Thiopurines (azathioprine and mercaptopurine) are widely used in patients with inflammatory bowel disease. In this paper, we review the main indications for their use, as well as practical aspects on efficacy, safety and method of administration. They are mainly used to maintain remission in steroid-dependent disease or with ciclosporin to control a severe ulcerative colitis flare-up, as well as to prevent postoperative Crohn's disease recurrence, and also in combination therapy with biologics. About 30–40% of patients will not respond to treatment and 10–20% will not tolerate it due to adverse effects. Before they are prescribed, immunisation status against certain infections should be checked. Determination of thiopurine methyltransferase activity (TPMT) is not mandatory but it increases initial safety. The appropriate dose is 2.5mg/kg/day for azathioprine and 1.5mg/kg/day for mercaptopurine. Some adverse effects are idiosyncratic (digestive intolerance, pancreatitis, fever, arthromyalgia, rash and some forms of hepatotoxicity). Others are dose-dependent (myelotoxicity and other types of hepatotoxicity), and their surveillance should never be interrupted during treatment. If therapy fails or adverse effects develop, management can include switching from one thiopurine to the other, reducing the dose, combining low doses of azathioprine with allopurinol and assessing metabolites, before their use is ruled out. Non-melanoma skin cancer, lymphomas and urinary tract tumours have been linked to thiopurine therapy. Thiopurine use is safe during conception, pregnancy and breastfeeding.
Las tiopurinas (azatioprina y mercaptopurina) se usan frecuentemente en pacientes con enfermedad inflamatoria intestinal. En este documento, revisaremos sus principales indicaciones, así como aspectos prácticos de seguridad, eficacia y modo de empleo. Sus usos principales son el mantenimiento de la remisión en la enfermedad corticodependiente o tras el control de un brote grave de colitis ulcerosa con ciclosporina, la prevención de la recurrencia posquirúrgica en enfermedad de Crohn y el empleo en terapia combinada junto con biológicos. El 30–40% de pacientes no responderá al tratamiento y un 10–20% no tolerará el tratamiento por efectos adversos. Antes de iniciarlas, se recomienda evaluar el estado de inmunización frente a ciertas infecciones; la determinación previa de la actividad de la tiopurina·metiltransferasa (TPMT) no es imprescindible, pero permite mayor seguridad inicial. La dosis adecuada es de 2,5mg/kg/día para azatioprina y de 1,5mg/kg/día para mercaptopurina. Algunos efectos adversos son idiosincrásicos (intolerancia digestiva, pancreatitis, fiebre, artromialgias, exantema y algunos casos de hepatotoxicidad). Otros son dosis-dependientes (mielotoxicidad y otros tipos de hepatotoxicidad) y su vigilancia debe mantenerse mientras dure el tratamiento. Si son ineficaces o aparecen efectos adversos, puede recurrirse al cambio de tiopurina, la reducción de dosis, combinar dosis bajas de azatioprina con alopurinol y determinar metabolitos antes de descartar su uso. Los tumores de piel distintos al melanoma, los linfomas y los tumores del tracto urinario se han relacionado con su administración. Las tiopurinas son fármacos seguros en la concepción, gestación y lactancia.
There really are few milestones in the treatment of inflammatory bowel disease (IBD) that mark a turning point. Obviously, the first milestone was surgery as resection of the diseased bowel was the only option in severe cases of both Crohn's disease (CD) and ulcerative colitis (UC). The first effective therapeutic approach was corticosteroids. After these, sulfasalazine was the first step to maintaining mild cases of UC, followed shortly by the use of thiopurines for the management of steroid dependence. These early contributions to the therapeutic arsenal were made prior to the international regulation of clinical research. The evidence available regarding drugs such as corticosteroids, sulfasalazine and thiopurines comes from studies that have certain limitations in their approach. At present, the design and statistical accuracy of studies is much better.
Thiopurines (azathioprine and mercaptopurine) are drugs used to treat patients with IBD. We know that their safety profile is not ideal, but that they are capable of achieving long-lasting remission at a low economic cost.
In this document we will review the reasons for using thiopurines in IBD management, and their efficacy and safety, and we will try to define whether their indication in the treatment of such diseases is still valid.
Efficacy of thiopurinesAre they effective in luminal/perianal Crohn's disease?Thiopurine monotherapy does not play a role in inducing remission in luminal CD. Although early clinical trials conducted more than 2 decades ago recognised that these drugs were somewhat effective for this indication, the latest meta-analyses no longer support this.1,2 The latest meta-analysis (5 studies, 380 patients) finds no differences between thiopurines (48%) and placebo (37%) when assessing the induction of clinical remission. There is only one controlled study comparing thiopurines and infliximab (SONIC) and, in this study, thiopurines are inferior to infliximab for induction of remission (32% vs 48%).3 Therefore, the use of thiopurine alone is not recommended for achieving remission.
Maintenance of remission in patients with corticosteroid-dependent luminal CD is the main indication for thiopurine monotherapy.2,4,5 In the latest systematic review, which includes 6 studies (n=-489), 73% of the patients treated with azathioprine remained in remission vs 62% of those treated with placebo.5 These figures underestimate the drug's effectiveness since the meta-analysis includes studies with doses considered subtherapeutic (1mg/kg/day of azathioprine). In the typical clinical scenario in which thiopurines are used to maintain remission in patients with IBD, the disease is corticosteroid-dependent and 2.5mg/kg/day of azathioprine is generally considered to be the effective dose.6
Finally, thiopurines are somewhat effective in perianal disease,7 although they are not indicated for use as monotherapy in the disease's complex forms. Even though there is little evidence, the 2 latest studies agree that early use of thiopurines reduces the need for perianal disease-related surgery.8,9
Are they effective in ulcerative colitis?Thiopurines are widely used in patients with UC and in various scenarios. There are few quality controlled trials that support their use for the maintenance of remission. In this clinical scenario, the meta-analysis by Gisbert et al. suggests a mean efficacy of azathioprine of 60% in controlled trials (NNT 5) and 76% in non-controlled trials.10 The most recently published meta-analysis includes 4 studies (n=232), although the quality of the evidence is poor, and concludes that patients treated with azathioprine experience a lower relapse rate than patients treated with placebo (44% vs 65%, respectively).11 It is necessary to mention the randomised, controlled trial conducted by Ardizzone et al. comparing azathioprine and mesalazine in the treatment of steroid-dependent UC, which showed that azathioprine is significantly more effective (53% vs 21%; OR: 4.78; 95% CI: 1.57–14.5) for inducing clinical and endoscopic remission and avoiding steroids in this group of patients.12
Another scenario in which thiopurines are used is in patients with acute flare-up of steroid-refractory UC managed with ciclosporin. We have observational studies analysing the effectiveness of azathioprine/mercaptopurine as maintenance therapy in this situation, and, based on these, we can conclude that it is a useful therapy for maintaining remission.13–19 However, the number of colectomies described in these studies is significant (between one-third and two-thirds of all cases), and therefore it seems that close monitoring of these patients is required for early detection of those patients requiring a step-up to biologics. Thiopurines can be introduced at the same time as switching from intravenous ciclosporin to oral ciclosporin. The GETECCU (Spanish Working Group on Crohn's Disease and Ulcerative Colitis) Guideline on UC recommends administering maintenance treatment with azathioprine/mercaptopurine (with or without oral ciclosporin for the first 3 months) after induction of remission with ciclosporin in patients with acute flare-up of steroid-resistant UC.20
Randomised clinical trials have shown no differences between infliximab and ciclosporin in steroid-refractory UC. This means that the choice of one drug or the other will depend on clinical factors, such as prior azathioprine intolerance or failure, or other aspects, such as the centre's experience and economic cost.21,22 In patients who were already being treated with thiopurines at the time of the acute flare-up, subsequent maintenance with azathioprine monotherapy after remission is very ineffective23; therefore, in this group of patients, direct use of infliximab instead of ciclosporin is more appropriate for treating the steroid-refractory condition followed by combined maintenance treatment with infliximab and thiopurines.
In a Spanish study involving patients in the ENEIDA database, which analyses the risk of colectomy in patients with UC being treated with thiopurines for any indication, almost one-tenth of patients required a colectomy and one of the factors associated with a higher risk was the need for therapy with ciclosporin or anti-TNF, as well as the need for early treatment with azathioprine/mercaptopurine.24 In another analysis involving this patient database, the cumulative colectomy rate was higher with ciclosporin than with infliximab, although in the patients treated after 2005, there were no differences in early and late colectomy rates between the two groups.25
Are they effective for preventing post-operative recurrence in Crohn's disease?Surgical resection is an effective method for treating CD. Unfortunately, it is not a curative procedure, and lesion recurrence on the ileal side of the anastomosis is the norm. At present, it is accepted that resection is an integral part of a more comprehensive work plan, and post-operative prophylactic treatment must be considered in all cases. In this regard, GETECCU recommendations for this therapeutic scenario26 have recently been published, and the reader should read these for a more complete analysis.
Thiopurines were one of the first drugs evaluated in clinical trials due to their affordability and ease of use. However, the different designs of the published studies make it difficult to compare overall results and obtain clear and solid recommendations. The meta-analysis evaluation of the results27 supports the effectiveness of thiopurines in post-operative endoscopic and clinical recurrence prevention. It is accepted that thiopurines should be used at a dose of 2–2.5mg/kg/day of azathioprine, with metronidazole added for the first 3 months after surgery to optimise results.28,29 Under these conditions, the post-operative endoscopic recurrence rates one year after resection are around 40%.28–30 We only have one controlled clinical trial that has compared an anti-TNF agent with thiopurines in this context. López-Sanromán et al., in a study sponsored by GETECCU, observed no y in endoscopic recurrence rates at 54 weeks between adalimumab (induction with 160mg, 80mg and maintenance with 40mg every 2 weeks) and azathioprine (2–2.5mg/kg/day), both in combination with a course of metronidazole for the first 3 months after surgery (42% vs 59% according to an intention-to-treat analysis, p=0.12).30 However, this lack of significant differences must be considered with caution due to the limited sample size.
Finally, the use of azathioprine in patients who develop recurrent endoscopic lesions may improve or even make lesions disappear,31 although we only have evidence for patients who developed such lesions without receiving any active treatment.
Start time, dose and duration of treatment with thiopurinesThiopurines have proven to be effective in IBD; however, 30–40% of patients do not respond to them, including 10–20% of cases that will not even tolerate them,32 although there are no known predictors of response to these drugs. In addition, steroid-free clinical remission is achieved in 30–50% of patients with mucosal healing in one-third of them. Therefore, these drugs should not be used in all patients. They are probably not indicated for patients with mild disease, but should be used in steroid-dependent patients with moderate disease33 as they help maintain remission in these patients, as mentioned before. They are also indicated in patients with severe disease in combination with biologics.33
Whether thiopurines should be used at an early stage in CD, i.e. within the first few months of diagnosis, remains controversial. As mentioned before, we know that these drugs do not induce remission when used as monotherapy. It has been suggested that early introduction of thiopurines could change the clinical course of CD based on data obtained in a placebo-controlled study conducted in a paediatric population.34 The Spanish AZTEC study was conducted in an adult population with a similar approach in order to evaluate the efficacy of azathioprine in an unscreened population of adult patients with newly diagnosed CD. The authors concluded that early treatment with azathioprine was not superior to placebo in adult patients with newly diagnosed uncomplicated CD.35 The RAPID study did not show that early treatment with azathioprine was superior to conventional management in a group of patients with newly diagnosed CD at high risk of developing disabling disease either, except for those at risk of developing complex perianal disease.8 In light of the above, conventional management using early intervention with immunomodulators when the disease shows steroid-dependence criteria seems to be the most appropriate.8,35
Both the dose and the duration of treatment determine its success. The recommended dose is 2.5mg/kg of azathioprine or 1.5mg/kg of mercaptopurine.7
In UC, the role of mesalazine in maintenance therapy is one of the determining factors of the reduced need for immunosuppressant therapy in this disease in comparison with CD. Concomitant prescription of thiopurines and 5-aminosalicylates (5-ASA) is common in steroid-dependent UC in an attempt to optimise the efficacy of therapy.36 When the severity of the disease requires the administration of thiopurines, there may be doubts regarding the efficacy and need for this concomitant prescription. The potential protective role of 5-ASA against colorectal cancer may argue in favour of such use37; however, thiopurines also play a protective role in relation to the onset of colon dysplasia,38 and, therefore, adding 5-ASA for that sole purpose does not seem a convincing argument. Likewise, the potential synergism between the two drugs because of the inhibition of thiopurine methyltransferase (TPMT) enzyme activity by 5-ASA39 has been discussed, as has an increase in 6-thioguanine (6-TGN) nucleotide levels.40 However, in vivo studies have been unable to show significant changes in TPMT levels in association with 5-ASA therapy.41 On the other hand, other authors have shown that the addition of 5-ASA does not increase the efficacy of azathioprine/mercaptopurine in steroid-dependent UC, but may increase the risk of myelotoxicity.42 A systematic review of the efficacy of concomitant thiopurine and 5-ASA prescription in UC concluded that the existing evidence obtained from clinical trials is insufficient to demonstrate whether concomitant treatment with 5-ASA and azathioprine improves the results of azathioprine alone.43 Furthermore, an increase in the number of drugs administered as long-term maintenance therapy may make therapeutic adherence more difficult, and good adherence is a key factor for treatment success.44 Based on the available data, we can state that the concomitant prescription strategy does not appear to be superior to monotherapy with azathioprine/mercaptopurine.45
Finally, the optimal duration of therapy is unknown. In the study conducted by the French group GETAID on azathioprine withdrawal in patients with CD after long-term treatment, 80% of the patients who continued with the treatment were still in remission for more than 5 years compared with 40% of patients who discontinued treatment.46 This aspect was also analysed in a prospective, placebo-controlled clinical trial involving a group of patients with CD in clinical remission after 3.5 years of treatment with azathioprine. Clinical relapse at 18 months in patients who continued with azathioprine treatment was 8±4%, while in the placebo group it was 21±6%.47 In UC, an Italian retrospective study showed that withdrawal of azathioprine is associated with a high risk of relapse (one-third of patients within 12 months, half within 2 years and two-thirds within 5 years).48 Therefore, when considering the potential withdrawal of thiopurine therapy, we must take into account the high risk of relapse in patients with IBD, the low individual absolute risk of lymphoma and the fact that the presence of persistent inflammation increases the probability of relapse.46,48 It is generally not advisable to withdraw thiopurine therapy. However, in cases where the decision is made to withdraw the drug, if relapse occurs, we must recommend resuming therapy early and maintaining it indefinitely.
Mucosal healing with thiopurinesThiopurines are effective drugs for maintaining clinical remission, as described before, and also induce endoscopic remission in a percentage of patients. However, according to data available from the SONIC study in CD, combined treatment with infliximab and azathioprine seems to be more effective in the short-medium term than either of these drugs alone. This clinical trial included 508 patients who had not previously been exposed to these drugs, and, at week 26, 56.8% of the patients receiving combined therapy were in steroid-free clinical remission compared with 44.4% of the infliximab group and 30% of the azathioprine group. The mucosal healing rate at 26 weeks was also higher in the combined therapy group (44%) vs the infliximab and azathioprine monotherapy groups (30% and 16.5%, respectively). There are methodological reasons for why these differences could have been less if the study design had been different. Thiopurines, as already mentioned, are not remission inducers and, although the use of steroids at the start of the study was allowed, more than 75% of patients receiving azathioprine did not take them. The same fact may also have affected the mucosal healing rate with azathioprine monotherapy.3 On the other hand, when patients were selected for the azathioprine arm of the SONIC study, including only those with at least a 7 fl rise in mean corpuscular volume (as the appropriate azathioprine dosing marker), the efficacy of thiopurine monotherapy for achieving mucosal remission was similar to that of infliximab monotherapy.49 However, the mucosal healing rate in clinically active IBD is generally higher with biologics than with azathioprine monotherapy.
The SUCCESS study compared the same 3 regimens as the SONIC study, but in patients with UC (n=236). Mucosal healing was higher in the combination therapy or infliximab monotherapy groups than in the azathioprine monotherapy group (63% vs 55% vs 37%).50 Although these rates are somewhat better than those obtained for patients with CD, the fact that results were evaluated early at 16 weeks may have had a negative effect on the final remission and mucosal healing rates of the azathioprine monotherapy group.
Based on the available data, we can assert that thiopurines achieve mucosal healing in a percentage of patients, although their efficacy is lower than that of biologics for achieving mucosal healing when used in clinically active IBD and as monotherapy.
Table 1 summarises the most relevant points in relation to the efficacy of thiopurine therapy in IBD.
Most relevant points regarding the efficacy of thiopurine therapy in inflammatory bowel disease.
| • Thiopurines are effective drugs for maintaining clinical remission, and also achieve endoscopic remission in a percentage of patients |
| • Maintenance of remission in UC or CD is the main indication for thiopurine monotherapy, especially in patients with steroid-dependent disease |
| • In patients with acute flare-up of steroid-resistant UC, thiopurines are useful as maintenance therapy after achieving remission with ciclosporin only when patients have not received prior maintenance therapy with thiopurines |
| • Thiopurines are effective drugs for the prevention of post-operative relapse in CD |
| • Although they are somewhat effective in perianal disease, they are not the drugs of choice for complex fistulas |
| • The strategy of concomitant thiopurine and 5-ASA prescription in steroid-dependent UC does not appear to be superior to thiopurine monotherapy |
| • It is generally not advisable to withdraw thiopurine therapy in patients with good inflammatory bowel disease control with these drugs due to the high risk of relapse |
5-ASA: 5-aminosalicylates; CD: Crohn's disease; UC: ulcerative colitis.
In patients with IBD, given the high probability of requiring immunosuppressant therapy during the course of the disease and the corresponding increased risk of opportunistic infections, we recommend reviewing scheduled immunisation compliance whenever possible, and always assessing whether patients’ have been immunised against specific infections.51–53 This assessment should be performed at diagnosis or, at the latest, prior to starting any immunosuppressant therapy in order to allow live vaccines (contraindicated if treatment has already started) to be administered, if required, and to ensure better immunisation, since decreased response rates to vaccines have been reported in patients receiving immunosuppressant therapy.52,53 Of the many microorganisms that could lead to opportunistic infections (uncommon and/or less severe in immunocompetent subjects), the immunisation status of the following should be assessed:
Hepatitis B virus (HBV) and hepatitis C virus (HCV). The prevalence of HBV and HCV infection in patients with IBD in our study is similar to that described for the general population.54 It has been shown that the probability and severity of liver dysfunction in patients with IBD infected with HBV and receiving immunosuppressant therapy is higher than in those infected with HCV. Furthermore, receiving 2 or more immunosuppressants is an independent predictor of HBV reactivation in these patients.54 Therefore, we recommend performing HBV tests before starting treatment with thiopurines. If the patient has no anti-HBs titre, we recommend continuing with immunisation. Since the efficacy of HBV vaccination in patients with IBD is lower than in the general population, we recommend using intensive vaccination regimens.53–56 In the case of HBsAg+ patients, antiviral therapy should be started 2 weeks prior to starting thiopurine therapy, regardless of the level of viraemia, and should be continued while the patient is receiving such treatment and for up to 6 months after withdrawing the immunosuppressant, if applicable.51,53 With regard to detection of HCV infection, there is no consensus regarding the need for assessment prior to starting thiopurine therapy since there is no HCV vaccine. Positive results in marker screening tests (anti-HCV and HCV-RNA) are not a contraindication to thiopurine therapy. However, it seems reasonable to determine baseline levels in view of the potential for thiopurine-induced hepatotoxicity and nodular regenerative hyperplasia.
Varicella-zoster virus (VZV). More frequent and more severe primary and reactivated VZV infections have been reported in patients treated with thiopurines.57 An Israeli study showed a positive predictive value of 93% and a negative predictive value of 0% for the reported history of VZV exposure.58 Based on these premises, it seems reasonable to perform VZV serological testing and proceed with vaccination (at least 3 weeks prior to starting immunosuppressant therapy since it is a live attenuated vaccine) if results are negative.51,53,57 An inactivated herpes zoster vaccine that is likely to be useful in these patients59 is in the final phases of clinical trial testing.
Epstein–Barr virus (EBV). Two studies conducted in Canada and Portugal have shown EBV seropositivity rates higher than 95% in patients with IBD over the age of 25.58,60 In the paediatric population with IBD, EBV seropositivity rates of 30–40% in children under the age of 10, 43–55% in the 10–15 years age bracket and 81% in children over the age of 1561 have been reported. Primary EBV infection in individuals receiving immunosuppressant therapy (and particularly thiopurines and anti-TNF) may be a major risk factor for haemophagocytic activation syndrome (with estimated mortality rates of 30%) and post-infection lymphoproliferative syndromes.62 Based on these premises, it seems reasonable to perform EBV serological testing, particularly in paediatric patients, immediately before starting thiopurines and considering other therapeutic options if results are negative. However, it is a mistake to think that this haemophagocytic syndrome only occurs in patients with no prior exposure to EBV and as a result of EBV infection. In fact, its development has been described in cases of cytomegalovirus infection and in immunosuppressed patients with no intercurrent viral infection.
Finally, it is important to remember that if live or attenuated vaccines are to be administered during treatment with thiopurines, these must be discontinued at least 3 months before vaccination and resumed at least 3 weeks after vaccination.51–53
Thiopurine metabolism study: thiopurine methyltransferase (TPMT) assayThiopurine pharmacokinetics exhibits a wide inter-individual variation that is largely due to the existence of several TPMT genetic polymorphisms.63 TPMT is the main enzyme responsible for the formation of 6-thioguanine (6-TGN) nucleotides, metabolites responsible for thiopurine-induced myelotoxicity, but also for its immunosuppressant effect.64 In the Caucasian population, TPMT activity (genetically determined) follows a trimodal distribution; 89% is homozygous for the high-activity allele, 11% is heterozygous and only 0.3% is homozygous for the low-activity allele.65,66 The latter group has a high probability of developing severe myelotoxicity (bone marrow aplasia) early and, therefore, thiopurine therapy should be avoided in these subjects. Consequently, high concentrations of 6-TGN are observed in patients with low TPMT activity, while low concentrations of 6-TGN are observed in those with high TPMT activity.64 Two procedures are available for determining TPMT activity: quantitative analysis or phenotypic testing and genotyping. The phenotypic test measures red blood cell TPMT activity,67 with thiopurine therapy being contraindicated if red blood cell TPMT activity is <5U/RBC.68 There are no studies showing any advantage (in efficacy or safety) of dosing the drug according to red blood cell TPMT activity.69 The degree of red blood cell TPMT activity seems to be directly related only to the time between the start of treatment and onset of myelotoxicity; it would be short (approximately 1.5 months) in patients who are homozygous for the low-activity allele, while it would be longer in heterozygous patients and even longer in patients who are homozygous for the high-activity allele.
Therefore, the benefit of determining TPMT activity lies in the fact that it prevents early bone marrow aplasia in 0.3% of individuals with TPMT deficiency. However, it should be remembered that intermediate or high activity does not exclude the possibility of myelotoxicity in any way. Therefore, TPMT activity assays are not essential for starting thiopurine therapy. Nevertheless, if such assays are possible, they will allow greater safety at the start of treatment. Several studies have shown a high degree of consistency between genotyping and phenotyping,70–72 but there is no consensus as to which is better. It is necessary to remember, however, that phenotyping of TPMT activity may be limited if the patient has received blood transfusions within the last 2–3 months (as TPMT activity is partly due to the donor's blood) and genotyping has a high number of polymorphisms that may increase the cost of testing.
How to start treatment? Full or initial low dose? Single or split dose?Given that the molecular weight of azathioprine is 55% of the molecular weight of mercaptopurine and 88% of azathioprine is metabolised to mercaptopurine, the thiopurine dose to be administered will depend on whether we choose one drug or the other. The recommended dose for treating IBD is 2.5mg/kg/day for azathioprine and 1.5mg/kg/day for mercaptopurine based on the results of a meta-analysis7 which showed that the effectiveness in maintaining remission depended on the dose (OR of effectiveness of azathioprine compared with placebo was 4.13 with 2.5mg/kg/day and only 1.2 with 1mg/kg/day). Typical recommendations were to start treatment with low doses of 50mg/day, with weekly dose increases following blood tests.73 The theoretical advantage of this treatment strategy was early identification of adverse effects; however, for idiosyncratic or dose-independent adverse effects (the most common during the first few months of treatment), this strategy would not make any sense. On the other hand, dose-dependent adverse effects tend to occur at doses above 50mg/day and, therefore, initial low doses do not seem to have any advantages, but only delay reaching therapeutic doses.74 Furthermore, some authors recommend using lower doses in patients with intermediate TPMT activity, decreasing azathioprine doses to 1–1.5mg/kg/day or mercaptopurine doses to 0.5–0.75mg/kg/day.75,76 However, regular blood tests makes it possible for full doses to be administered to this group of patients from the start of treatment with reductions in azathioprine or mercaptopurine dose if dose-dependent adverse effects should arise.
Another debated issue is whether to administer the drug as a single daily dose or as split doses. Split doses have been described as a limiting factor for therapy adherence, particularly in the case of chronic medication, as is the case of thiopurine therapy, which supports its administration as a single full dose from the start of treatment. Split doses should only be used in patients suffering from specific side effects. In this regard, a potential benefit of split doses has been reported in patients with digestive intolerance, elevated transaminase levels or leukopaenia as an adverse effect of thiopurines.77
Table 2 summarises the most relevant points in relation to pre-assessment and start of thiopurine therapy.
Most relevant points in relation to pre-assessment and start of thiopurine therapy.
| • Before starting thiopurine therapy, scheduled vaccination compliance should be checked and HBV and varicella-zoster serological tests should be performed. If the patient does not have antibodies against either of these viruses, vaccination should be given |
| • In the case of HBsAg+ patients, antiviral therapy should be started 2 weeks prior to starting thiopurine therapy and should be continued while the patient is receiving such treatment and for up to 6 months after withdrawing the immunosuppressant |
| • TPMT activity assays (phenotyping or genotyping) are not essential for starting thiopurine therapy. If such assays are possible, they will allow greater initial safety |
| • The recommended dose for treating inflammatory bowel disease is 2.5mg/kg/day for azathioprine and 1.5mg/kg/day for mercaptopurine, as a single full dose from the start |
HBsAg: hepatitis B virus surface antigen; HBV: hepatitis B virus; TPMT: thiopurine methyltransferase.
The onset of adverse events results in withdrawal of the drug in 10–20% of patients receiving thiopurines.78 Their frequency varies according to different definitions, but in one study involving the ENEIDA database (3931 treated patients), the cumulative incidence of adverse events was 26%, with an annual risk of 7% per patient-year of treatment. Seventeen per cent of patients had to withdraw from treatment due to adverse events.79
Thiopurine-related adverse events can be divided into idiosyncratic adverse events (dose-independent, such as digestive intolerance, pancreatitis, fever, arthralgia, myalgia, exanthema and some cases of hepatotoxicity) and dose-dependent adverse events, such as myelotoxicity.
Idiosyncratic adverse events tend to occur in the short term, at the start of treatment, within 3 months. Digestive intolerance occurs relatively frequently (in up to 8% of cases) and sometimes leads to withdrawal of treatment.79 Acute pancreatitis occurs in around 3% of cases (pain and lipase levels elevated to 3 times the upper limit of normal) and is usually mild.80 There is a series of poorly defined symptoms, such as fever and flu-like illness, arthralgia, erythema nodosum and neutrophilic dermatosis, which may occur as adverse events, but are sometimes difficult to recognise as such. These reactions may present in up to 1% of patients, also at the start of treatment. All idiosyncratic reactions stop quickly (48–72h) after withdrawing the drug,81 a fact that aids diagnosis in case of any doubt.
Some dose-dependent adverse events (myelotoxicity, hepatotoxicity) may also occur at the start of treatment. Blood tests will be more regular at that time, although they may become more infrequent later on, but never stopped. The incidence rate of myelotoxicity (leukopaenia, leucocytes <3×109/l; neutropaenia, neutrophils <1.5×109/l; anaemia, Hb <10mg/dl; thrombocytopaenia <100×109/l)82 is approximately 3% per patient and year of treatment and the incidence rate of severe myelotoxicity is 0.9% (neutrophils <0.5×109/l).83 Leukopaenia/neutropaenia is the most common haematological adverse effect, and the treatment regimen may need to be adjusted. Other haematological adverse effects, such as significant lymphopenia, thrombocytopaenia or anaemia, are less common.83 There is a relationship between TPMT activity and myelotoxicity: myelotoxicity is significantly more common in homozygous patients with low or undetectable TPMT activity (0.3% of patients) or, to a lesser degree, in heterozygous patients (5–10% of patients).84 However, in most cases, myelotoxicity is independent of TPMT and may arise due to reasons such as genetic factors or viral infections.
Hepatotoxicity occurs in at least 1.4% of cases per patient and year of treatment.85,86 It may follow an idiosyncratic or dose-dependent pattern, and may lead to withdrawal of therapy. Liver damage is more common during the first few months. It is defined as elevated transaminase levels >2 times the upper limit of normal, or alkaline phosphatase >2 times the upper limit of normal.82 Furthermore, it is not uncommon to observe slight increases in indirect bilirubin due to ineffective myelopoiesis. Slight and transient increases in transaminase levels are observed with some frequency without clinical implications.85
Three basic types of hepatotoxicity can occur: (1) hypersensitivity syndrome, which occurs within the first 2–3 weeks of treatment and can be controlled by decreasing the dose in some patients; (2) idiosyncratic cholestatic reaction, in which bilirubin and alkaline phosphatase, along with transaminase, levels increase significantly, requiring withdrawal of treatment; and (3) dose-dependent secondary to endothelial cell injury, which comprises the development of nodular regenerative hyperplasia, resulting more in portal hypertension with thrombocytopaenia than in abnormal liver function tests, which may be normal, veno-occlusive disease and peliosis hepatis,85,87,88 which require withdrawal of treatment. There are several risk factors for developing nodular regenerative hyperplasia, such as male gender, having CD and small bowel resection >50cm.89
Other potential adverse effects of thiopurine therapy include infections, some severe, and treatment-associated tumours (an aspect addressed in another section). Myelotoxicity predisposes patients to infections, with viral infections being most common (herpes zoster virus, herpes simplex virus, cytomegalovirus, EBV and human papillomavirus).90,91 The risk increases when neutrophil counts are less than 1×109/l. These types of adverse effects should be monitored throughout treatment.
What clinical–analytical tests should we perform and how often?Analytical tests should include a full blood count and biochemistry tests with liver function tests. Amylase and lipase tests are not required unless pancreatitis is suspected. We suggest performing the first clinical-analytical test 15 days after the start of treatment, a second test at one month and a third test at 2 months. Thereafter, tests may be performed every 3 months. In stable patients with no adverse effects, analytical tests may be performed every 4–6 months after more than one year of treatment with azathioprine/mercaptopurine.92
We recommend limiting sun exposure and using sunscreens with a high sun protection factor to minimise the risk of dermatological lesions due to photosensitivity.
In the event of the onset of fever in a patient receiving azathioprine/mercaptopurine therapy, the patient should see a doctor so that the cause of the fever may be investigated. Based on the diagnosis and the severity of the fever, temporary withdrawal of treatment may be required.
How can I manage adverse effects in order to be able to continue with thiopurine therapy?In patients with gastrointestinal intolerance to azathioprine, alternatives such as splitting the dose into 2 divided doses, administering the drug after meals and/or taking part of the dose at bedtime, may be tried. If the adverse effect persists, it is possible to successfully switch to equivalent doses of mercaptopurine in approximately half of patients.90,93–95 However, for the remaining idiosyncratic adverse effects (pancreatitis or febrile syndrome), recurrence of the adverse effect in case of re-exposure to the drug or a change of thiopurine is the norm. In some patients, minor increases in pancreatic enzymes not associated with symptoms may occur, which do not require therapeutic modifications; therefore, as indicated above, these tests should not be performed unless pancreatitis is suspected because this could result in inappropriate withdrawal of treatment.
In patients with hepatotoxicity, particularly at the start of treatment, a dose reduction (by 50%) with strict controls over subsequent weeks may be sufficient before trying to achieve tolerance of the initial dose.86 Patients with more severe hepatotoxicity (there is no specified limit) or jaundice, and those in whom liver enzymes do not normalise after reducing the dose of thiopurines, will require withdrawal of the drug. If liver function tests do not improve after withdrawing the drug, a comprehensive study is required. The administration of split doses (every 12h) of thiopurines may be useful for managing some cases of hepatotoxicity.77 In the event of azathioprine-induced hepatotoxicity, mercaptopurine can be successfully used instead, before ruling out the use of thiopurines, in a high percentage of cases.90,96,97 In some patients (‘shunters’), there is preferential metabolism to methylmercaptopurine which is displayed as dyspepsia and hepatotoxicity.77 If thiopurine metabolite testing is available, a smaller dose of thiopurines (25%) together with 100mg of allopurinol can be tried, which increases blood 6-thioguanine levels,98 as explained in more detail in another section of this document. Experience with this strategy involving the administration of allopurinol with low doses of thiopurines is limited to a few sites and has also been used in indications other than hypermethylation, where it has been described that a proportion of patients with side effects other than hepatotoxicity, such as myalgia, arthralgia and nausea and vomiting (idiosyncratic adverse effects), could respond to concomitant prescription of allopurinol.99,100 If this strategy is chosen, closer monitoring is recommended due to risk of myelotoxicity. On the other hand, in patients with cholestatic hepatotoxicity reactions or nodular regenerative hyperplasia, withdrawal of thiopurines is essential.
The risk of significant myelotoxicity is related to the neutrophil count: in patients with mild neutropaenia (neutrophils between 1.5 and 1×109/l), the azathioprine/mercaptopurine dose should be reduced by 50%. Once the cell count has returned to normal, it is often possible to administer the full dose of the drug again.83,85,101 In cases of moderate neutropaenia (neutrophils <1×109/l), it is necessary to withdraw thiopurine therapy. Once the neutrophil count has returned to normal, attempts can be made to improve treatment tolerance by using lower doses (50% of the initial dose), and, if neutropaenia returns, the drug should be withdrawn permanently.102 In the event of severe neutropaenia (neutrophils <0.5×109/l), treatment should be withdrawn permanently.
Table 3 summarises the most relevant points in relation to adverse effects of thiopurine therapy.
Most relevant points in relation to adverse effects of thiopurine therapy.
| • Idiosyncratic adverse events tend to occur at the start of thiopurine therapy, with gastrointestinal intolerance being the most common. Acute pancreatitis is usually mild |
| • Some dose-dependent adverse events, such as myelotoxicity or hepatotoxicity, may occur at any time during treatment, which requires periodic analytical controls, including a full blood count and biochemistry tests with liver function tests |
| • Other potential adverse effects of thiopurine therapy are infections, sometimes severe |
| • Some adverse effects can be managed using measures such as dose reduction, split doses or switching from azathioprine to mercaptopurine. Pancreatitis and febrile syndrome require permanent withdrawal of thiopurine therapy |
Non-melanoma skin cancers have been associated with thiopurine therapy in several studies.103,104 In one meta-analysis published in 2014, which included 60,351 patients, a hazard ratio of 2.28 (95% CI: 1.50–3.45) was observed for the development of non-melanoma skin cancer in patients exposed to thiopurines.105 Although an increased risk of melanoma has been observed in patients with IBD, there is no evidence of a higher risk in patients treated with thiopurines.106
Patients receiving thiopurines for IBD have a higher risk of developing lymphoproliferative disorders, especially non-Hodgkin's lymphoma. In the study by the French cohort CESAME, an increased risk of lymphoma was observed in patients being treated with thiopurines compared to patients who did not take thiopurines (hazard ratio: 5.08; 95% CI: 2.01–13.9).107 Furthermore, a recent meta-analysis observed an increased risk of lymphoma in patients treated with thiopurines (standardised incidence ratio: 4.9; 95% CI 3.1–7.8), which increased from the first year of treatment, although the risk did not persist after withdrawal of the drug.108 This meta-analysis describes that the absolute risk is higher in patients over the age of 50, and the relative risk is significantly higher in men younger than 30. Primary or reactivated EBV infection has been associated with this increased risk of lymphoma in patients exposed to thiopurines, with a 1/10,000 risk of developing fatal post-mononucleosis lymphoproliferative disorder in men younger than 35 undergoing treatment with thiopurines.107 An increased risk of myelodysplastic syndrome and acute myeloid leukaemia has also been observed in patients with prior exposure to thiopurines.109
In a recent study by the French cohort CESAME, a significantly increased risk of urinary tract tumours was observed in patients treated with thiopurines compared with those who did not receive these drugs (hazard ratio: 2.82; 95% CI: 1.04–7.68), especially in men over the age of 65.110 Control of thiopurine-induced inflammatory activity has been associated with a lower risk of colorectal cancer.38,111 While some data suggest an increased risk of cervical cancer in women with IBD treated with thiopurines, these are not conclusive.112
Based on currently available data, the risk of lymphoma and urinary tract tumours must be taken into account when prescribing thiopurine therapy to elderly patients.
What preventive measures should the patient take?Given the increased risk of non-melanoma skin cancer in patients with IBD treated with thiopurines, it is recommended that sun exposure be limited and sunscreens be used on a regular basis. It could be useful to include patients with other added risk factors, such as those with increased sun exposure due to their profession, patients with multiple ephelides or individuals with high-risk skin types (phototypes I and II), in a periodic follow-up programme offered by the Dermatology department.112 While there are no conclusive data on the synergistic effects of tobacco consumption and thiopurine therapy on the risk of cancer, data from the Eneida registry have shown an increased risk of extracolonic tumours in smokers and, therefore, all patients are advised to avoid tobacco.113 Women with IBD are advised to undergo annual cervical cancer screening and be vaccinated against human papillomavirus if applicable.112,114
What is the most appropriate attitude towards tumour diagnosis in patients receiving thiopurine therapy?Non-melanoma skin cancer allows thiopurine therapy to be continued in very specific cases.104 An increased risk of tumours in patients who have had previous malignancies treated with thiopurines compared to patients not treated with thiopurines has not been observed in several recent studies.115–117 However, if a tumour is detected in a patient receiving thiopurine therapy, the Oncology department's opinion regarding the potential impact of thiopurines on the clinical course of the tumour is critical. Taking into account the type of tumour and its characteristics, the potential association with thiopurine therapy, the patient's characteristics and the course and treatment of IBD, each case should be managed individually.
In the case of patients who develop lymphoma, permanent withdrawal of thiopurine therapy is recommended. Temporary withdrawal of thiopurines is recommended during treatment of an invasive cancer, with the exception of long-term adjuvant hormonal therapy which may be administered concomitantly with thiopurine therapy, according to the individualised recommendations of the Oncology department.118 In patients with tumours with good prognosis or with no clear association with thiopurines, it may be possible to continue thiopurine therapy, although this must be discussed with the Oncology department and the patient.
In patients in whom thiopurine therapy is withdrawn after diagnosis of a tumour, a specific time for resuming thiopurine therapy has not been defined. Based on data from post-transplantation patients, a period of 2–5 years has been suggested for resuming treatment.118 However, an individual assessment of each case with the Oncology department is currently recommended, taking into account the risk of tumour recurrence and alternative IBD therapies.
Table 4 summarises the most relevant points in relation to tumour risk and attitude towards thiopurine therapy.
Most relevant points in relation to tumour risk and attitude towards thiopurine therapy.
| • Thiopurine therapy has been associated with an increased risk of non-melanoma skin cancer, lymphoma and urinary tract tumours |
| • Primary or reactivated Epstein–Barr virus infection has been associated with the onset of lymphoma in patients exposed to thiopurines |
| • Permanent withdrawal of thiopurines is recommended if lymphoma is diagnosed and temporary withdrawal is recommended in the case of invasive cancer; an agreement should be reached with the Oncology department on a case-by-case basis as to whether this therapy can be resumed, taking into account the risk of tumour recurrence and alternative treatments for inflammatory bowel disease |
Thiopurines are safe for use during conception, pregnancy and breastfeeding. This summarises the following section, but it is important to reiterate this central idea. This is because, due to their mechanism of action, they are still included in FDA pregnancy category D since there is evidence of teratogenicity in animals. However, the FDA document assumes that there are no controlled studies in humans. Therefore, the recommendation would be to not use them unless the benefits outweigh the risks. More regular use of the ‘Pregnancy and Lactation Labeling Rule (PLLR)’, which includes detailed information on risks and benefits, is advised.
This rule should be read and understood since the first reaction of any doctor who is not accustomed to using thiopurines, and who is doing a superficial search of traditional manuals or the Internet, would be to discontinue thiopurines in the case of a woman taking thiopurines who is or is trying to get pregnant. We must warn all our patients of childbearing age, even in writing, that this attitude is incorrect and that clinical recurrence of IBD during gestation could in fact harm the foetus.119
Conception and gestationInformation on the safety of thiopurine use during conception and gestation comes from extensive experience with women who are primarily solid organ transplant recipients. This has resulted in reviews or clinical guidelines119,120 recommending that thiopurine therapy be continued during gestation.121,122 Although uncommon, there is nothing strictly preventing patients from starting thiopurine therapy during this period. However, given that most adverse events occur at the start of treatment, deferring therapy for as long as possible is recommended.
Moreover, increased rates of infection have not been reported in the first year of life of children born to mothers treated with thiopurines.119
Data relating to the potential influence of these drugs on male gametogenesis and the paternal component of potential cases of teratogenicity are limited. Initially, very fragmented evidence was available with isolated cases and a study involving a small sample of patients. In a Spanish national study123 involving a greater number of pregnancies during which fathers were exposed to thiopurines, no adverse events were detected in newborns. Likewise, in another study conducted in Spain, treatment with thiopurines was not observed to be associated with poor sperm quality or impaired fertility.124 Therefore, if a patient is having difficulty conceiving, we must firmly assure the exposed patients that thiopurines are unlikely to be the cause.
BreastfeedingExpert opinion (always uncontrolled) on the use of thiopurines during breastfeeding has also varied. Today, thiopurines are considered to probably be safe and their withdrawal is not recommended119,120 since excretion into breast milk occurs 4h after oral administration in very small amounts, which are lower than therapeutic levels.
Table 5 summarises the most relevant points in relation to the safety profile of thiopurines in terms of reproduction.
Most relevant points in relation to the safety profile of thiopurines in terms of reproduction.
| • Thiopurines are safe for use during conception, pregnancy and breastfeeding |
| • Thiopurine therapy is not associated with adverse events in newborns or poor sperm quality in men receiving treatment |
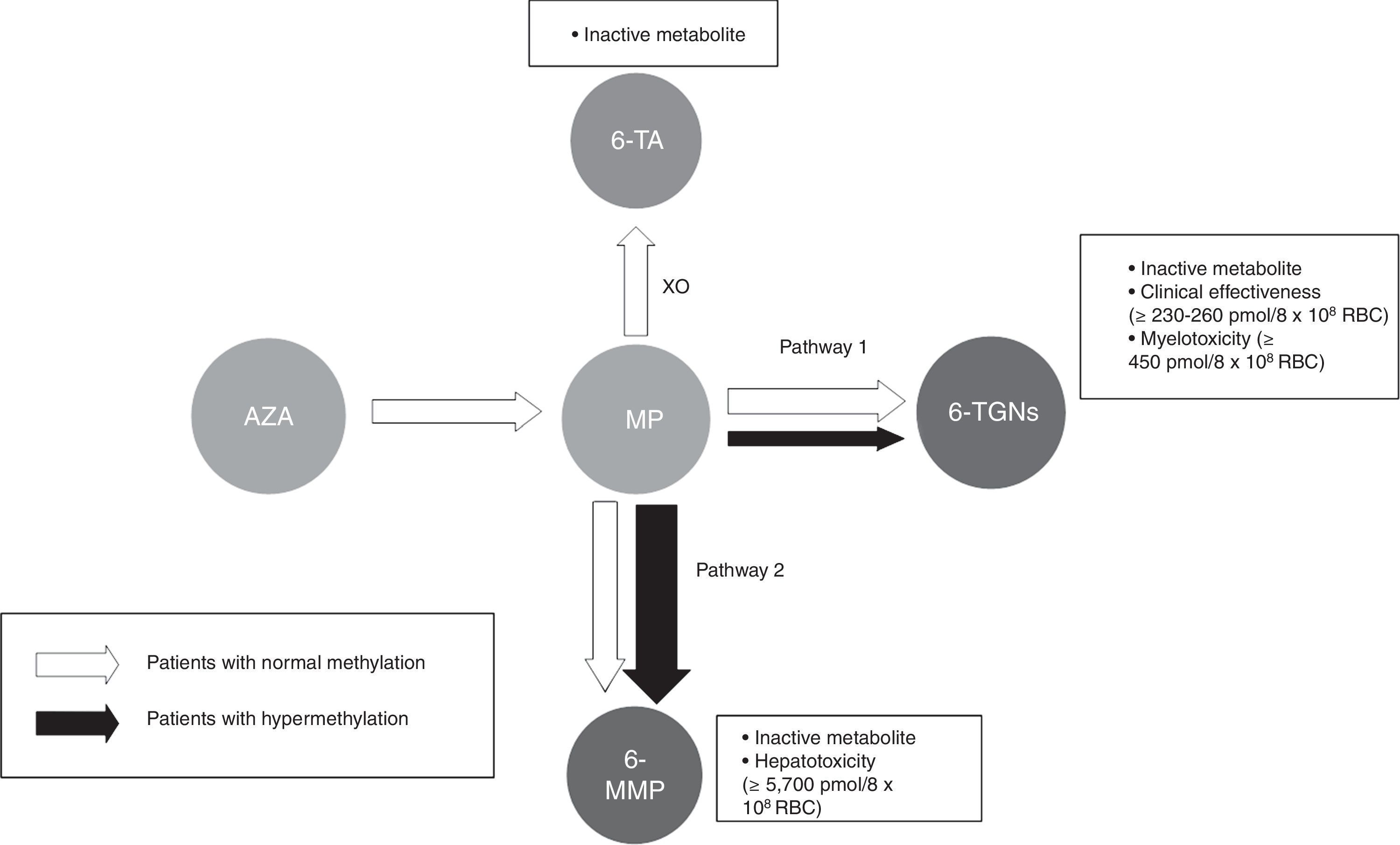
Thiopurine metabolism is complex and varies extensively between individuals. It can generally be said that 2 main metabolites are produced (Fig. 1): those derived from 6-methylmercaptopurine (6-MMP) and those from 6-TGN. The former are responsible for many of the adverse events, especially hepatotoxicity. The latter determine therapeutic action and influence the onset of myelotoxicity.
Flow chart of thiopurine metabolism. Patients with normal methylation and hypermethylation with preferential metabolism through TPMT (pathway 2). Thiopurines. AZA: azathioprine; MP: mercaptopurine. Metabolites. 6-MMP: 6-methylmercaptopurine; 6-TA: 6-thiouric acid; 6-TGN: 6-thioguanine nucleotides. HPRT: hypoxanthine-guanine phosphoribosyltransferase; TPMT: thiopurine methyltransferase; XO: xanthine oxidase.
Most people preferentially metabolise thiopurines to 6-TGN. However, some people, classed as ‘shunters’ or hypermethylators, preferentially metabolise thiopurines through the other pathway, with accumulation of 6-MMP and, therefore, less efficacy and more potential toxic effects.
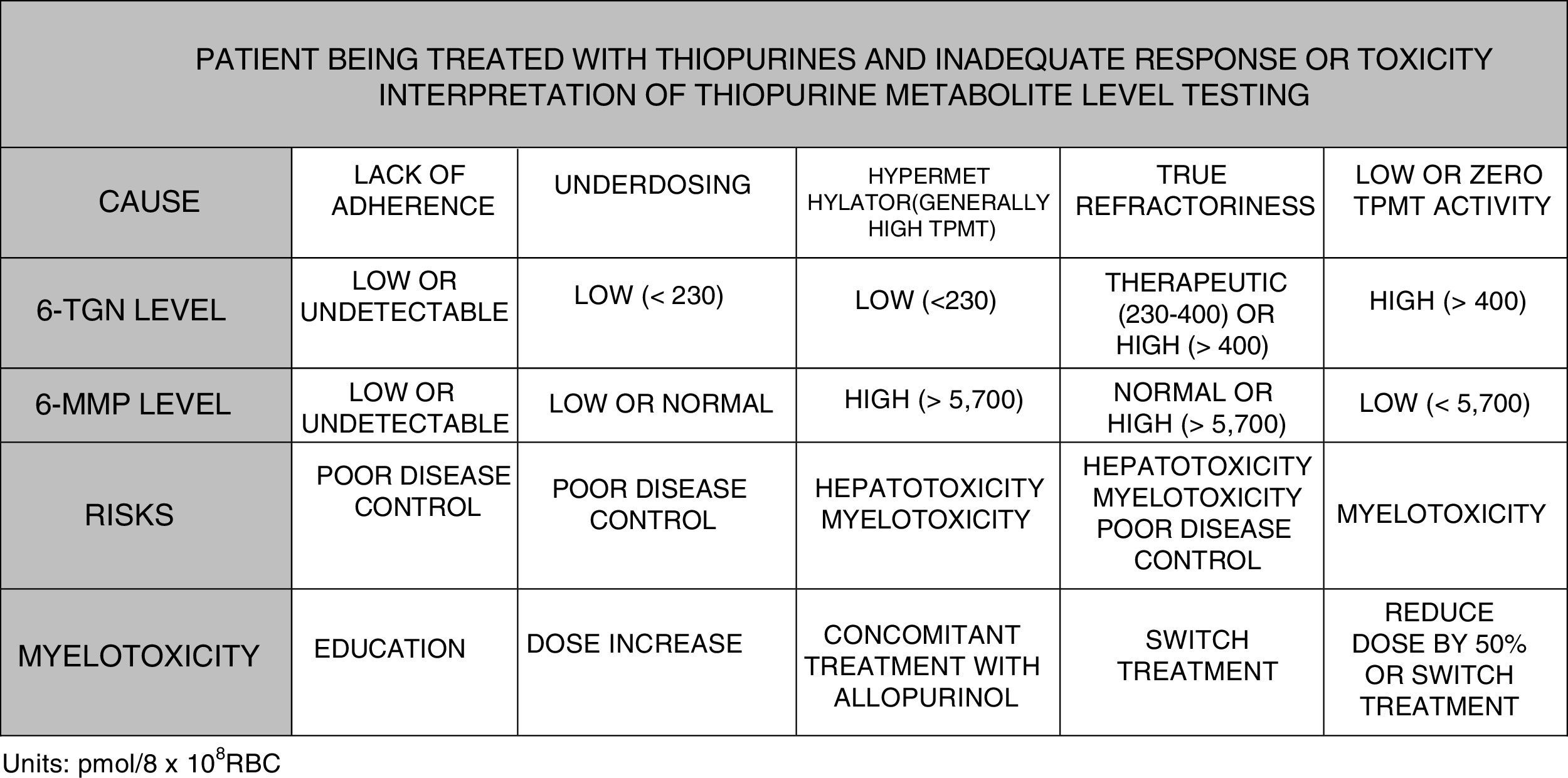
It is possible to determine blood 6-TGN and 6-MMP concentration, which allows subjects with hypermethylation to be identified and underdosing and lack of treatment adherence to be detected. Some scientific societies, such as the European Crohn's and Colitis Organisation (ECCO) or the American Gastroenterological Association (AGA), contemplate use of such tests during thiopurine therapy; testing could even be considered before labelling a patient as thiopurine-refractory.125–127 In paediatric disease guidelines (ECCO/ESPGHAN/NASPGHAN),128,129 its use is also considered in the event of side effects (cytopaenia, hepatotoxicity). More specifically, it could be said that TGN levels may help predict response to treatment since 84% of patients with levels greater than 230pmol/8×108 red blood cells (RBC) achieve a clinical response compared with 18% of cases with lower levels (OR: 3.27). Very high TGN levels (>450pmol/8×108 RBC) are associated with the onset of myelotoxicity and cytopaenia. A reduced dose in the event of TGN levels >550pmol/8×108 RBC has not been followed with regard to loss of therapeutic efficacy. However, individuals with hypermethylation produce high levels of methylated metabolites (6-MMP) and low levels of active metabolites (6-TGN) and, therefore, lower therapeutic efficacy and a higher incidence of hepatotoxicity have been described.130–134
When to measure metabolites?Stable metabolite levels in blood are achieved 4 weeks after the start of treatment, which in theory should allow early dose adjustment and later optimisation prior to clinical response. However, early optimisation cannot be recommended in clinical practice since better results have not been shown over the course of the disease with this practice; in fact, the results of the METAZA study, which was designed and conducted in Spain in order to clarify this matter, concluded that metabolite testing at the start of thiopurine therapy did not help determine clinical response.69
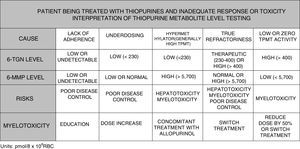
To summarise, in view of the above, the testing of metabolite levels may be considered in various situations130–135: patients with no response or partial response after 3–6 months of treatment; patients with suspected poor adherence; and cases with side effects such as cytopaenia or hepatotoxicity (Fig. 2).
Benefit of modulating thiopurine metabolism using concomitant allopurinol treatmentPatients with hypermethylation or high TPMT activity (Fig. 1) use this pathway preferentially, producing a higher concentration of methylated metabolites (6-MMP) and a lower concentration of 6-TGN (6-MMP/6-TGN ratio >11).136 These patients show diminished treatment effectiveness and an increased possibility of hepatotoxicity, particularly when the 6-MMP level exceeds 5700pmol/8×108 RBC.
Allopurinol is a xanthine-oxidase inhibitor that is toxic in normal use when combined with full doses of thiopurines. However, its use with thiopurines at low doses may enhance metabolism of thiopurines to 6-TGN metabolites, thus reducing methylated metabolites, which results in increased effectiveness and decreased liver toxicity.136 It is necessary to point out once more that the dose of thiopurines must be reduced to 25–33% of the previous dose as otherwise it would result in serious adverse events.133,136
This combination of allopurinol and thiopurines may be used in certain circumstances, but always with close monitoring and metabolite testing. It may also be useful in cases of hepatotoxicity associated with thiopurine therapy and in partial response in patients with hypermethylation.94
The dose of allopurinol that is usually used in combination therapy is low (100mg/day) and is associated with few side effects. The most common side effect is a skin rash, which is generally self-limiting, but others, such as dyspepsia, headache, vertigo, impaired taste and vision, hypertension, alopecia, hepatotoxicity or gynecomastia, may occur. Rare cases of toxic epidermal necrolysis have been reported, especially in individuals from China and Southeast Asia.136
Combined treatment with thiopurines and biologicsWithin the therapeutic options for IBD, combination therapies with biologics (especially anti-TNF) and immunosuppressants (thiopurine, but also methotrexate) are one option associated with higher efficacy, but significantly more toxicity. The combination of a thiopurine and an anti-TNF, especially infliximab, is considered to be more effective than infliximab monotherapy in terms of clinical response and mucosal healing. The underlying reason for this is that concomitant use with thiopurines results in increased serum concentrations of infliximab and decreased immunogenicity with the formation of less anti-infliximab antibodies.137,138 Although the clinical effectiveness of thiopurine monotherapy is associated with TGN levels >230pmol/8×108 RBC, TGN levels associated with decreased immunogenicity (TNG levels >125pmol/8×108 RBC have been suggested) are not defined in combined treatment. A lower dose of azathioprine may be sufficient to achieve this effect.139 However, recent studies using combined adalimumab and thiopurine therapy suggest greater therapeutic benefit and lower antibody formation when TGN levels >222–235pmol/8×108 RBC are achieved.140,141
Although it is possible that patients receiving combined maintenance therapy require sufficient levels of thiopurine metabolites to maintain an adequate response,137,138 definitive recommendations cannot be given. Specific prospective studies comparing clinical effectiveness results based on observed levels of anti-TNF and TGN could be useful for answering these questions.
Table 6 summarises the most relevant points in relation to controlling treatment using thiopurine metabolite testing.
Most relevant points in relation to controlling treatment using thiopurine metabolite testing.
| • Thiopurine metabolite testing may be useful in some situations, such as suspected poor treatment adherence or underdosed patients, and in the event of hepatotoxicity or myelotoxicity |
| • Concomitant treatment with low doses of thiopurines and allopurinol, monitored by observing metabolite levels, may be an option in patients with hypermethylation who develop hepatotoxicity or show a lack of response to treatment |
| • Combined therapy with thiopurines and an anti-TNF (especially infliximab) decreases immunogenicity and is associated with higher effectiveness, but also causes more significant toxicity |
The authors declare that they have no conflicts of interest regarding publication of this article.
We would like to thank Alicia Algaba for her assistance in sorting and reviewing the article's references.
Please cite this article as: Bermejo F, Aguas M, Chaparro M, Domènech E, Echarri A, García-Planella E, et al. Recomendaciones del Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU) sobre el uso de tiopurinas en la enfermedad inflamatoria intestinal. Gastroenterol Hepatol. 2018;41:205–221.