Although most patients with ulcerative colitis should be given topical treatment, different studies have shown that they are underused in clinical practice. The purpose of this article is to answer 10 specific questions about which drugs are available for topical use in the treatment of ulcerative colitis, and their characteristics in terms of formulation, dosage, presentation, application and proximal distribution of rectal-administered drugs. The efficacy of available topical drugs and the benefits of combining different formulations and routes of administration, and their usefulness during disease remission are evaluated. Finally, a series of recommendations addressed to patients are given on the correct application of topical treatment.
Aunque un elevado porcentaje de pacientes con colitis ulcerosa debería recibir tratamiento tópico por vía rectal, los estudios de práctica clínica han demostrado que éste está infrautilizado. El propósito de este artículo es el de responder a 10 preguntas concretas sobre qué fármacos están disponibles para uso tópico, su forma de presentación, formulación, y métodos de aplicación, así como cuál de ellos es más eficaz e idóneo en los distintos escenarios clínicos de la colitis ulcerosa. Asimismo, se evalúa la posibilidad de combinar diferentes formulaciones y vías de administración, y la utilidad en la fase de remisión de la enfermedad. Por último, se hacen una serie de recomendaciones para una mejor información de los pacientes acerca de una correcta aplicación y administración.
Ulcerative colitis (UC) is chronic inflammation of the colonic mucosa that continuously affects the rectum and a variable extension of the colon; it is generally characterised by a clinical course with flare-ups of activity and periods of remission.1 The Montreal classification phenotypically classifies UC according to its extension, either as proctitis (limited to the rectum), left colitis (up to the splenic flexure) or extensive colitis (beyond the splenic flexure).2 Distal colitis, alone or in the context of a more extensive UC, is responsible in many patients for symptoms such as urgency, tenesmus, "rectal sputum" or sensation of incomplete bowel movement, which lead to worse quality of life. The macroscopic extension of the inflammation determines patient management and the route of administration of some treatments. The fact that some of the drugs used to treat UC do not reach maximum concentrations in the colonic mucosa when administered orally, or even parenterally, makes the possibility of an effective, safe treatment directly on the mucosa very attractive. Frieri et al. found an inverse correlation between the concentration of 5-aminosalicylic acid in rectal biopsies and severity of inflammation in UC.3
Topical rectal treatment is defined as anal administration of a drug to obtain a high drug concentration in the mucosa that it covers, with little systemic availability. Different presentations in the form of suppositories, foam and enemas, with a variety of doses and volumes, enable us to adapt to different disease extensions, tolerances and patient preferences.4,5
According to the European Crohn’s and Colitis Organisation's consensus guidelines, most patients with active UC should receive topical treatment.6 Topical mesalazine is the treatment of choice in mild or moderate proctitis and combined oral and topical mesalazine treatment is recommended in mild or moderately active left and extensive UC. In severe flare-ups, topical therapy can be used as adjuvant therapy when tolerated by the patient, to minimise distal symptoms. Topical therapy is also indicated as maintenance treatment in proctitis and left UC.7,8
Although the recommendations advise topical therapy in most patients with active UC, this treatment is underused. In a study conducted in patients with poorly controlled UC referred for a second opinion, it was found that 75% of those who had distal colitis had never received topical treatment.9 These data have been confirmed in our setting, with topical use among patients in whom it was indicated of just 41%.10 In another study conducted in Switzerland, only 25% of the patients with proctitis received topical treatment in monotherapy and 13% in combination with systemic therapy.11 This low use could be explained by their lack of prescription by doctors due to unfamiliarity, doubts about their utility or bad experiences in the past, or because of the patient rejecting topical treatment or presenting poor adherence.4 A prospective study of 70 patients with topical treatment evaluated adherence based on the drug withdrawal rate from the pharmacy, finding that 71% were non-adherent (possession of <60% of the medication). The main reasons provided by patients for non-adherence were method of administration (65%) and very busy lives (40%).12
This review of topical treatment in UC is an update of that published in Enfermedad Inflamatoria Intestinal al Día in 2015.13 Its structure in question and answer form has been maintained in order to optimise its use, from the perspective of indications, efficacy and applicability in clinical practice. A series of recommendations and information for the patient are also included to facilitate the correct administration and completion of topical treatment, in order to improve its acceptance, adherence and, hence, its effectiveness. A video made by GETECCU about the administration of topical treatment, which may be very useful for patients, can be found at the following link: www.youtube.com/watch?v=oWyfExo8Zj8&t=79s.
Which topical drugs can we use to treat ulcerative colitis?Various topical treatments have been used in UC for more than 50 years. Anecdotally, enemas of silver nitrate, potassium permanganate, ciclosporin, butyrate, nicotine or arsenic have been used.14
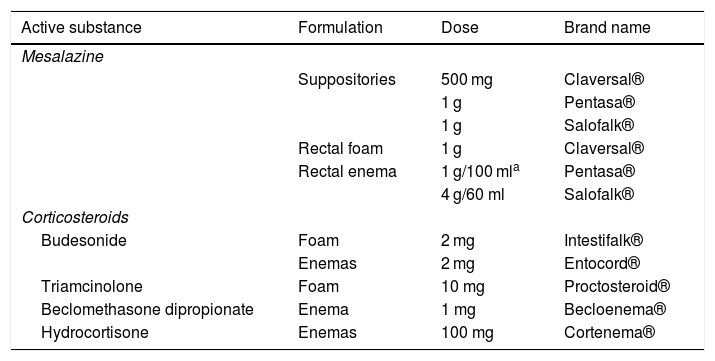
We now have mesalazine and different corticosteroids in suppository, rectal foam or enema form.14Table 1 summarises the formulations marketed in Spain.
Presentations for topical treatment in ulcerative colitis.
| Active substance | Formulation | Dose | Brand name |
|---|---|---|---|
| Mesalazine | |||
| Suppositories | 500 mg | Claversal® | |
| 1 g | Pentasa® | ||
| 1 g | Salofalk® | ||
| Rectal foam | 1 g | Claversal® | |
| Rectal enema | 1 g/100 mla | Pentasa® | |
| 4 g/60 ml | Salofalk® | ||
| Corticosteroids | |||
| Budesonide | Foam | 2 mg | Intestifalk® |
| Enemas | 2 mg | Entocord® | |
| Triamcinolone | Foam | 10 mg | Proctosteroid® |
| Beclomethasone dipropionate | Enema | 1 mg | Becloenema® |
| Hydrocortisone | Enemas | 100 mg | Cortenema® |
Mesalazine suppositories are probably the most used topical formulation, especially in ulcerative proctitis, which accounts for more than 30% of all UC cases in Spain.15 Rectal foam is characterised by greater viscosity than enemas, less volume and easy dispersion in and significant adhesion to the colonic mucosa.16 Enemas enable the administration of greater volume to reach higher parts of the colon.17 However, the ability to reach more proximal portions of the colon can also lead to worse tolerance of the treatment.
When choosing from the different formulations, we have to consider the poor adherence of many patients, especially younger patients, to these topical treatments,4,12 and the fact that a single daily administration has been shown to be as effective as a twice-daily dose.18
Data is available from a randomised, double-blind study with rectal tacrolimus in patients with refractory proctitis that shows high efficacy rates. The product is not marketed and it was compounded in pharmacies,19 available as a suppository or enema.
Which topical drugs are the most effective in ulcerative colitis?Two meta-analyses compared the efficacy and safety of topical mesalazine with that of topical corticosteroids (including budesonide, hydrocortisone, fluticasone propionate and beclomethasone) in the treatment of distal UC. The first included seven clinical trials that directly compared conventional corticosteroids with 5-aminosalicylates in different topical forms (most of them directly compared enemas of the two formulations with products of similar volume to prevent bias). It concluded that topical mesalazine was more effective than topical steroids for obtaining clinical remission (odds ratio [OR] 2.42; 95% confidence interval [CI] 1.72–3.41), endoscopic remission (OR 1.89; 95% CI 1.29–2.76) and histological remission (OR 2.03; 95% CI 1.28–3.20).20 This meta-analysis included two clinical trials with a smaller number of patients and directly compared topical forms of budesonide with mesalazine, finding no statistically significant differences (OR 0.95; 95% CI 0.43–2.10) between the two drugs. The second meta-analysis,21 which included a larger number of studies, confirms significantly higher response rates in patients treated with topical 5-aminosalicylates (77%) than in those treated with topical steroids (52%) (p = 0.02).
A study was subsequently published with 237 patients with mild-moderate UC randomised to receive topical budesonide (n = 118) or mesalazine enemas (n = 119) for eight weeks, confirming the results obtained in previous meta-analyses.22 The proportion of patients in clinical remission was significantly greater in those treated with mesalazine than in those treated with topical budesonide, in both week 4 and week 8 (77 vs 63.5%, p < 0.05, and 75.3 vs 59.5%, p < 0.02, respectively).
In view of this, the European Crohn’s and Colitis Organisation's Guidelines6 consider topical mesalazine as the first choice of treatment in proctitis and left UC, with level of evidence 1b and grade of recommendation A, with topical corticosteroids relegated to second line treatment. Likewise, the GETECCU clinical guidelines on UC treatment based on the GRADE23 methodology strongly recommend the use of rectal mesalazine at a minimal dose of 1 g/day versus rectal corticosteroids in the induction of remission in patients with mild-moderate flare-ups of left UC. In these guidelines, the authors not only highlight its greater efficacy but also mesalazine's lower toxicity in comparison with topical steroids.
Although there are no comparative data, a recently published retrospective series of 43 patients found high efficacy of tacrolimus suppositories in patients refractory to mesalazine and topical corticosteroids.24
Which extension is treated with each formulation?The objective with topical treatment is for each pharmaceutical form to adapt to different extensions of UC. Although the Montreal classification classifies patients as proctitis, left colitis and extensive colitis2, in daily clinical practice the pharmaceutical form to be used may differ depending on whether the portion affected is up to the sigmoid or the splenic flexure.
Scintigraphy studies conducted with Tc-99 m confirm that suppositories only release their contents in the rectum, while foam can reach the sigmoid and liquid enemas reach the splenic flexure,25–27 although this can also vary depending on their viscosity and the volume of liquid administered.
Suppositories are therefore the pharmaceutical form of choice in proctitis. Foam can be used in patients in whom only the rectum and sigmoid are affected; it is the second most commonly used pharmaceutical form in proctitis as more than 40% is released in the rectum.26 Enemas are generally used in more extensive forms as only 10% of the drug is released in the rectum.
Which patients with ulcerative colitis could benefit from topical therapy?Any patient with UC can potentially benefit from topical treatment as the rectum is affected in practically all patients with this disease.
In patients with mild to moderate proctitis or left colitis, topical treatment with mesalazine can be considered the first therapeutic option, alone or in combination, both in induction and maintenance of remission.28
In mild to moderate flare-ups of extensive colitis, topical treatment added to oral therapy with mesalazine obtains a greater and faster clinical response.29
Even in patients with severe flare-ups of colitis, adding topical treatment improves their symptoms, primarily distal symptoms such as rectal urgency or tenesmus.6
Which is the treatment of choice to induce remission in proctitis and left ulcerative colitis?The recent update of the European Crohn’s and Colitis Organisation's Consensus Guidelines6 clearly describe the optimal treatment for each clinical status manifested by patients with UC. The most important aspects of the recommendations in these guidelines for clinical practice are described below.
Proctitis: in patients with active proctitis, 1 g daily of mesalazine in suppository form is the treatment of choice in mild-moderately active cases. Mesalazine foam is an alternative for patients with poor tolerance to suppositories. In some cases, the use of topical mesalazine can be combined with oral mesalazine, or added to topical steroids, which may be more effective than any of these treatments alone. The use of corticosteroids, immunosuppressants or biological drugs may be necessary in cases of refractory proctitis.30
Left colitis: mild-moderate left UC should be initially treated with mesalazine enema (1−4 g/day) together with oral mesalazine (doses of 2.4 g/day or more). Like monotherapy with oral aminosalicylates, topical treatment with steroids or aminosalicylates in monotherapy is less effective than combined oral and topical mesalazine treatment.23,31 On the other hand, topical mesalazine is more effective than treatment with topical steroids.22,32 Regarding posology, it has been shown that a single daily administration of mesalazine is as effective as its division into multiple daily doses18 and can improve treatment adherence in clinical practice. Topical systemic or oral corticosteroids should be started if the symptoms do not improve. Hospitalisation and treatment with intravenous systemic steroids should be considered in patients with severe flare-ups of left colitis.
What role does topical therapy play in the treatment of active extensive ulcerative colitis?Mild-moderate extensive UC should initially be treated with oral mesalazine at doses of 2.4 g/day or more, which should be combined with topical mesalazine (doses of 1 g/day or more), if tolerated, to increase the efficacy of the treatment.6 As explained earlier, a single daily dose should be favoured, which is as effective as dividing into several doses, with potential for better adherence.
The benefit of combining oral and rectal mesalazine treatment for patients with extensive UC was shown in a clinical trial that included 127 patients randomised to receive oral mesalazine at a dose of 4 g/day in combination with mesalazine enemas of 1 g/day, versus a group receiving the same oral mesalazine, but in combination with placebo enemas.33 In this study, the remission and response rates were greater in the group treated with combined oral and rectal mesalazine. Remission was achieved in 64% of the patients in the mesalazine enema group and in 43% of the placebo group (p = 0.03) in week 8. Also striking was the good patient acceptance of the topical therapy: 84% of the mesalazine enema group and 85% of the placebo enema group were willing to receive the combined treatment in the future if prescribed by their doctor.
How long does it take to evaluate the results of topical treatment?Rapid resolution of the rectal syndrome (urgency, tenesmus, rectal bleeding) is an essential objective in the treatment of UC. Few studies in the literature specifically evaluate response time after starting treatment with topical or oral mesalazine.33–37 Sandborn et al.34 performed a post hoc analysis of two prior studies in order to evaluate bleeding cessation and mucosa healing time from start of treatment with topical mesalazine, both alone and in combination with oral mesalazine. The first study included 76 patients with distal UC treated with a rectal suspension of 4 g of mesalazine and 77 patients treated with placebo. On day 2, 31% of the patients treated with mesalazine presented no rectal bleeding, versus 5.5% of the patients treated with placebo (p < 0.001). Median bleeding cessation time was eight days. In week 3, the proportion of patients in clinical and endoscopic remission was significantly greater in the group treated with topical mesalazine compared with placebo (49 vs 9.6%, p < 0.0001, and 25% vs 7.8%, p < 0.005, respectively).35 The second study included 22 patients with distal UC treated with oral mesalazine (2.4 g/day), 18 patients treated with topical mesalazine (4 g/day) and 20 patients treated with both in combination. On day 8, the patients treated with topical mesalazine or a combination of topical and oral mesalazine presented a greater rate of rectal bleeding cessation than the patients treated with oral mesalazine alone. In week 3, the combined treatment patients presented a greater clinical remission rate than those treated with oral mesalazine alone (58% vs 18%, p < 0.005). Mean time to bleeding cessation was 25 days in the group treated with oral mesalazine and 12 days in the group treated with topical mesalazine or with combined topical and oral mesalazine.36 In a more recently published randomised, double-blind, placebo-controlled study, it was found that the endoscopic remission rate after four weeks of treatment with mesalazine 1 g or placebo suppositories was 84% and 36%, respectively (p < 0.0001). The proportion of patients with bleeding cessation was significantly greater in the group treated with mesalazine compared to placebo after only three days of treatment.37
The data obtained from these studies show that the clinical benefit obtained with topical mesalazine is fast, appearing from two to 14 days after starting the treatment, although some responses do not manifest until week 8.33
Is it useful to combine topical treatment with mesalazine and corticosteroids?Published scientific evidence on the efficacy of the combination of topical mesalazine and corticosteroids is very limited. A Dutch double-blind study included 60 patients randomised to treatment with beclomethasone enemas (3 mg/100 ml), mesalazine enemas (2 g/100 ml) or a combination of both (3 mg/2 g/100 ml) for four weeks.38 Response evaluated as "subjective improvement" was 100% in the patients on combined treatment, 76% in patients treated with mesalazine and 70% in patients treated with corticosteroids (p < 0.01), with no significant differences in the onset of adverse effects.
More recently, a prospective, randomised, multicentre study has been published, which included 337 patients with ulcerative proctitis who were randomly assigned to four groups of daily topical treatment with suppositories: budesonide 2 mg, budesonide 4 mg, mesalazine 1 g or the combination of budesonide 2 mg and mesalazine 1 g.39 The study's primary endpoint was to evaluate time to resolution of symptoms, defined as the first three consecutive days with an index of 0 for rectal bleeding and frequency of bowel movements. Mean time to resolution of symptoms was similar in the groups treated with suppositories of budesonide 4 mg (30 days), mesalazine 1 g (29 days), the combination of budesonide 2 mg and mesalazine 1 g (29 days), and significantly greater in the group treated with budesonide 2 mg suppositories (35 days) (p < 0.046). Budesonide 4 mg suppositories are not available on the market, so this study's results have no practical implications in our setting.
Therefore, despite a lack of evidence, in clinical practice it is not uncommon to add treatment with topical corticosteroids in patients with distal UC who do not achieve remission with topical mesalazine alone.
When should topical therapy be used in the maintenance of remission?A recent meta-analysis included seven studies with a total of 555 patients that compared the efficacy of topical mesalazine with placebo in the maintenance of remission of quiescent distal UC.40
In all the studies, mesalazine was significantly superior to placebo in maintaining remission with a relative risk of clinical recurrence of 0.60 (95% CI 0.49-0.73) and a necessary number of patients to be treated to prevent a recurrence of three. The posology varied in the different studies and included mesalazine 500 mg, 1 g or 4 g/day in suppositories or enemas and 1 g or 4 g/every three days in suppositories or enemas. Although not statistically significant, there was a dose-response effect with lower recurrence rates in the patients receiving daily treatment versus those receiving intermittent treatment. The therapeutic effect was also greater when the weekly doses of mesalazine were ≥7 g. No differences were found in the incidence of adverse effects among the patients treated with topical mesalazine or placebo.
In the maintenance treatment of UC, especially patients with distal UC in whom topical treatment is indicated, it is essential to consider the patient's preferences to ensure good adherence. In general, all the pharmaceutical forms of topical mesalazine have been shown to be effective and there is no evidence at this time to recommend one or the other or the best application regimen (daily versus intermittent).
Is topical treatment safe in ulcerative colitis?Topical therapies can have unpleasant side effects, such as drug leaks or losses, retention and distension problems. Serious complications such as rectal perforation by the application cannula have been reported but are extremely rare.14 A Swiss study evaluated the side effects of the different treatments received in a cohort of 779 patients with UC. Twenty-five of the 334 patients (7.5%) with topical treatment (mesalazine or corticosteroids) experienced side effects versus 13% of the patients treated with oral mesalazine, 48% of those treated with immune modulators (p < 0.001) and 24% of those treated with anti-TNF alpha (p < 0.001).11 The most common side effects of topical treatment with mesalazine were pain during the application, incontinence diarrhoea and skin rash.
No significant systemic side effects were observed in patients receiving topical mesalazine. The 127 patients of one study received oral mesalazine and were randomised to receive mesalazine enemas of 1 g/day versus placebo enemas.33 The percentage of patients with at least one side effect at eight weeks was 34% in the mesalazine enema group compared to 50% in the placebo group. Most of the side effects were of mild or moderate intensity. A meta-analysis included four studies with 214 patients, comparing the efficacy and safety of topical versus oral mesalazine in the treatment of mild-moderate UC. In total, 21% of the patients with topical treatment experienced at least one adverse effect versus 33% of the patients on oral treatment (relative risk 0.61; 95% CI 0.24–1.52).41 The idiosyncratic effects of treatment with topical mesalazine are anecdotal, with two published cases of secondary pancreatitis.14
Topical budesonide has low bioavailability (10–15 %), with fewer adverse effects than systemic corticosteroids. In a meta-analysis that compared topical budesonide and placebo, no significant differences were found in the rate of side effects (relative risk 2.06; p = 0.203) or suspension of treatment (relative risk 1.38; p = 0.648),42 and it was considered to be a safe treatment, with few adverse effects. Although intestinal absorption can case drops in cortisol levels, it does not generally cause significant clinical effects.39
Table 2 summarises the practical recommendations for patients to learn basic skills for the use of topical therapies, and advice to increase tolerance and adherence to treatment.
Practical recommendations for patients concerning topical treatment in ulcerative colitis.
| 1. Position of the patient and method of administration: |
| • Enema: it is recommended to lie on your left side with both legs bent or the left leg straight and the right knee bent. Shake the enema container for about 30 s and remove the cap. Lubricate generously and then gently insert the applicator into the anus as far as it will go (about 3−6 cm). Squeeze the enema container slowly until all its contents have been deposited in the rectum. While administering small volumes, if you feel the urge to defecate, rest for a few seconds until it passes and try again. After inserting the entire volume, wait for 15−30 s and slowly remove the applicator. It is recommended to remain comfortably lying down for as long as possible after the administration of the enema (at least 30 min). |
| • Foam: stand with one foot on a chair or stool. The containers marketed for the administration of foam are metal and have disposable applicators that are tightly fitted to the container. Each container contains enough for several applications (14–16). After shaking the container and removing the safety tab, attach the applicator and lubricate it. Then hold the container's dome with your index finger. The container must always be held so that the dome is facing downwards. In this position, slowly insert the applicator into the anus as far as it will go. When you feel comfortable and sure of the position, firmly press the dome and the foam will enter the rectum. Wait for 10−15 s and slowly remove the applicator. Remove the applicator from the tube and dispose of it in a plastic bag. Hold in the foam as long as possible. The foam container has a safety system that locks the dome to prevent the foam from accidentally spilling. |
| • Suppository: it can be applied in either of the two previously described positions (standing with a raised foot or lying on your left side). It is recommended to wear gloves (if you are allergic to latex, use gloves made of a different material), although suppositories slip less when directly in contact with the skin, which is why gloves are not always used. Check that the suppository is hard before removing it from its wrapping. If it is soft, put it in the fridge for a few minutes or submerge in cold water. After removing the wrapping, hold it with your fingers so that the pointy end stops at your index finger and the flat end is by your anus. Some suppositories are the same shape at both ends, so no precaution is required when choosing the end to insert into the anus. It is recommended to lubricate the anus, the suppository or both prior to insertion. In this position, insert the suppository through the anus to the rectum. Then tighten your buttocks and try to hold the suppository in for as long as possible. |
| 2. If you have mobility problems or do not believe that you can administer the treatments yourself (obesity, old age, etc.), ask for help. |
| 3. Ideal time: when you go to bed is a good time as it helps to hold the product in the intestine for longer. |
| 4. Topical treatment (in any form) can give rise to an urgent need to defecate. Try to hold it for a few minutes, as the urgency generally slowly disappears without the need for a bowel movement. If this occurs with enemas, apply as much as you can hold and you will find that in successive enemas, you will be able to apply the entire volume as the inflammation in your intestine reduces. If this happens with suppositories, try reducing their size. This can be done by cutting them in half lengthwise. |
| 5. Be patient waiting for the symptoms to improve. In general, the time between start of treatment and symptom relief can range from two to 14 days. Constancy is therefore recommended, unless your clinical status worsens during this time, in which case you should contact your doctor as soon as possible. |
| 6. Do not worry about the amount of drug prescribed. It is often necessary to administer mesalazine in high doses through the anus (from 1 to 4 g) and also orally. The safety of these doses is generally very high apart from in exceptional cases. |
| 7. Tell your doctor if you are intolerant to oral mesalazine or have experienced any adverse effects. |
| 8. It is recommended to store the suppositories in a cool place but not in the fridge. |
| 9. The safety and efficacy of topical therapy has been proven in numerous clinical studies and is supported by routine clinical practice. The treatment benefits you not only when you have symptoms (urgency, bleeding, etc.), but also when you are asymptomatic, as it reduces the likelihood of the symptoms returning. So try to be disciplined and apply the treatment (which will generally be less often than when the disease is active), even if you feel well. |
| 10. As there are different presentations and methods of application of the drugs (suppositories, enemas and foam), and you may have preferences or problems with some of them, do not hesitate to talk to your doctor about anything that could improve their administration. The method of application is often changed (from enemas to suppositories, for instance) for occupational, social or availability reasons, without this affecting the clinical course of your disease. |
Daniel Ginard is a consultant and member of advisory boards, and gives conferences or has received research funding from AbbVie, Adacyte Therapeutics, Amgen, Takeda, Janssen, Chiesi, Otsuka Pharmaceutical and Fresenius Kabi.
Ignacio Marín-Jiménez is a consultant and member of advisory boards, gives conferences or has received research funding from MSD, AbbVie, Amgen, Hospira, Takeda, Janssen, Pfizer, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Chiesi, Gebro Pharma, Otsuka Pharmaceutical, Sandoz and Tillotts Pharma.
Manuel Barreiro-de Acosta is a consultant and member of advisory boards, gives conferences or has received research funding from MSD, AbbVie, Hospira, Takeda, Janssen, Pfizer, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Chiesi, Gebro Pharma, Otsuka Pharmaceutical and Tillotts Pharma.
Elena Ricart is a consultant and member of advisory boards, and gives conferences or has received research funding from MSD, AbbVie, Takeda, Janssen, Pfizer, Ferring, Otsuka Pharmaceutical and Fresenius Kabi.
Eugeni Domènech has received fees for giving conferences or expert advice, his attendance at courses or congresses has been facilitated, or his unit has received donations or grants for events or research projects from MSD, AbbVie, Takeda, Kern Pharma, Pfizer, Janssen, Celgene, Otsuka Pharmaceutical, Adacyte, Ferring, Shire Pharmaceuticals, Tillotts Pharma, Thermofisher, Grifols and Gebro.
Javier Gisbert has received scientific advice, support for research or training activities from MSD, AbbVie, Hospira, Pfizer, Kern Pharma, Biogen, Takeda, Janssen, Roche, Sandoz, Celgene, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical and Vifor Pharma.
María Esteve has received personal fees from Janssen, Pfizer, Menarini, Tillotts Pharma and Takeda, and research funding from MSD and AbbVie.
Miguel Mínguez has collaborated as a consultant with MSD, AbbVie, Janssen, Takeda and Shire Pharmaceuticals.
Please cite this article as: Ginard D, Marín-Jiménez I, Barreiro-de Acosta M, Ricart E, Domènech E, Gisbert JP, et al.; en representación de GETECCU. Recomendaciones del Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU) sobre el tratamiento tópico en la colitis ulcerosa. Gastroenterol Hepatol. 2020;43:97–105.