts and higher diagnostic specificity. However, in terms of vascular invasion and lymph node (LN) metastasis, MRI and MRCP are less feasible.
Endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous biliary transhepatic cholangiography (PTC) are helpful in assessing the extent of proximal tumor infiltration, albeit the low sensitivity and specificity. ERCP can be combined with biliary brushings for further cytological evaluation. Fluorescence in situ hybridization (FISH), which targets pericentromeric regions of chromosomes 3, 7 and 17, can significantly enhance the sensitivity of brush cytology from 21% to 58%.7 Given the invasiveness and complications, direct cholangiography for diagnostic purposes is rarely performed.
Fluorine-18 fluoro-D-glucose positron-emission tomography has a high accuracy in detecting LN and distant metastases. However, it is difficult to popularize because of the high price. Endoscopic ultrasound (EUS) with fine needle aspiration (FNA) has been used in PHC patients to biopsy the primary tumor and regional LNs, which can even detect metastatic LNs of patients with negative abdominal imaging.8 Considering a higher rate of peritoneal spread with FNA, patients who are candidates for liver transplantation or accept non-surgical therapy should be avoided9 (Table 1).
Comparison of different examinations.
| Examinations | Diagnosis rate | Resectability | Involvement |
|---|---|---|---|
| Ultrasonography | 84.48%54 | 40%54 | Ductal involvement: 59%–82% for sensitivity 55 Portal vein: 75%–83% for sensitivity and 93%–100%55 Hepatic artery involvement: 43% for sensitivity and 100% for specificity55 |
| HRCT | 88–92%55 | 74.5%–91.7%56 | Ductal involvement: 86% for sensitivity55 Portal vein involvement: 89% for sensitivity and 92% for specificity55 Hepatic artery involvement: 84% for sensitivity and 93% for specificity55 Lymph node metastasis: 61% for sensitivity and 88% for specificity55 Distant metastasis: 67% for sensitivity and 94% for specificity55 |
| MRI | 86%57 | 94%58 | Ductal involvement: 71%–80% for sensitivity55 Portal vein involvement: 79% for sensitivity55 |
| MRCP | 100%59 | 80%60 | Portal vein involvement: 70%–92% for sensitivity61,62 Hepatic artery involvement: 76%–99% for sensitivity61,62 Lymph node metastasis: 74%–77% for sensitivity61,62 |
| PET/CT | 58.8%63 | Lymph node metastasis: 42% for sensitivity and 80% for specificity55 Distant metastasis: 56% for sensitivity and 88% for specificity55 | |
| ERCCP/PTC | 75%–85%64 |
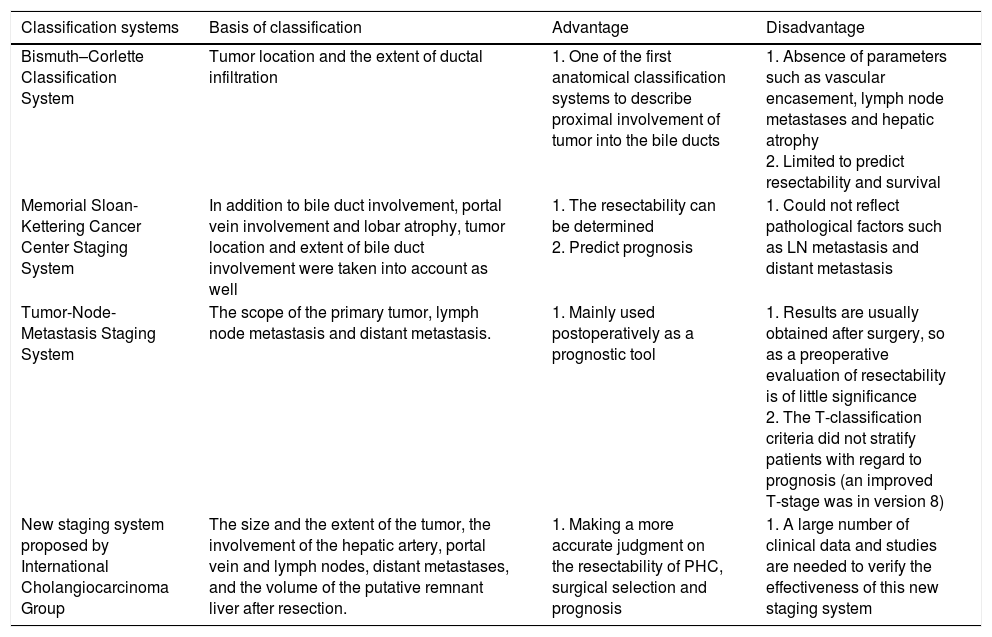
The significance of tumor staging is to guide the treatment plan and support the decision of whether to take surgical treatment or not. Besides, different classifications can also be used as a basis for predicting the prognosis of patients. There are many clinical classifications for PHC, including Bismuth–Corlette Classification System, Memorial Sloan-Kettering Cancer Center Staging System, American Joint Committee on Cancer (AJCC) Tumor-Node-Metastasis (TNM) staging system and New staging system proposed by International Cholangiocarcinoma Group. Table 2 compares the different staging systems in detail.
Comparison of different classification systems.
| Classification systems | Basis of classification | Advantage | Disadvantage |
|---|---|---|---|
| Bismuth–Corlette Classification System | Tumor location and the extent of ductal infiltration | 1. One of the first anatomical classification systems to describe proximal involvement of tumor into the bile ducts | 1. Absence of parameters such as vascular encasement, lymph node metastases and hepatic atrophy 2. Limited to predict resectability and survival |
| Memorial Sloan-Kettering Cancer Center Staging System | In addition to bile duct involvement, portal vein involvement and lobar atrophy, tumor location and extent of bile duct involvement were taken into account as well | 1. The resectability can be determined 2. Predict prognosis | 1. Could not reflect pathological factors such as LN metastasis and distant metastasis |
| Tumor-Node-Metastasis Staging System | The scope of the primary tumor, lymph node metastasis and distant metastasis. | 1. Mainly used postoperatively as a prognostic tool | 1. Results are usually obtained after surgery, so as a preoperative evaluation of resectability is of little significance 2. The T-classification criteria did not stratify patients with regard to prognosis (an improved T-stage was in version 8) |
| New staging system proposed by International Cholangiocarcinoma Group | The size and the extent of the tumor, the involvement of the hepatic artery, portal vein and lymph nodes, distant metastases, and the volume of the putative remnant liver after resection. | 1. Making a more accurate judgment on the resectability of PHC, surgical selection and prognosis | 1. A large number of clinical data and studies are needed to verify the effectiveness of this new staging system |
A more widely debated topic is the use and approach of preoperative biliary drainage (PBD) in patients with jaundice. In the case of jaundice and severe biliary obstruction, hepatectomy is associated with increased risk of postoperative complications such as hemorrhages, biliary fistulae, sepsis, and liver failure. Proponents advocate that PBD not only can reduce jaundice and postoperative complications, but also improves the ability of the liver to regenerate postoperatively.10,11 Opponents argue that PBD not only fails to reduce postoperative mortality, but also increases the risk of infection and delays treatments.12,13 Furthermore, neoplastic seeding along the track of percutaneous drains were reported up to 5.2% to 18.2%.14,15 Given the higher postoperative morbidity and higher risk of wound infections and vascular injuries,10,12,16 further prospective studies are needed to better define the optimal indications of PBD in PHC. For now, PBD is only recommended in patients with malnutrition, hypoalbuminemia, suspected cholangitis, long-term jaundice, planned preoperative anti-neoplastic therapy and future liver remnant (FLR)<30%.11,16,17 Besides, patients who required major hepatectomies, especially right lobectomy for Bismuth type IIIA or IV PHC, or preoperative portal vein embolization (PVE) with chemoradiation therapy are benefited from PBD.
The optimal drainage method is still a much-debated topic. Regardless of the location of the biliary obstruction, percutaneous transhepatic biliary drainage (PTBD), endoscopic drainage (endoscopic nasobiliary drainage [ENBD] or endoscopic retrograde biliary drainage [ERBD]), or surgical drainage can be used. Compared with PTBD, ERBD is more physiological and can improve nutritional status and immune function, reduce endotoxemia and normalize dyslipidemia.12 Because ERBD and ENBD are unlikely to cause duodenal bile duct reflux, the inflammatory reaction around the bile duct would be less severe. Compared with ERBD, ENBD was considered the most suitable method for initial PBD due to the less complications and higher success rate.18 For the selection of the site of bile duct drainage, unilateral drainage of the hepatic lobe is generally preferred, which can not only decrease the jaundice effectively, but also increase the function and volume of reserved liver leaves. However, for patients with uncertain surgical methods, slow bilirubin reduction or concurrent cholangitis, bilateral biliary drainage should be performed.
Despite the widely use of PBD in PHC, the optimal duration and preoperative bilirubin level has not been determined. Considering the possibility of tumors spread through the fistula, overlong time of drainage was associated with a lower R0 resection rate,19 therefore, duration of PBD <2 weeks is more appropriate. In terms of optimal preoperative bilirubin level, Lin et al.20 reported that patients with preoperative serum bilirubin level more than 5mg/dL had higher likelihood to acquire an infectious complication, and Farges et al.11 reported that preoperative bilirubin level more than 3mg/dL was associated with increased mortality. Prospective studies with large samples were needed to determine the optimal duration and level of preoperative bilirubin.
Portal vein embolizationFor major hepatic resection, Abdalla et al.21 described three benchmarks for safe resection: >20% FLR in patients with normal liver, >30% FLR in patients with diseased liver (steatosis or steatohepatitis), >40% FLR in patients with underlying cirrhosis. If FLR does not have enough volume or function for safe resection, PVE can increase the size of FLR by changing the local hemodynamics to release a range of interleukins and growth factors. Studies have proven that preoperative PVE can increase resectability rate without increasing mortality after hepatectomy.21,22 However, PVE may delay the surgical resection and correlate with rapid tumor growth or liver metastases. The incidence of complications in PVE were reported in 1.6%–10%, including extensive portal thrombosis, hemorrhage, transitory fever, pulmonary embolism and so on.23,24 The %FLR increase by PVE ranged from 33.8% to 43.8% in PHC patients.24 If FLR ≤20% or hypertrophy ≤5% after PVE, hepatectomy should be considered as high risk.22 For cases that the FLR is very small, a new surgical technique called associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) has been introduced. However, the 3-month mortality rate for ALPPS was extremely high, which up to 31%.25
Surgical managementSurgical resection is the best available therapy for PHCs. The type of resection depends on the location of the tumor and biliary anatomy at the confluence of the hepatic ducts. Moreover, relationships between the tumor and adjacent periductal structures, portal vein, hepatic arteries are also considered. Radical resection, which include hemihepatectomy plus lymphadenectomy, combined with excision of the liver hilum and extrahepatic biliary tree, can achieve a R0 resection rate of 76%–92%.17,26 Multiple studies have demonstrated that compared to R0 resection, R1 margins resection (microscopic disease is present at the operative margin) is a marker of worse prognosis.17,27
About 36%–50.2% of patients were found unresectable at the time of operative exploration.17,28 Unresectable may stem from the presence of a wide range of local conditions, distant metastases, or the presence of patients with complications. Massive extension of tumor into the liver parenchyma, absence of adequate parenchyma for recovery, bilateral segmental ductal extension, unilateral atrophy with either contralateral segmental ductal or vascular inflow involvement, and unilateral segmental ductal extension with contralateral vascular inflow involvement are all factors that lead to local unresectable. Furthermore, presence of metastases to LNs beyond the hepatoduodenal ligament (N2 nodes) is considered as unresectable as well.29 The existence of anatomic variations should be noted. For example, the anterior and posterior sectorial branches of the right ductal system drain directly into the main hepatic duct, once the right anterior, posterior sectorial branches and segmental involved by tumor, it is defined by the Bismuth–Corlette classification as type IV and considered as unresectable. However, a radical resection using an extended left hepatectomy may be useful in this situation.
Is hepatectomy necessary for Bismuth–Corlette I or II PHC?Even though combined major hepatic resection is widely accepted for Bismuth–Corlette III and IV PHCs, it is still controversial whether major hepatic resection is necessary for type I or II tumors. Launois et al.30 deemed that the type of surgery was closely related to tumor location, TNM classification, and staging. If tumor location is type I or II, primary tumor is Tis or T1, and in stage 0 or stage I tumors, tumor resection alone could achieve the 5-year survival of 27.3%. Ikeyama et al.31 concluded that for nodular and infiltrating tumors of type I or II, right hepatectomy is essential; while for papillary tumor, bile duct resection alone is adequate. However, studies reported that patients with Bismuth type I or II tumors who underwent bile duct resection alone often had local recurrence and poor 5-year survival rate (0%–15%), even though resections were negative margins.32,33 The assessment of the effectiveness of bile duct resection alone for type I or II tumors was limited, because the submucosal extension is predominant at the tumor's proximal border, diagnostic imagings, including cholangiography, are unable to provide precise information about longitudinal extension, which lead to the high rate of recurrence in patients underwent bile duct resection alone. Therefore, there are authors who recommend that regardless of the tumor classification, Bismuth type I and II PHCs should be considered concomitant liver resection for higher curative resections and increased postoperative survival rate.27 Nevertheless, multicenter trials are needed to manage the standard treatment for Bismuth types I and II PHCs.
Minor hepatectomy or extended hepatectomyPre- and intra-operative difficulties in accurately assessing the extent of proximal tumors have made it difficult to reach a consensus on the scope of hepatectomy. Despite the lower curability rate of minor hepatectomy (54.5%) than that of the major hepatectomy (76.7%), the 5-year survival rates had no significant intergroup difference (14.9% for minor resection vs. 25.2% for major resection).34 In addition to the same postoperative survival rates as extended hepatectomy, minor hepatectomy had the lower surgical morbidity rate.26 However, in these retrospective studies, patients presented in major or minor resection groups were not randomized. Therefore, to distinguish between the two surgical strategies in tumors with a certain bismuth type is difficult.
The bile duct from segment 1 mostly enters the left hepatic duct near the confluence of hepatic ducts, tumors can invade the biliary branches or directly infiltrate the parenchyma of the caudate lobe. In the early 1990s, Japanese scholars initially reported that combining hilar resection and partial hepatectomy with complete caudate lobe resection further improved the rate of marginal negative resection.35 For Bismuth type III and IV PHCs, caudate lobe resection not only decreased mortality, but also increased rate of R0 resection and 5-year survival. Considering the rate of caudate lobe involvement in PHCs up to 31% to 98%,35,36 routine caudate lobe resection should be carried out.
Right or left (extended) hepatectomyPerforming right or left hepatectomy depends on factors such as local tumor extension, involvement of portal vein and hepatic artery, FRL volume and so on. From the anatomical standpoint, firstly, biliary tract junction is located on the right side of the hepatoduodenal ligament, which have an anatomic advantage for right-sided hepatectomy (R-H). Moreover, if the extrahepatic part of the right hepatic duct is short (<1cm), the left hepatic duct would be a relatively long and straight line, which reaches the boundary of the left portal vein and branches into segments 2 and 3.37 In some cases, when the vertical tumor spreads to right hepatic artery, which is usually located at the back of the proximal common bile duct and close to the ductal confluence, the right hepatic artery and the surrounding tissue can be resected. In addition, the anatomical variation of the right intrahepatic bile ducts was more than that of the left. Therefore, R-H is often preferred if the tumor extent and FRL volume allow both options. For tumors of Bismuth-Corlette I, II, IIIa and IV, R-H is also preferred. R-H with the removal of the right hepatic artery and surrounding connective tissue clearly has an advantage in obtaining R0 resection and longer survival time.32,38 A disadvantage of R-H is that the inadequate FLR may lead to a higher morbidity and mortality (47.6%–59% and 8.2%–10.7%, respectively).38,39 The most frequent cause of death was liver failure, which associated with extensive parenchymal demolition. Furthermore, the sharp decrease of vascular bed in the operation of major hepatectomy may lead to acute portal hypertension.
In a review of 574 patients with PHCs who underwent resection, 51.8% patients were performed left-sided hepatectomy (L-H), while 38.3% performed R-H.40 Considering the fact that segment 4 is potentially invaded by tumor, some surgeons prefer L-H. L-H is applied to treat PHCs predominantly involving the left side of the hepatic hilum, such as Bismuth–Corlette IIIb or IV lesions. However, studies have been reported that compared with R-H, L-H not only had higher tumor-positive margin ratio and local metastase rate,38,39 but also had the lower 5-year survival rate.32,38 In order to solve this problem, some researchers tried to resect and reconstruct the right hepatic artery, its clinical significance still remains controversial. During the operation, the orifice of the right posterior sectionary duct is located behind the right portal vein, making biliary-enteric anastomosis technically more difficult. Hence, the incidence of bilioenteric anastomotic leakage was higher in L-H cases (9.5% and 18.2% respectively).39
Concomitant vascular resectionThe distance between the tumor and the portal vein is less than 1mm, vascular structures are often invaded by tumors. The reported proportion of tumors invading the vasculature ranged from 22% to 88%.32,41,42 It is technically feasible to perform related portal vein resection (PVR) in its bifurcation or main portal trunks and to have subsequent end-to-end anastomosis or vein grafts. Similarly, by using microsurgical techniques under magnification, hepatic artery resection (HAR) and reconstruction can be performed. Some authors concluded that compared with conventional hepatectomy, patients with PVR did not have any proven survival benefit.43,44 It should be noted that the incidence of advanced disease (T3 and T4) in patients undergoing PVR was significantly higher than that of patients without PVR. Although several meta-analyses reported that there was no strong evidence that could prove combined PVR leads to higher mortality for patients with PHCs,42,43 a large multi-institutional analysis of 305 patients who underwent hepatectomy combined with PVR demonstrated an increased 90-day mortality, which is up to 17.6%.41 A relatively long operation time of HAR may be associated with a higher morbidity, and patients who required HAR had a greater proportion of independent prognostic factors (portal vein invasion or perineural invasion), which worsened the prognosis. Therefore, in terms of risk of surgery and overall survival, PVR and HAR in patients with locally advanced PHCs remains controversy.
Based on the anatomical relationship, a no-touch technique including excision of portal vein bifurcation and the right hepatic artery, en bloc with tumor excision and hepatectomy was proposed. With this technique, the separation of the hilar vessels in the vicinity of the tumor was avoided. According to a study, 50 patients, even with advanced tumor stages or positive hilar LNs, who underwent curative hilar en bloc resection would have a better 5-year survival rate than that of 50 patients who underwent conventional major hepatectomy (58% and 29%, respectively).45 Due to the fact that the biliary anatomy with late segmental ramification of the left hepatic duct allows more radical resections in R-H, this technique is only feasible in R-H.37
LymphadenectomyThe incidence of LN metastasis of PHC is 30%–50%, and increases with the depth of primary tumor invasion.29,36 The actual lymphatic metastasis rate may be higher due to the inability to detect LN micrometastases in routine pathological examinations. The pericholedochal nodes were the most common sites of metastasis, and the metastasis rate could be up to 42.7%.29 The main pathway of lymphatic metastasis is from the station to the LN around the paraportal vein, the common hepatic artery and the pancreatic head, and then to the paraaortic LN.37 Most scholars recommend that the scope of lymphadenectomy includes resection of LNs around hepatoduodenal ligament, common hepatic artery and posterior pancreatic head. According to a study of 110 patients who underwent surgical resection for PHC with lymphadenectomy including both the regional and paraaortic LN, the 5-year survival rates of patients with paraaortic LN metastases was 12.3%, which had not been significantly different from that of patients with unresectable tumor.29 It is suggested that survival is not influenced by the extent of LN dissection, but rather by the presence of stage of LN. Therefore, aggressive paraaortic LN dissection is not recommended in patients with widespread nodal involvement on intraoperative inspection. Assessment of LN involvement is considered to be a key factor in the stratification of prognosis for PHC patients undergoing radical surgery. Compared to the location of LN metastasis, the number of metastatic lymph nodes seemed to more precisely indicate the prognosis after surgical resection. LN deficiency may lead to a large number of false-negative N0 patients, therefore, 3–7 LNs retrieved were recommended for correct staging.46,47
Liver transplantation and neoadjuvant optionsLiver transplantation has become a potential treatment option for patients with unresectable tumors or inability to reach R0 resection. Previously, performing liver transplantation alone was found to be a dismal failure in PHC, which associated with poor long-term survival (5-year survival rate was no more than 30%) and high recurrence rate (up to 84% within 2 years of transplantation).48 However, the majority of patients in these early studies had advanced disease and did not receive neoadjuvant chemoradiation. Based on the findings that patients with negative surgical resection margins and negative regional LNs in liver transplantation could achieve long survival,49 plus the 22% 5-year survival of patients treated with radiation, brachytherapy, and 5-fluorouracil (without resection),50 University of Nebraska and Mayo Clinic proposed a protocol for orthotropic liver transplantation in highly selected patients with early stage, unresectable PHC. These criteria include: (1) positive or strongly suspicious intraluminal brush or biopsy; (2) an ERCP demonstrating a malignant appearing stricture along with polysomy on FISH analysis on brushings of the stricture or a serum CA 19-9 of greater than 100U/mL in the absence of cholangitis; (3) a well-defined mass with the diameter <3cm on cross-sectional imaging. Eligible patients receive neoadjuvant chemoradiation. External beam radiation therapy (EBRT) is administered to a total dose of 4500cGy (in 30 fractions of 150cGy twice daily for 3 weeks) with a continuous infusion of 5-fluorouracil (5-FU) at 500mg/m2 daily given for the duration of EBRT. And then brachytherapy boost is delivered through transcatheter iridium-192 seeds (total dose of 2000–3000cGy). Patients are treated with 5-FU 225mg/m2 daily during brachytherapy. Subsequently, patients remain on oral 5-FU or capecitabine at 2000mg/m2 of body surface area in two divided doses for 2 weeks in every 3-week cycle until transplantation. Near the anticipated time of transplantation, patients undergo staging laparotomy with biopsy of hepatic artery and pericholedochal lymph nodes plus any suspicious lesion. Patients with negative staging operations proceed with liver transplantation.
When combined with neoadjuvant chemoradiation, liver transplantation evolved to represent a promising option in patients with unresectable tumors. A study of 24 patients with unresectable, stage I and II PHC who treated with neoadjuvant chemoradiation prior to liver transplantation had a 5-year survival of 82%.51 81 patients who enrolled in the PHC protocol at Mayo Clinic underwent liver transplantation after preoperative chemoradiation therapy with 73% 5-year survival and 18% recurrence rate.52 In addition, studies have demonstrated that compared with resection alone, patients with PSC-associated PHC have better outcomes with transplantation. According to Croome et al.53 the 5-year overall survival rates of 54 patients who underwent liver transplantation combined with neoadjuvant chemoradiation were greater than that of 99 patients who underwent liver resection (59% and 36%, respectively), especially for patients with PSC-associated Bismuth–Corlette IV PHC. Despite liver transplantation combined with a standardized protocol including external beam and transluminal radiation, as well as systemic chemotherapy, become the standard care for unresectable PHC, it is still difficult to generalize the results to all patients with PHCs cause patients who receive the transplant are highly selective and require strict inclusion criteria. The role of liver transplantation in patients with resectable PHC is unclear. As discussed, 5-year survival ranges are similar for both margin-negative resection and neoadjuvant therapy combined with liver transplantation. Given the scarcity of donor resources and limited high-level clinical studies of liver transplantation for PHC, resection should still be considered as the standard treatment for resectable PHC.
ConclusionsPHC is a rare and fatal gastrointestinal malignancy, which occurs at or near the biliary confluence. The diagnosis and treatment of PHC is challenging. By imaging such as CT, MRCP, PET/CT, ERCP and PTC, PHC can be properly diagnosed. Surgical resection is the only way for cure. Preoperative managements including PBD and PVE could reduce mortality. Negative tumor margins with major liver resection can improve the survival. LN metastasis over N2 nodes preclude long-term survival. The benefit of concomitant vascular resection is uncertain. For highly selected patients with unresectable tumors, liver transplantation combined with neoadjuvant chemoradiation is a promising option.
Conflict of interestNone.