The regular arrangement of collecting venules (RAC) refers to the appearance of multiple regular tiny veins in the body of the stomach and is considered to be very effective for identifying gastric mucosa with non-Helicobacter pylori infection. This meta-analysis was conducted to systematically evaluate the value of the sign in predicting a Helicobacter pylori-negative stomach and the relevant factors that may affect the performance of this prediction.
MethodsTwo biomedical databases (PubMed and EMBASE) were systematically searched through April 20, 2020. The pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR) and area under the SROC curve (AUC) were calculated.
ResultsFourteen articles with 4070 patients were included. The pooled sensitivity, specificity, PLR, NLR, DOR and AUC for the RAC in predicting non-Hp infection were 0.80 (0.67–0.89), 0.97 (0.93–0.98), 24.8 (12.2–50.8), 0.21 (0.12–0.36), 120 (47–301) and 0.97 (0.19–1.00), respectively.
ConclusionsThe RAC is a valuable endoscopic feature for the prediction of patients without Hp infection.
La disposición regular de vénulas colectoras (RAC, en inglés) se refiere a la aparición de múltiples venas minúsculas regulares en el cuerpo del estómago y se considera muy eficaz para identificar la mucosa gástrica con infección no causada por Helicobacter pylori. Este metaanálisis se llevó a cabo para evaluar sistemáticamente el valor del signo en la predicción de un estómago negativo para Helicobacter pylori (Hp) y los factores relevantes que pueden afectar a la obtención de esta predicción.
MétodosSe realizaron búsquedas sistemáticas en dos bases de datos biomédicas (PubMed y EMBASE) el 20 de abril de 2020. Se calcularon la sensibilidad, la especificidad, el cociente de probabilidad positiva (PLR), el cociente de probabilidad negativa (CPN), el cociente de probabilidad de diagnóstico (NLR) y el área bajo la curva SROC (AUC) agrupados.
ResultadosSe incluyeron 14 artículos con 4.070 pacientes. La sensibilidad, especificidad, PLR, NLR, DOR y AUC agrupados para la RAC en la predicción de la infección no debida a Hp fueron de 0,80 (0,67-0,89), 0,97 (0,93-0,98), 24,8 (12,2-50,8), 0,21 (0,12-0,36), 120 (47-301) y 0,97 (0,19-1,00), respectivamente.
ConclusionesLa RAC es una característica endoscópica útil para la predicción de pacientes sin infección por Hp.
Helicobacter pylori (Hp) is considered to be a major risk factor for gastric cancer. Other risk factors1 include age, sex, smoking, ethnicity, family history, and so on. At present, it is recommended that once a Hp infection is found, unless for some special reason, it needs to be treated or eradicated.2 In the past, Japanese endoscopists found that the appearance of a large number of red spots in the stomach body was a characteristic discovery in the normal stomach, indicating that the gastric mucosa was not infected with Hp. The discovery was called the “regular arrangement of collecting venules” (RAC).3 Therefore, Japanese endoscopists began to use the RAC to make a preliminary diagnosis of whether the patient was infected with Hp. Many studies have shown that the RAC has good diagnostic efficacy for a normal stomach without an Hp infection.4,5 However, some studies suggest that the effectiveness of this prediction may be affected by the age of the patient or other factors.6 Therefore, we conducted this meta-analysis to systematically evaluate the value of RAC in predicting Hp-negative stomach, and to analyze the value of this predictive efficiency in different ages and ethnicities.
MethodsData sources, search strategy and study selectionThe study was conducted in accordance with Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies (PRISMA-DTA).7
We defined a systematic search strategy and searched 2 electronic databases, PubMed and EMBASE, through April 20, 2020. The following comprehensive search terms were used: (“H pylori” or “Hp” or Helicobacter pylori) and (“RAC” or “IRAC” or “Regular arrangement of collecting venules” or “Irregular arrangement of collecting venules”). Document screening was independently carried out by two authors (Lunan Li and Huiqin Gao). Any inconsistencies were discussed in our group to obtain an accurate result. In the process, no search limits or filters were used. EndNote X9 was used for data management.
Studies were included when they fulfilled both of the following conditions: 1. The study had a negative control group. 2. Sufficient data was included to calculate the true positive (TP), true negative (TN), false positive (FP) and false negative (FN). However, conference papers were excluded even if they met the above conditions. We included the first published article when multiple studies focused on the same population.
Data extraction and quality assessmentData extraction and quality assessment were independently completed by two individuals (Lunan Li and Huiqin Gao). Any inconsistencies were carried out by a panel discussion to maintain a consistent result.
The following information about the studies was recorded: year of publication, first author of the study, study type, country, characteristics of included patients (number, gender) and whether the study provided TP, TN, FP and FN. The Quality Assessment of Diagnostic Accuracy Studies II (QUADAS-II) 8tool was used to evaluate the included articles.
Data analysisWe performed a bivariate random-effects model9 to calculate the pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), area under the SROC curve (AUC) and corresponding 95% confidence intervals (CIs). The heterogeneity was calculated by the I2 statistic, and I2<50% indicated no significant heterogeneity.10 Subgroup analysis was used to analyze the statistical significance of the RAC in predicting an Hp-negative stomach among different ages and ethnicities. A p-value less than 0.05 was considered statistically significant. Publication bias was assessed by Deeks’ asymmetry test, and p<0.05 was considered significant.11 Stata 13 was used to perform the statistical analysis.
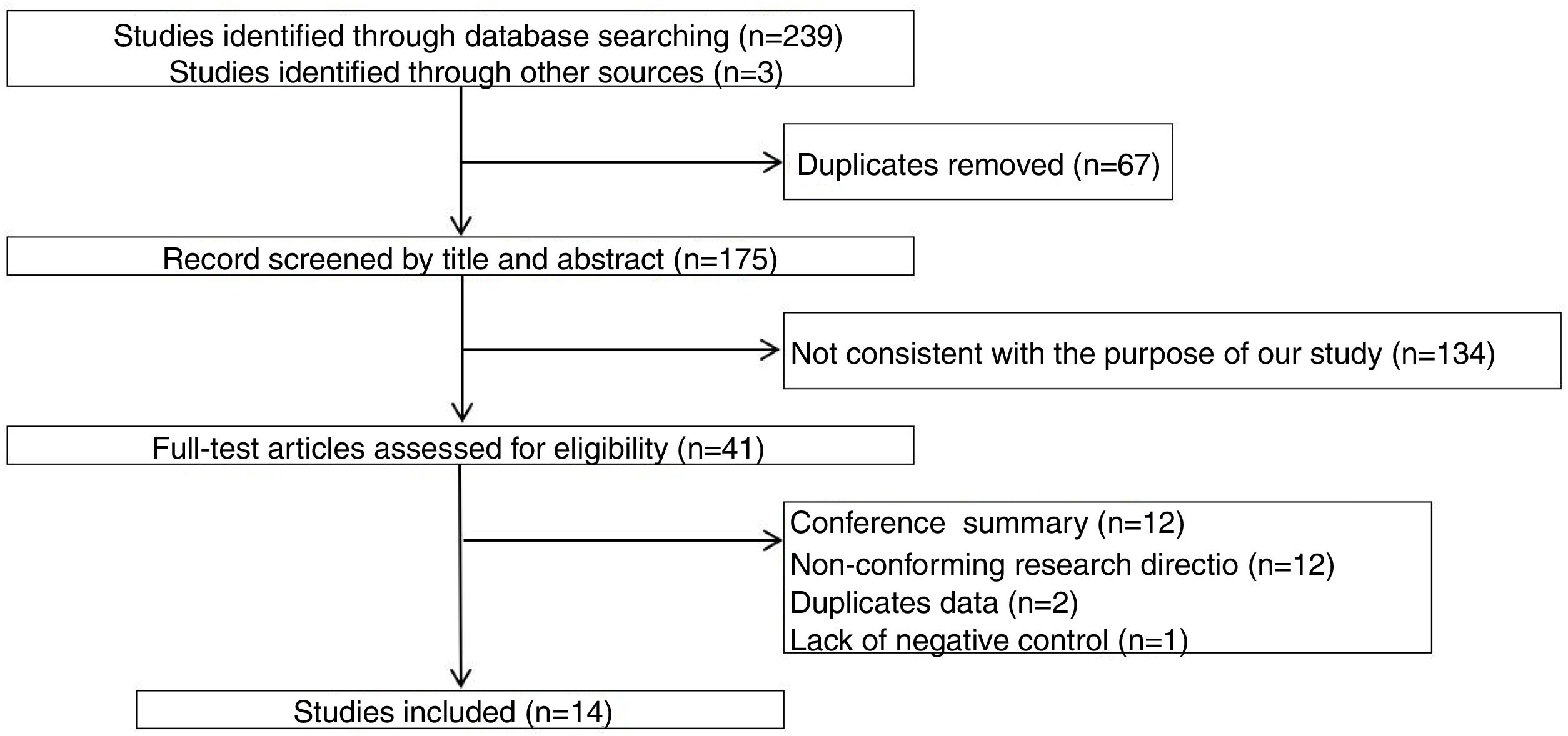
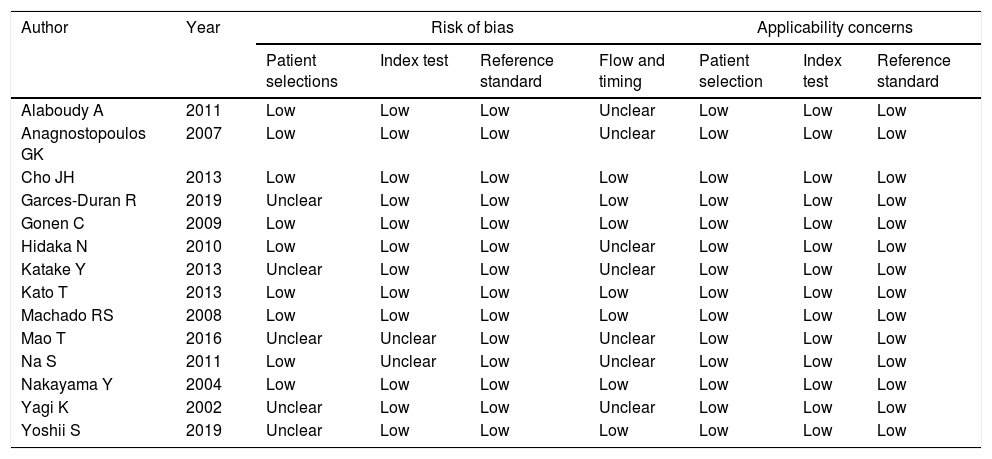
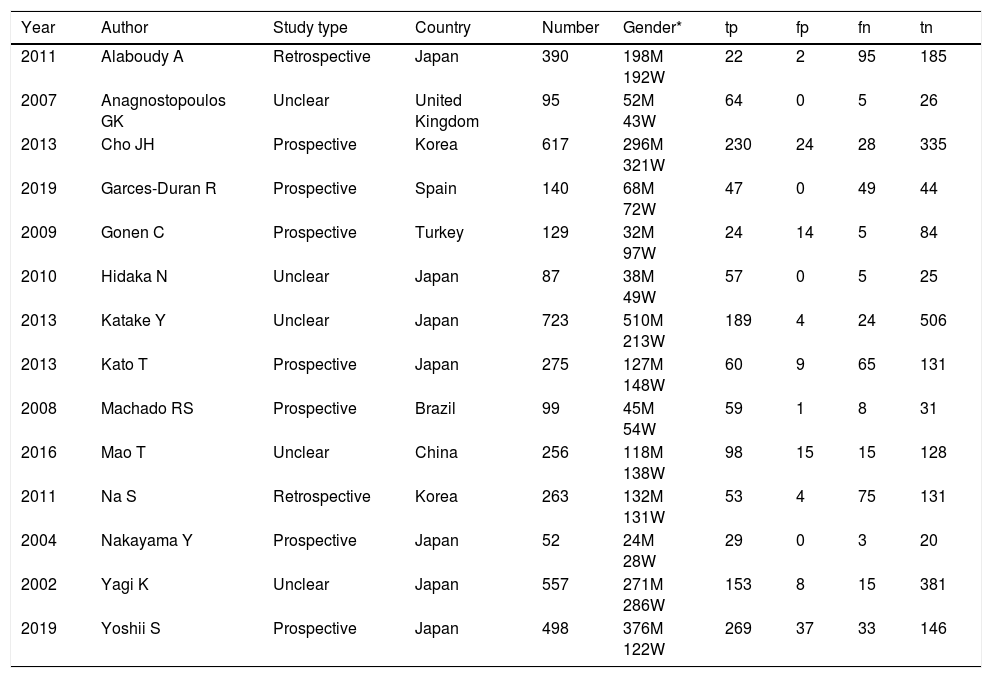
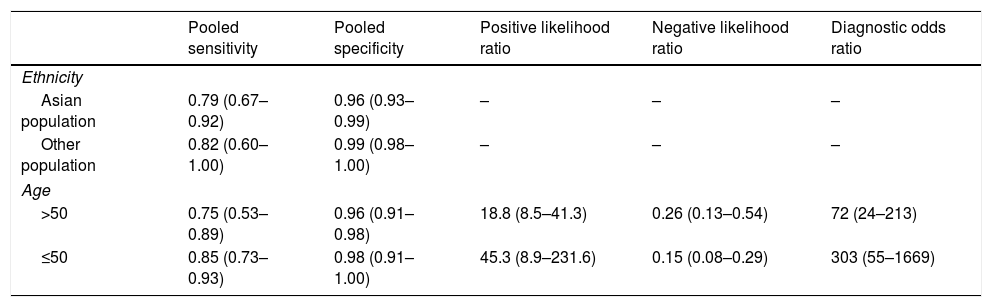
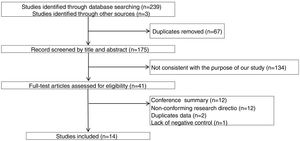
ResultsThe study selection process is shown in Fig. 1. A total of 242 studies were identified by the database search. After removing 67 duplicates and 134 studies that were not consistent with our purpose, 41 studies were assessed. Among these, 12 studies were conference summaries, 2 studies were duplicates, 12 studies did not provide relevant information, and one study lacked a negative control group. Ultimately, 14 studies with 4070 patients 3–6,12–21were included. The QUADAS-II quality assessment for each study is presented in Table 1. There was unclear bias in our included studies, but none of them were excluded from our meta-analysis. The TP, FP, FN, TN and characteristics of each study are presented in Table 2. Subgroup analysis for pooled sensitivity, pooled specificity, PLR, NLR and DOR are presented in Table 3.
Quality evaluation for included articles using the QUADAS-II tool.
| Author | Year | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|---|
| Patient selections | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | ||
| Alaboudy A | 2011 | Low | Low | Low | Unclear | Low | Low | Low |
| Anagnostopoulos GK | 2007 | Low | Low | Low | Unclear | Low | Low | Low |
| Cho JH | 2013 | Low | Low | Low | Low | Low | Low | Low |
| Garces-Duran R | 2019 | Unclear | Low | Low | Low | Low | Low | Low |
| Gonen C | 2009 | Low | Low | Low | Low | Low | Low | Low |
| Hidaka N | 2010 | Low | Low | Low | Unclear | Low | Low | Low |
| Katake Y | 2013 | Unclear | Low | Low | Unclear | Low | Low | Low |
| Kato T | 2013 | Low | Low | Low | Low | Low | Low | Low |
| Machado RS | 2008 | Low | Low | Low | Low | Low | Low | Low |
| Mao T | 2016 | Unclear | Unclear | Low | Unclear | Low | Low | Low |
| Na S | 2011 | Low | Unclear | Low | Unclear | Low | Low | Low |
| Nakayama Y | 2004 | Low | Low | Low | Low | Low | Low | Low |
| Yagi K | 2002 | Unclear | Low | Low | Unclear | Low | Low | Low |
| Yoshii S | 2019 | Unclear | Low | Low | Low | Low | Low | Low |
“Low” indicate low risk of bias; “unclear” represent unclear risk of bias; QUADAS II=Quality Assessment of Diagnostic Accuracy Studies.
Summary of the characteristic in the meta-analysis.
| Year | Author | Study type | Country | Number | Gender* | tp | fp | fn | tn |
|---|---|---|---|---|---|---|---|---|---|
| 2011 | Alaboudy A | Retrospective | Japan | 390 | 198M 192W | 22 | 2 | 95 | 185 |
| 2007 | Anagnostopoulos GK | Unclear | United Kingdom | 95 | 52M 43W | 64 | 0 | 5 | 26 |
| 2013 | Cho JH | Prospective | Korea | 617 | 296M 321W | 230 | 24 | 28 | 335 |
| 2019 | Garces-Duran R | Prospective | Spain | 140 | 68M 72W | 47 | 0 | 49 | 44 |
| 2009 | Gonen C | Prospective | Turkey | 129 | 32M 97W | 24 | 14 | 5 | 84 |
| 2010 | Hidaka N | Unclear | Japan | 87 | 38M 49W | 57 | 0 | 5 | 25 |
| 2013 | Katake Y | Unclear | Japan | 723 | 510M 213W | 189 | 4 | 24 | 506 |
| 2013 | Kato T | Prospective | Japan | 275 | 127M 148W | 60 | 9 | 65 | 131 |
| 2008 | Machado RS | Prospective | Brazil | 99 | 45M 54W | 59 | 1 | 8 | 31 |
| 2016 | Mao T | Unclear | China | 256 | 118M 138W | 98 | 15 | 15 | 128 |
| 2011 | Na S | Retrospective | Korea | 263 | 132M 131W | 53 | 4 | 75 | 131 |
| 2004 | Nakayama Y | Prospective | Japan | 52 | 24M 28W | 29 | 0 | 3 | 20 |
| 2002 | Yagi K | Unclear | Japan | 557 | 271M 286W | 153 | 8 | 15 | 381 |
| 2019 | Yoshii S | Prospective | Japan | 498 | 376M 122W | 269 | 37 | 33 | 146 |
Gender* M=man; Gender* W=women; tp=true positive; tn=true negative; fp=false positive; fn=false negative.
The subgroup analysis results.
| Pooled sensitivity | Pooled specificity | Positive likelihood ratio | Negative likelihood ratio | Diagnostic odds ratio | |
|---|---|---|---|---|---|
| Ethnicity | |||||
| Asian population | 0.79 (0.67–0.92) | 0.96 (0.93–0.99) | – | – | – |
| Other population | 0.82 (0.60–1.00) | 0.99 (0.98–1.00) | – | – | – |
| Age | |||||
| >50 | 0.75 (0.53–0.89) | 0.96 (0.91–0.98) | 18.8 (8.5–41.3) | 0.26 (0.13–0.54) | 72 (24–213) |
| ≤50 | 0.85 (0.73–0.93) | 0.98 (0.91–1.00) | 45.3 (8.9–231.6) | 0.15 (0.08–0.29) | 303 (55–1669) |
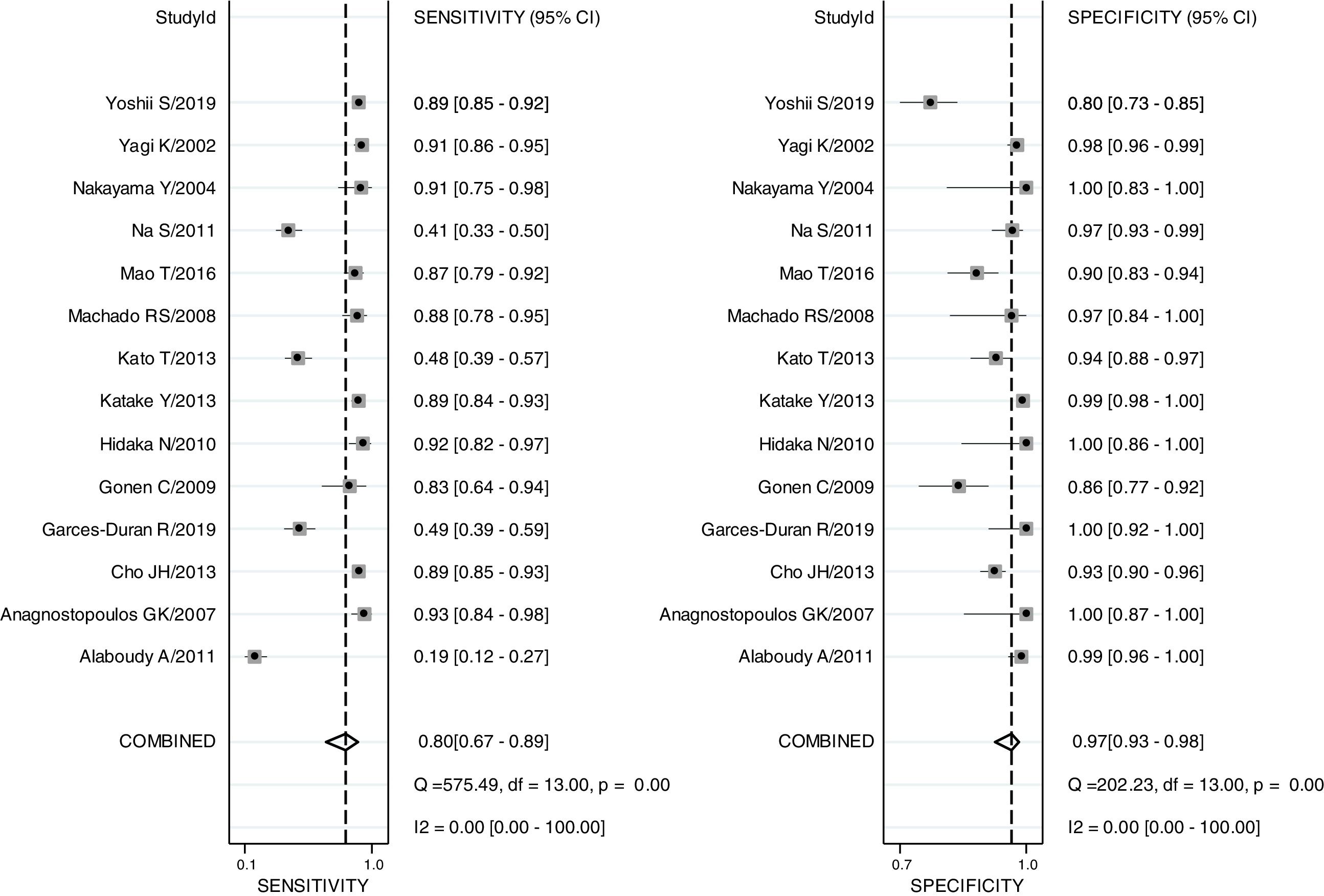
The pooled sensitivity, pooled specificity, PLR, NLR, DOR and AUC for RAC in predicting an Hp-negative stomach were 0.80 (0.67–0.89), 0.97 (0.93–0.98), 24.8 (12.2–50.8), 0.21 (0.12–0.36), 120 (47–301) and 0.97 (0.19–1.00), respectively. The I2 value was 0.00 (0.00–100.00) for the summary sensitivity and 0.00 (0.00–100.00) for the summary specificity (Fig. 2). Subgroup analysis was conducted for age and ethnicity. The p values of age and ethnicity were greater than 0.05 (Table 3). The pooled estimates for Asian population were as follows: sensitivity, 0.79 (0.67–0.92); specificity, 0.96 (0.93–0.99); Similarly, the corresponding values for other ethnicity were 0.82 (0.60–1.00), 0.99 (0.98–1.00). The RAC had a sensitivity of 0.75 (0.53–0.89), specificity of 0.96 (0.91–0.98), PLR of 18.8 (8.5–41.3), NLR of 0.26 (0.13–0.54) and DOR of 72 (24–213) for patients older than 50. The corresponding values were 0.85 (0.73–0.93), 0.98 (0.91–1.00), 45.3 (8.9–231.6), 0.15 (0.08–0.29) and 303 (55–1669) for patients younger than 50 years of age.
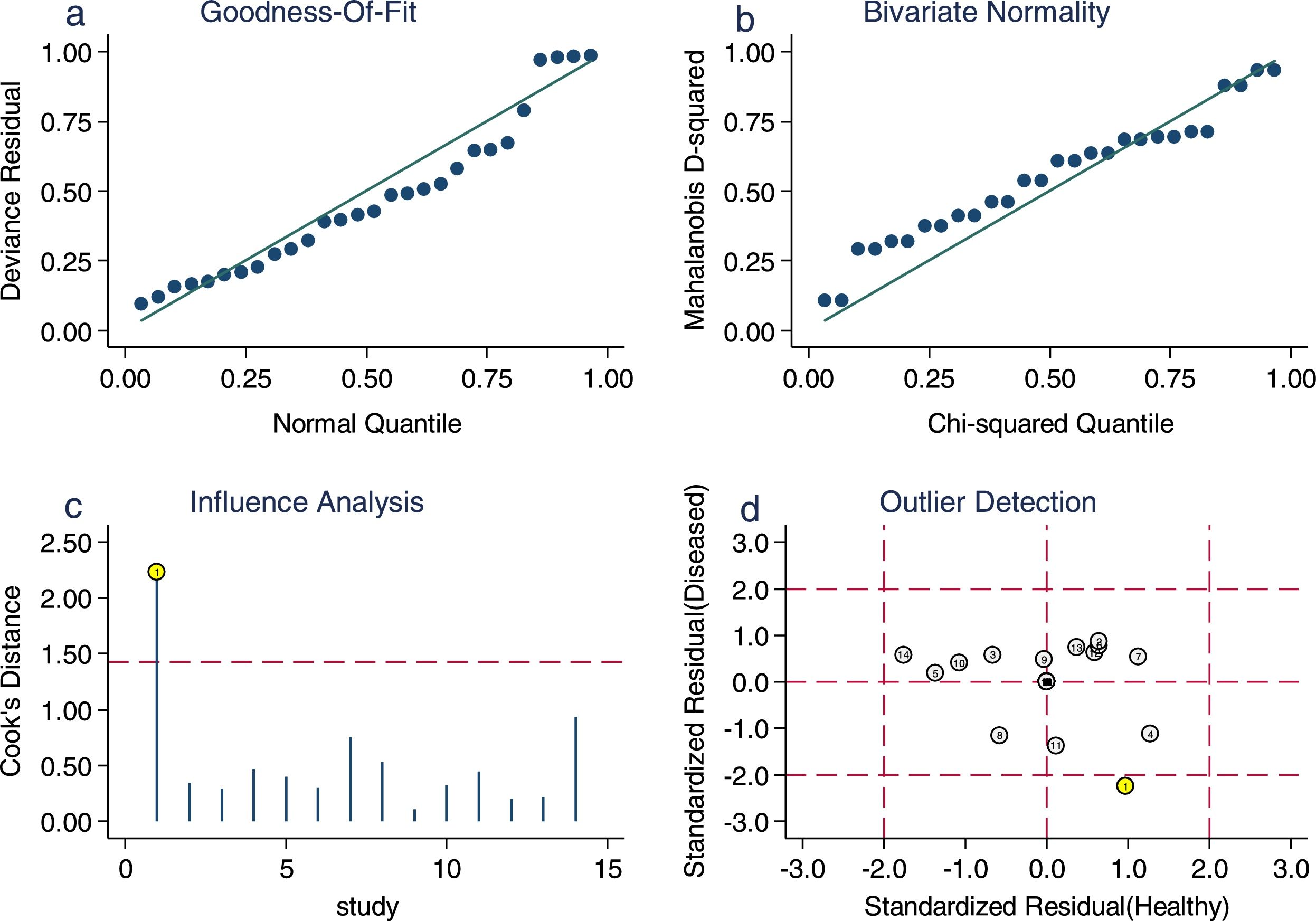
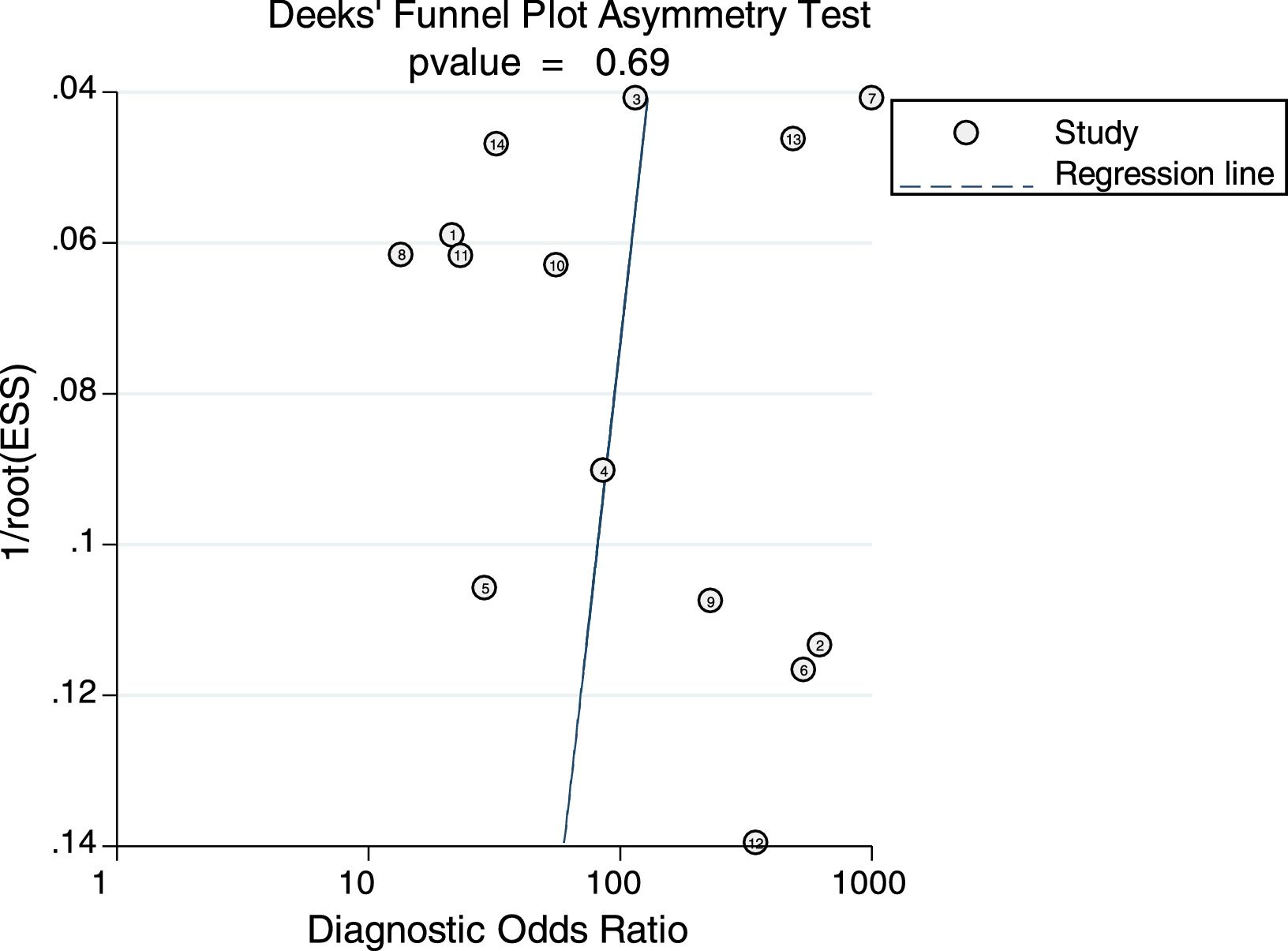
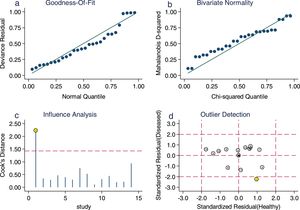
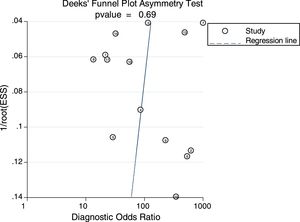
Sensitivity analysis (Fig. 3) was performed to ensure that our study results were not overly affected by any single included study. Results with and without outliers were compared. The results showed that removing a study19 increased the pooled sensitivity from 0.80 to 0.83 and the DOR from 120 to 136 but reduced the PLR from 24.8 to 23.8 and the NLR from 0.21 to 0.18. The pooled specificity in both cases was 0.97. The I2 for heterogeneity for the pooled sensitivity and specificity in both cases was still 0.00. These results show that our meta-analysis was reliable. Deeks’ funnel plots (Fig. 4) suggest that no evidence of publication bias was found in this meta-analysis.
DiscussionAccording to our systematic meta-analysis, the RAC is a valuable sign for predicting patients without an Hp infection, and the summary result did not show significant heterogeneity. When subgroup analysis was conducted, the performance between Asian and other ethnicities and between the two age groups did not show significant differences. Na S 20showed that the sensitivity and specificity of the RAC in the diagnosis of non-HP infection may be affected by the patient's age. We only divided the age group into two parts; thus, the results of this study need to be further confirmed. In addition, the low H. pylori infection rates in Western countries, combined with the high predictive efficiency of the RAC in predicting an HP-negative stomach, suggest that the RAC can be used to reduce the number of gastric mucosal biopsies performed in this population.
The RAC is considered to be a very simple and effective sign for determining gastric mucosa with non-HP infection. We found that endoscopic physicians observed the RAC from different locations, although a gastritis study in Kyoto, Japan showed that the RAC was generally observed at the lesser curvature and the lower part of the stomach.22 However, Cho14 suggested that it is best to observe the RAC in the corpus at the greater curvature. There is no unified point of view about this matter. Therefore, the best observation position for the RAC remains to be determined. Machado18 suggested that any disease with mucosal infiltration caused by inflammatory cells can lead to the absence of the RAC. However, there is a lack of research to confirm whether this claim is correct, whether it is related to IBD, whether it is affected by the degree of gastritis and the kind of drugs used (PPI, H2 receptor inhibitors, and antibiotics). Machado18 showed that the RAC can be recovered in some patients after the removal of the Hp infection. However, this phenomenon has not been explained. Therefore, it is worth discussing the relationship between the recovery of the RAC and the risk of gastric cancer. Perhaps through further prospective studies, the model for the prediction and treatment of HP infections can be improved.
Our article is the first to systematically evaluate the value of the sign in predicting a Helicobacter pylori-negative stomach. However, the limitations of our study should be recognized. First, we excluded conference abstracts, which may have led to some bias. Second, some detailed descriptions were not found, leading to an “unclear” assessment in QUADAS-II. Third, given the limited information, including multiple age groups and some other factors were not analyzed. Further studies should focus on the patient-relevant characteristics with or without the RAC and whether this sign is associated with gastric cancer.
ConclusionsThe RAC is a valuable sign for predicting patients without an Hp infection. No significant differences were found between ethnicities and ages with regard to the predictive ability of the RAC.
Conflict of interestThey are no conflict of interest.
This work was supported by the Zhejiang medicine key scientific and technology project (grant number: 2018258924) and the Zhejiang medicine scientific and technology project (grant number: No. 2019RC094).