There is an opportunity for improvement in the recording and measuring of quality indicators. However, no previous experiences exist in our field in terms of their compliance in esophagogastroduodenoscopy (EGD).
ObjectiveTo analyse compliance with EGD quality criteria and evaluate improvement after conducting a training programme.
Patients and methodsComparative study of 2 cohorts: one retrospective (control group) and one prospective (intervention group), before and after a training programme consisting of an information session and the report writing improvement programme. The quality indicators proposed by the American Society for Gastrointestinal Endoscopy and the American College of Gastroenterology were used.
ResultsA total of 1200 EGDs were included in a sequential manner (600 in each group). Following the training programme, a significant improvement was observed in the following indicators: documented indication (93 vs 99.8%; p<0.01), documented full examinations (94.7 vs 97.3%; p<0.01), correct performance (63.7 vs 87.9%; p<0.01), appropriate biopsies according to protocols (57.9 vs 83.8%; p<0.01), photo-documentation of described lesions (84.1 vs 94.9%; p<0.01), photo-documentation per segment (52.9 vs 70.5%; p<0.01) and correct overall assessment (56.9 vs 90.5%; p<0.01). Biopsies for coeliac disease, documented indication, full examination and correct performance, if it went ahead, exceeded the recommended standard.
ConclusionA very simple training programme improves EGD quality indicators, with the majority reaching the standards recommended by the American Society for Gastrointestinal Endoscopy and the American College of Gastroenterology.
El registro y la medición de los indicadores de calidad suponen una oportunidad de mejora. Sin embargo, no existen experiencias previas en nuestro medio sobre su cumplimiento en la esofagogastroduodenoscopia (EGD).
ObjetivoAnalizar el cumplimiento de los criterios de calidad de las EGD y evaluar la mejora tras la realización de un programa de formación.
Pacientes y métodosEstudio comparativo de 2 cohortes: una, retrospectiva (grupo control) y otra, prospectiva (grupo intervención), antes y después de un programa de formación que consistió en una sesión informativa y la mejora del programa de elaboración de informes. Se utilizaron los indicadores de calidad propuestos por la American Society for Gastrointestinal Endoscopy y el American College of Gastroenterology.
ResultadosSe incluyeron un total de 1.200 EGD de forma secuencial (600 en cada grupo). Tras el programa de formación se observó una mejoría significativa en los siguientes indicadores: indicación documentada (93 vs. 99,8%; p<0,01), exploraciones completas documentadas (94,7 vs. 97,3%; p<0,01), actuación correcta (63,7 vs. 87,9%; p<0,01), toma adecuada de biopsias según protocolos (57,9 vs. 83,8%; p<0,01), fotodocumentación de lesiones descritas (84,1 vs. 94,9%; p<0,01], fotodocumentación por segmentos (52,9 vs. 70,5%; p<0,01) y valoración global correcta (56,9 vs. 90,5%; p<0,01). La toma de biopsias para celiaquía, la indicación documentada, la exploración completa y la actuación correcta, si procedía, consiguieron superar el estándar recomendado.
ConclusiónUn programa de formación muy sencillo mejora los indicadores de calidad de la EGD, alcanzando la mayoría los estándares recomendados por la American Society for Gastrointestinal Endoscopy y el American College of Gastroenterology.
Oesophagogastroduodenoscopy (OGD) is widely used for the diagnosis and treatment of oesophageal, gastric and duodenal lesions and is considered a safe and well-tolerated procedure. It is indicated for a multitude of conditions, including dysphagia, gastrointestinal bleeding, peptic ulcer, refractory gastroesophageal reflux disease, anaemia testing and coeliac disease. The examination may involve diagnostic biopsies, as well as treatment to remove lesions, obtain haemostasis and dilatation or to place prostheses in the event of stenosis.1
The quality of healthcare can be measured by comparing the work of an individual or group with a reference standard. The parameter used for comparison is called a quality indicator. Quality indicators may be recorded as the correlation between the rate and the likelihood of correct operation, or as the proportion of procedures that meet a predefined objective. The quality indicators can be divided into three categories: (1) structural, which assess all the characteristics of the healthcare provided (for example, the participation of a doctor or other clinical personnel in a clinical data registry that includes approved consensus of colorectal cancer screening colonoscopy quality measures); (2) process, which assess performance during the procedure or examination (for example, the prescribing frequency of prophylactic antibiotics before gastrostomy feeding tube insertion); and (3) assessment of the outcome of care provided (for example, OGD rate of adverse events).1,2
The quality indicators should be objective, feasible, easy to measure and result in improved patient management. The purpose of measuring and recording quality indicators is to identify those aspects where there is a lack of compliance and to focus on making the necessary improvements in order to offer our patients care of the highest quality.3
Assessing endoscopy quality is a current priority, and, over the last decade, significant effort has been made to identify colonoscopy quality indicators (examination duration, adenoma detection rate, interval cancer, etc.). However, the selection of quality indicators for OGD represents a significant challenge as there is no well-established procedure.
In 2006, the American Society for Gastrointestinal Endoscopy (ASGE) and the American College of Gastroenterology (ACG) published the first version of gastroscopy quality indicators. These were recently updated in 2015 to include new process quality indicators divided into three periods: before the procedure, during the procedure and after the procedure.1,2 However, no studies have been conducted that use these indicators and no OGD quality data have been published in Spain.
The aim of our study was to assess the quality of the OGDs performed at our centre, and to evaluate improvement after the implementation of a training programme.
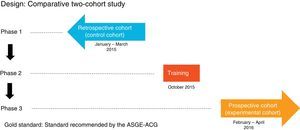
Patients and methodsDesignComparative study of two cohorts: one retrospective cohort (control group) and one prospective cohort (experimental group), before and after a training programme. The protocol was approved by the Hospital Clinic Independent Ethics Committee and the patients participating in the prospective study signed the informed consent form.
Study subjectsAll OGDs performed at Hospital Clínic de Barcelona during the assessment period.
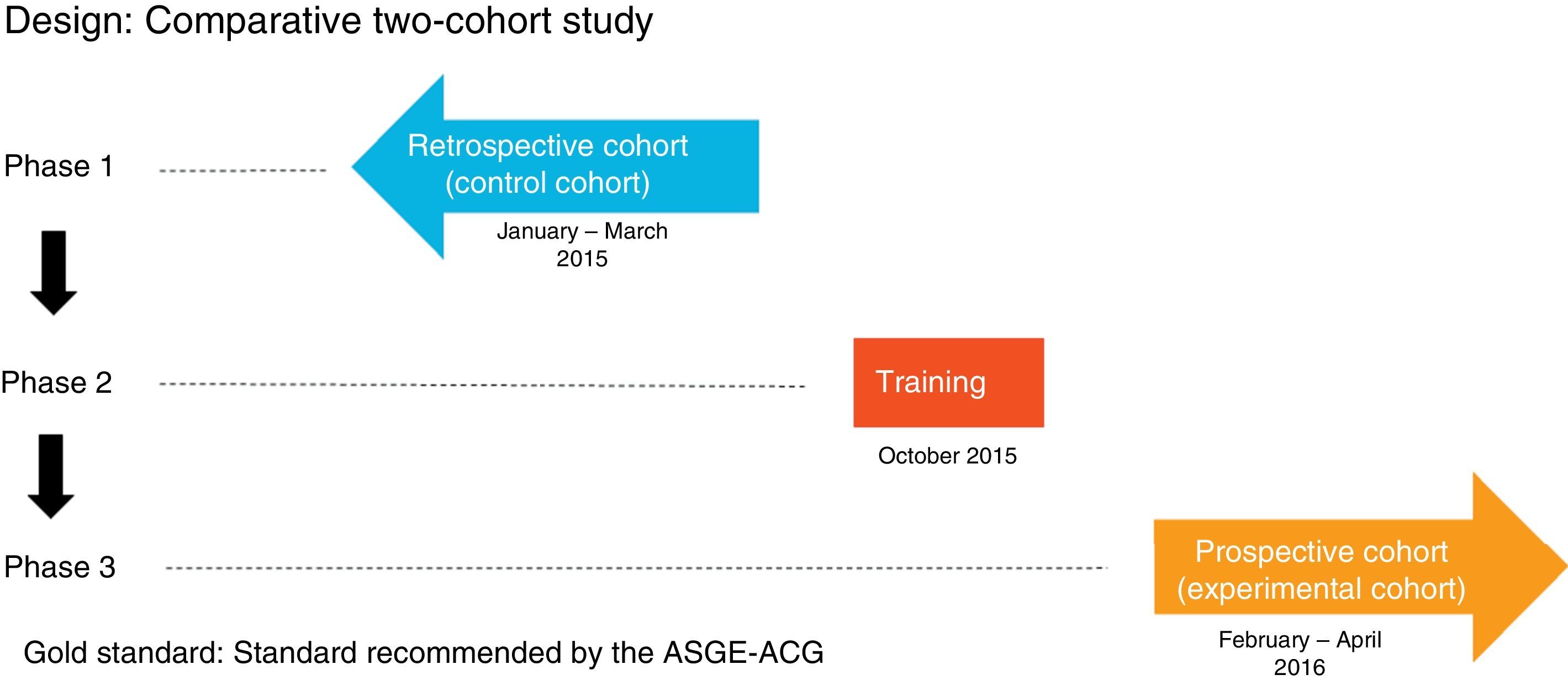
MethodIt comprised three phases (Fig. 1):
- -
Phase one: review of 600 consecutive OGDs between 1 January and 31 March 2015 (control group) by means of the Endobase® endoscopy reporting programme and the electronic medical records, collecting the data referred to in the case report forms.
- -
Phase two: OGD quality indicators training programme attended by the gastroenterologists responsible for endoscopy at the hospital, comprising an informative session during which the currently accepted indications, the classifications used in the various diseases and the updated protocols for obtaining biopsies for each indication were discussed, and all the points stipulated in the ASGE-ACG quality indicators document were reviewed. These protocols were then updated in the Endobase® programme.
- -
Phase three: review of 600 OGDs performed consecutively after the training programme from 1 February to 30 April 2016 (experimental group) by means of the Endobase® programme and the electronic medical records.
All doctors working at the Hospital Clínic Endoscopy Unit who dedicate all of their time to endoscopy were defined as expert endoscopists, while gastroenterologists who dedicate part of their time to gastrointestinal endoscopy were defined as non-expert endoscopists. The OGDs performed by residents were not included in this study.
The quality indicators were measured by calculating the proportion of procedures that met a predefined objective before and during the OGD.
Conduct was deemed to be correct when a procedure was performed when indicated (either a biopsy or a therapeutic procedure), or when no biopsy was taken or other procedure performed when not necessary.
The procedure was deemed to be completely correct if it met the examination criteria in full, if conduct was correct and if there was photodocumentation of the lesion (when applicable).
Statistical analysisThe qualitative variables are expressed in absolute values and their percentages, while the continuous variables are expressed as mean±standard deviation. The chi-square test was used to compare the proportions before and after the training programme, while the Student's t-test was used to compare the quantitative variables. Data analysis was performed with the SPSS® version 22 statistical package.
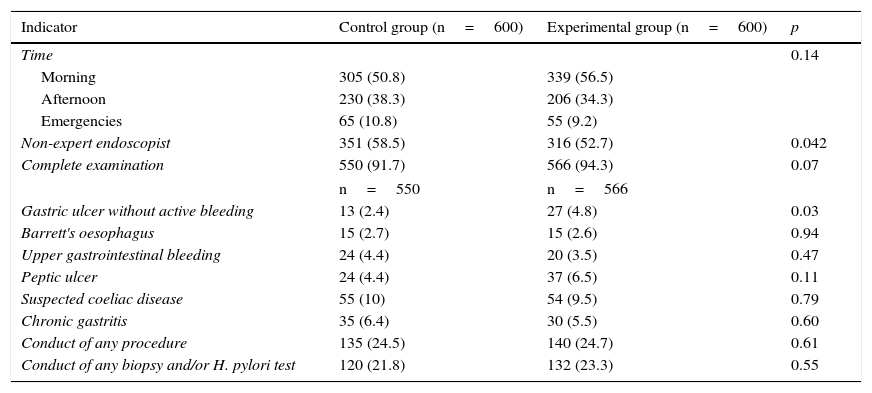
ResultsA total of 1200 OGDs were included (600 in each group). Most OGDs were performed during the morning shift and more than 50% by non-expert endoscopists (control 351 [58.5%] vs procedure 316 [52.7%]; p=0.04). No differences were observed in terms of indication or number of complete examinations between the two groups, with the reasons for incomplete examination being the presence of food, clots, intolerance of the test and the simultaneous conduct of an endoscopic ultrasound. In terms of pathological findings, more gastric ulcers with no clinical signs of bleeding were found in the experimental group than in the control group (27 [4.8%] vs 13 [2.4%]; p=0.03). No differences in the diagnosis of other diseases, the conduct of any therapeutic procedure or the taking of biopsies were found between the two groups (Table 1).
Characteristics of the two groups.
| Indicator | Control group (n=600) | Experimental group (n=600) | p |
|---|---|---|---|
| Time | 0.14 | ||
| Morning | 305 (50.8) | 339 (56.5) | |
| Afternoon | 230 (38.3) | 206 (34.3) | |
| Emergencies | 65 (10.8) | 55 (9.2) | |
| Non-expert endoscopist | 351 (58.5) | 316 (52.7) | 0.042 |
| Complete examination | 550 (91.7) | 566 (94.3) | 0.07 |
| n=550 | n=566 | ||
| Gastric ulcer without active bleeding | 13 (2.4) | 27 (4.8) | 0.03 |
| Barrett's oesophagus | 15 (2.7) | 15 (2.6) | 0.94 |
| Upper gastrointestinal bleeding | 24 (4.4) | 20 (3.5) | 0.47 |
| Peptic ulcer | 24 (4.4) | 37 (6.5) | 0.11 |
| Suspected coeliac disease | 55 (10) | 54 (9.5) | 0.79 |
| Chronic gastritis | 35 (6.4) | 30 (5.5) | 0.60 |
| Conduct of any procedure | 135 (24.5) | 140 (24.7) | 0.61 |
| Conduct of any biopsy and/or H. pylori test | 120 (21.8) | 132 (23.3) | 0.55 |
Data expressed as n (%).
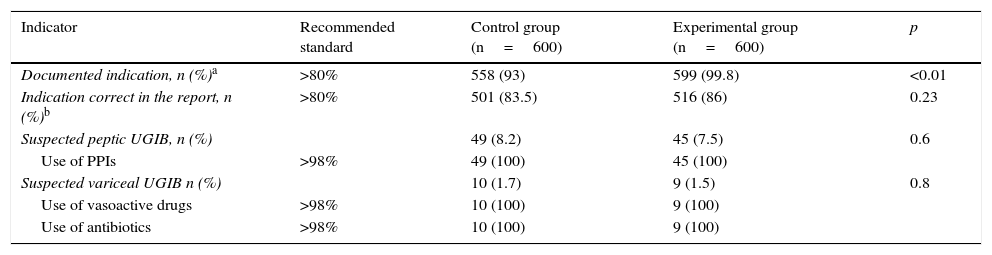
All pre-procedure indicators were above the recommended values, and a significant improvement in the documented indication after the training programme was observed (558 [93%] vs 599 [99.8%]; p<0.01) (Table 2).
Pre-procedure quality indicators.
| Indicator | Recommended standard | Control group (n=600) | Experimental group (n=600) | p |
|---|---|---|---|---|
| Documented indication, n (%)a | >80% | 558 (93) | 599 (99.8) | <0.01 |
| Indication correct in the report, n (%)b | >80% | 501 (83.5) | 516 (86) | 0.23 |
| Suspected peptic UGIB, n (%) | 49 (8.2) | 45 (7.5) | 0.6 | |
| Use of PPIs | >98% | 49 (100) | 45 (100) | |
| Suspected variceal UGIB n (%) | 10 (1.7) | 9 (1.5) | 0.8 | |
| Use of vasoactive drugs | >98% | 10 (100) | 9 (100) | |
| Use of antibiotics | >98% | 10 (100) | 9 (100) |
PPI: proton-pump inhibitors; UGIB: upper gastrointestinal bleeding.
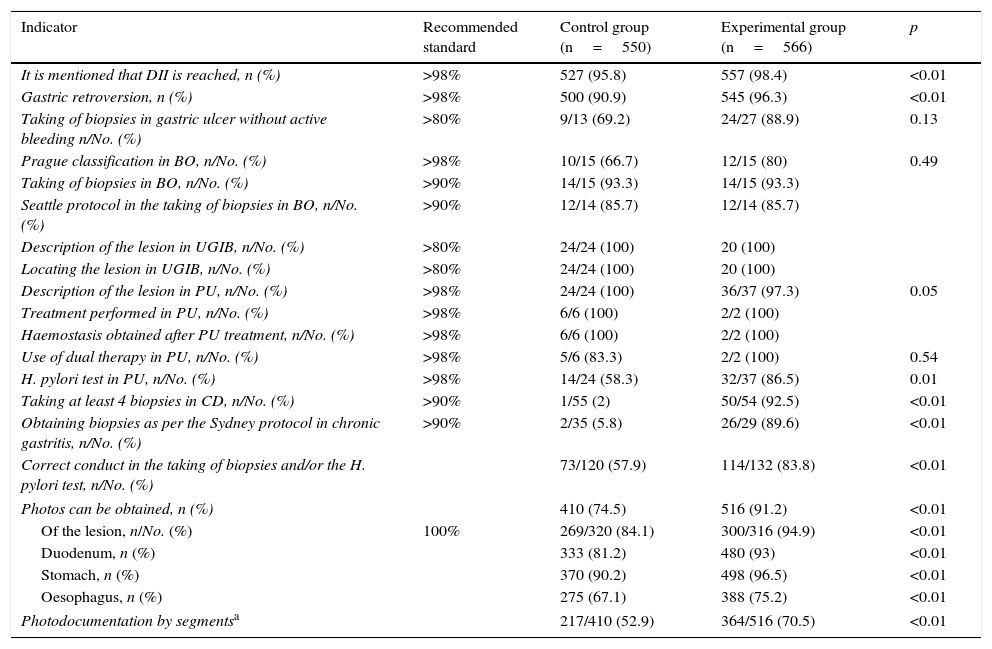
The results of the intra-procedure indicators are shown in Table 3. Reporting of reaching the second portion of the duodenum (527 [95.8%] vs 557 [98.4%]; p<0.01) and gastric retroversion (500 [90.9%] vs 545 [96.3%]; p<0.01) increased after the training programme, although only the former was above the recommended value. Other indicators that saw a significant improvement were biopsies taken according to the protocols for the study of coeliac disease (1/55 [2%] vs 50/54 [92.5%]; p<0.01), chronic gastritis (2/35 [5.8%] vs 26/29 [89.6%]; p<0.01) and H. pylori testing in gastroduodenal peptic ulcer disease (73/120 [57.9%] vs 114/132 [83.8%]; p<0.01), although only coeliac disease was above the recommended value. In many cases where no improvement was seen after the training programme, the initial values were already above the recommended standard. The indicators that did not exceed the recommended standard also failed to do so when taking into account the level of experience, emergency versus scheduled endoscopy and morning versus afternoon shift, except in the case of biopsying gastric ulcers with no active bleeding. This indicator increased from 50% to 100% (p=0.007) for non-expert endoscopists.
Intra-procedure quality indicators.
| Indicator | Recommended standard | Control group (n=550) | Experimental group (n=566) | p |
|---|---|---|---|---|
| It is mentioned that DII is reached, n (%) | >98% | 527 (95.8) | 557 (98.4) | <0.01 |
| Gastric retroversion, n (%) | >98% | 500 (90.9) | 545 (96.3) | <0.01 |
| Taking of biopsies in gastric ulcer without active bleeding n/No. (%) | >80% | 9/13 (69.2) | 24/27 (88.9) | 0.13 |
| Prague classification in BO, n/No. (%) | >98% | 10/15 (66.7) | 12/15 (80) | 0.49 |
| Taking of biopsies in BO, n/No. (%) | >90% | 14/15 (93.3) | 14/15 (93.3) | |
| Seattle protocol in the taking of biopsies in BO, n/No. (%) | >90% | 12/14 (85.7) | 12/14 (85.7) | |
| Description of the lesion in UGIB, n/No. (%) | >80% | 24/24 (100) | 20 (100) | |
| Locating the lesion in UGIB, n/No. (%) | >80% | 24/24 (100) | 20 (100) | |
| Description of the lesion in PU, n/No. (%) | >98% | 24/24 (100) | 36/37 (97.3) | 0.05 |
| Treatment performed in PU, n/No. (%) | >98% | 6/6 (100) | 2/2 (100) | |
| Haemostasis obtained after PU treatment, n/No. (%) | >98% | 6/6 (100) | 2/2 (100) | |
| Use of dual therapy in PU, n/No. (%) | >98% | 5/6 (83.3) | 2/2 (100) | 0.54 |
| H. pylori test in PU, n/No. (%) | >98% | 14/24 (58.3) | 32/37 (86.5) | 0.01 |
| Taking at least 4 biopsies in CD, n/No. (%) | >90% | 1/55 (2) | 50/54 (92.5) | <0.01 |
| Obtaining biopsies as per the Sydney protocol in chronic gastritis, n/No. (%) | >90% | 2/35 (5.8) | 26/29 (89.6) | <0.01 |
| Correct conduct in the taking of biopsies and/or the H. pylori test, n/No. (%) | 73/120 (57.9) | 114/132 (83.8) | <0.01 | |
| Photos can be obtained, n (%) | 410 (74.5) | 516 (91.2) | <0.01 | |
| Of the lesion, n/No. (%) | 100% | 269/320 (84.1) | 300/316 (94.9) | <0.01 |
| Duodenum, n (%) | 333 (81.2) | 480 (93) | <0.01 | |
| Stomach, n (%) | 370 (90.2) | 498 (96.5) | <0.01 | |
| Oesophagus, n (%) | 275 (67.1) | 388 (75.2) | <0.01 | |
| Photodocumentation by segmentsa | 217/410 (52.9) | 364/516 (70.5) | <0.01 | |
BO: Barrett's oesophagus; CD: coeliac disease; DII: second portion of the duodenum; PU: peptic ulcer; UGIB: upper gastrointestinal bleeding.
In terms of photodocumentation, more photos were taken in the experimental group (410 [74.5%] vs 516 [91.2%]; p<0.01), both of the lesions (269/320 [84.1%] vs 300/316 [94.9%]; p<0.01) and the different segments examined (217/410 [52.9%] vs 364/516 [70.5%]; p<0.01), although the quality standard was not reached for any of the indications (Table 3).
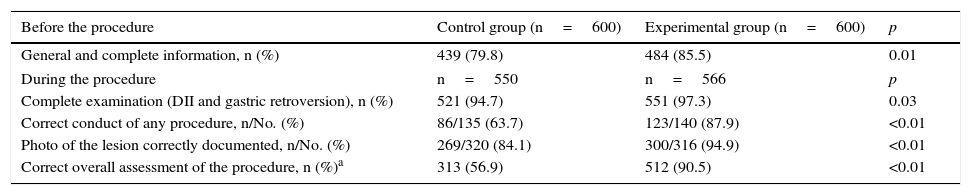
As a general assessment of the improvement programme, significant progress in the number of complete examinations, correct conduct and taking of photos was recorded, resulting in an increased number of procedures that were completed correctly (316 [56.9%] vs 512 [90.5%]; p<0.01) (Table 4).
General assessment after the improvement programme.
| Before the procedure | Control group (n=600) | Experimental group (n=600) | p |
|---|---|---|---|
| General and complete information, n (%) | 439 (79.8) | 484 (85.5) | 0.01 |
| During the procedure | n=550 | n=566 | p |
| Complete examination (DII and gastric retroversion), n (%) | 521 (94.7) | 551 (97.3) | 0.03 |
| Correct conduct of any procedure, n/No. (%) | 86/135 (63.7) | 123/140 (87.9) | <0.01 |
| Photo of the lesion correctly documented, n/No. (%) | 269/320 (84.1) | 300/316 (94.9) | <0.01 |
| Correct overall assessment of the procedure, n (%)a | 313 (56.9) | 512 (90.5) | <0.01 |
DII: second portion of the duodenum.
This is the first study to describe the quality of OGDs performed at a tertiary hospital in Spain together with a methodology to achieve a substantial improvement in such a way that the majority of the indicators comply with the standards recommended by the ASGE-ACG after conducting a simple procedure.
The recording and assessment of quality indicators represents an opportunity for improvement for both endoscopists and endoscopy units.4–6 A marked improvement in quality indicators in both colorectal cancer population screening colonoscopies7,8 and the early detection of gastric cancer9 has been observed after the implementation of training and educational programmes. However, very few studies have assessed the quality of OGDs10 and none has evaluated the effects of a training programme.
Endoscopy quality is directly related to the quality of the endoscopy report and the use of standardised terminology, which ensures better communication between professionals and complete clarity regarding the pathological findings described.5,11 Various nomenclatures, including the Los Angeles classification of oesophagitis and the Paris endoscopic classification of superficial neoplastic lesions, are used to describe different endoscopic findings.12,13 Steps have been taken in recent years to standardise the description of Barrett's oesophagus and the use of the Prague classification.14 Despite their use increasing in our study, this was not statistically significant and the recommended standards were not reached. In any case, we feel that a strategy as simple as including these classifications in the mandatory tabs of reporting programmes like Endobase® may significantly help to improve compliance with this indicator.
The taking of biopsies and compliance with the well-established biopsy protocols for certain diseases15,16 is another crucial facet of endoscopy quality assessment as it is closely correlated with early cancer detection. Indeed, taking biopsies that contravene the protocols may reduce endoscopy quality.17–19 The intestinal metaplasia–dysplasia sequence has been hypothesised to be part of the natural history of oesophageal and gastric adenocarcinoma.20 The identification of cancer precursor lesions is vital as it has been reported that 10–19% of patients with invasive gastric cancer underwent a negative OGD in the three years prior to diagnosis.21,22 Use of the Sydney protocol in cases of initial suspicion or follow-up of chronic atrophic gastritis with intestinal metaplasia improved significantly in our study, almost meeting the recommended standard. Despite our endoscopists not applying the Prague classification correctly, they were au fait with the biopsy protocol and exceeded the recommended standard even before following the training programme. In terms of gastric ulcers, despite not reaching statistical significance probably due to the small number of cases, our study met the recommended standard after the training programme. A more intensive and specialised training programme for endoscopists such as that implemented by Zhang et al.,9 which focused on early gastric cancer diagnosis together with attendance at conferences led by Japanese experts, could lead to significant improvements in this indicator.
The development of clinical guidelines for non-variceal upper gastrointestinal bleeding has led to significant benefits in the management of this condition in recent years.23 Because of the widespread use of these guidelines in our study, all the standards recommended by the ASGE-ACG were duly met, even before the procedure: description and location of the offending lesion causing the upper gastrointestinal bleeding, description of the clinical signs of peptic ulcer bleeding, endoscopic treatment indicated for peptic ulcers, use of dual therapy and obtaining haemostasis.
Poor implementation of the H. pylori test in cases of gastroduodenal peptic ulcer is a surprising finding of this study. Although recommended in more than 98% of cases, this target was not achieved despite a significant improvement after the training programme. One possible explanation could be the fact that an H. pylori test is not recommended in our local guidelines for duodenal ulcer, as direct treatment is advocated without prior testing.24
Evidence is lacking concerning the need to take photographs of the normal anatomical points of reference, or whether these photographs might improve the diagnostic performance of the endoscopy. While the ASGE-ACG recommends photodocumentation of all pathological findings and abnormalities reported, no mention is made of the number of photos required or which segments should be photographed. Appropriate photodocumentation is important as it could facilitate the diagnosis of certain diseases, primarily early gastric cancer. Photodocumentation recommendations vary from society to society, with the European Society of Gastrointestinal Endoscopy recommending eight photos, while Japanese authors recommend between 20 and 40 photographs (for example, for the systematic screening protocol for the stomach).25–28
The ASGE-ACG recommendations do not include other potential quality indicators, such as duration of the examination or the use of staining in patients at risk of oesophageal or stomach cancer. However, these indicators were proposed in a recently published document as minor OGD performance measures to improve quality.29 Diagnostic performance has been shown to be correlated to examination time, with twice as many premalignant and malignant gastric lesions detected in examinations lasting seven minutes or longer.30 In the case of Barrett's oesophagus, more dysplasia is identified with an examination time of one minute per centimetre.31 There is also currently no indicator on the use of the Sydney protocol in the initial suspicion or follow-up of chronic gastritis with intestinal metaplasia. Nevertheless, its inclusion in the future seems reasonable given that the location, extension and severity of intestinal metaplasia and gastric atrophy are risk factors associated with the onset of gastric cancer.18,20
The main limitation of our study is the retrospective nature of one of the cohorts, which precluded the assessment of outcome indicators (immediate and delayed adverse effects) and post-examination indicators (such as patient satisfaction). The lack of improvement in some quality indicators could be explained by the low number of cases and/or the time elapsed (four months) between the training session and the start of the data collection period. Finally, as a result of the study design it was impossible to ascertain whether the improvements seen were due to the training undertaken by the endoscopists or to the support offered by the computer programme. Given that the changes made to the reporting programme were minimal and the programme was used after completing the endoscopy, we believe that the improvements found reflect the endoscopists’ greater knowledge of the protocols.
In conclusion, a very simple training programme for endoscopists improves the quality indicators of OGDs, meeting the standards recommended by the ASGE-ACG in the majority of cases.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Córdova H, Sánchez-Montes C, Delgado-Guillena PG, Morales VJ, Sendino O, González-Suárez B, et al. Indicadores de calidad en la esofagogastroduodenoscopia: estudio comparativo de los resultados tras un programa de mejora en un hospital terciario. Gastroenterol Hepatol. 2017;40:587–594.