Intrahepatic cholestasis of pregnancy is a temporary, pregnancy-specific disease that resolves with delivery, characterized by itching (pruritus), as well as high transaminase and serum bile acid levels in the third trimester of pregnancy. Due to the effects of Autotaxin on the physiology of pregnancy, we aimed to investigate Autotaxin activity in patients with intrahepatic cholestasis of pregnancy.
Patients and methodsSixty-nine patients diagnosed with intrahepatic cholestasis of pregnancy and 20 healthy pregnant women were enrolled in the study. Fasting serum bile acid, pruritus intensity, serum parameters, gestational week of the patients at the time of diagnosis were recorded, and birth week and birth weight were monitored. Autotaxin serum level was measured enzymatically.
ResultsThe mean serum bile acid level (n=69; 38.74±35.92μmol/L) in patients with intrahepatic cholestasis of pregnancy (n=69) was detected to be higher than healthy pregnant women (n=20; 5.05±1.88μmol/L) (p<0.001). Weak correlation was detected between serum bile acid level and itch intensity (p=0.014, r=0.295), while no relation was detected between Autotaxin and itch intensity (p=0.446, r=0.09). Although mean Autotaxin (intrahepatic cholestasis of pregnancy: 678.10±424.42pg/mL, control: 535.16±256.47pg/mL) levels were high in patients with intrahepatic cholestasis of pregnancy, it was not statistically significant (p=0.157).
ConclusionIn our study, we observed that the serum Autotaxin level did not make a significant difference in patients with intrahepatic cholestasis of pregnancy compared to healthy pregnant women. These findings suggest that larger clinical studies are required to reveal the physio-pathological effects of Autotaxin on pregnancy.
La colestasis intrahepática del embarazo es una enfermedad temporal específica del embarazo caracterizada por picazón (prurito), niveles elevados de transaminasas y ácidos biliares séricos elevados en el tercer trimestre del embarazo que se resuelve con el parto. Debido a los efectos de la autotaxina en la fisiología del embarazo, nuestro objetivo fue investigar la actividad de la autotaxina en pacientes con colestasis intrahepática del embarazo.
Pacientes y métodosEn el estudio se incluyeron 69 pacientes con diagnóstico de colestasis intrahepática del embarazo y 20 mujeres embarazadas sanas. Registramos los ácidos biliares séricos en ayunas, la intensidad del prurito, los parámetros séricos y la semana de gestación de las pacientes en el momento del diagnóstico, y controlamos la semana del parto y el peso al nacer. Los niveles séricos de autotaxina se midieron de forma enzimática.
ResultadosSe observó que el nivel medio de ácidos biliares en suero era mayor en pacientes con colestasis intrahepática del embarazo (n=69; 38,74±35,92μmol/l) que en mujeres embarazadas sanas (n=20; 5,05±1,88μmol/l) (p<0,001). Se detectó una correlación débil entre el nivel de ácidos biliares en suero y la intensidad del prurito (p=0,014; r=0,295), mientras que no se observó ninguna relación entre la autotaxina y la intensidad del prurito (p=0,446; r=0,09). Aunque los niveles medios de autotaxina fueron altos en pacientes con colestasis intrahepática del embarazo (678,10±424,42 frente a 535,16±256,47pg/ml en los controles), la diferencia no fue estadísticamente significativa (p=0,157).
ConclusiónObservamos que el nivel de autotaxina sérica no supuso una diferencia significativa en pacientes con colestasis intrahepática del embarazo en comparación con las mujeres embarazadas sanas. Estos hallazgos sugieren que se requieren estudios clínicos más amplios para determinar los efectos fisiopatológicos de la autotaxina en el embarazo.
Intrahepatic cholestasis of pregnancy (ICP) is a disease that usually occurs in the third trimester and presents with pruritus and elevation in liver enzymes. Pruritus disappears on its own shortly after birth and serum biochemical parameters return to their normal range.1 The etiology of ICP has still not been elucidated today, and its incidence varies greatly depending on ethnicity and geographic location.2 An increase in maternal serum bile salts is observed in ICP3 and therefore, ursodeoxycholic acid (UDCA) has been the most frequently used medication among treatment options.1 The diagnosis is confirmed by the exclusion of diseases that can explain pruritus, elevated levels of liver enzymes, and fasting serum bile acid (SBA) level being above 10μmol/L.4 Severe ICP is the case if the SBA level is greater than 40μmol/L and is associated with an increased risk of fetal complications.5,6
Autotaxin (ATX) was first defined in various pathological mechanisms such as tumor cell proliferation and metastasis formation as an autocrine cell motility factor in melanoma cells.7,8 ATX plays a crucial role in Lysophosphatidic acid (LPA) production by converting lysophosphatidylcholine to LPA.9 LPA is a small but powerful bioactive phospholipid with a wide range of effects on a multitude of cell types, from cell migration to cytokine production and platelet activation through the inter-cell signal transmission associated with the G protein.8,10,11 It has been shown that in vitro LPA signaling contributes to the production of chemokine from human trophoblast cells, and these chemokines regulate the migration, proliferation, and angiogenesis of human endometrial epithelial cells.12
It has also been shown that LPA plays an important role in a wide variety of reproductive processes, including functional maturation of the endometrium to support embryonic growth, also known as decidualization, as well as implantation, and continuation of pregnancy.13 It has been found that ATX and LPA concentrations in the peripheral blood of pregnant women gradually increase during pregnancy and rapidly return to their pre-pregnancy level following birth.14 There is a high degree of correlation between blood LPA level and ATX levels, and it has been stated that it could be required for the continuation of pregnancy.15 In parallel, it is assumed that placental trophoblasts and syncytiotrophoblasts are the sources of elevated ATX levels.16 Besides, it has been stated that fatty tissue, enteroendocrine cells, and liver may also contribute to ATX synthesis.17 While examining the effects of ATX on the physiology of pregnancy, a cause-effect relationship was attempted to be established with pregnancy-specific diseases such as pre-eclampsia (PE) and ICP. This relationship has been tried to be explained in various studies,18–22 but the results contradict each other.
In this study, we aimed to investigate the relationship between pregnancy complications (such as preterm delivery, pruritus) and serum ATX levels in intrahepatic cholestasis of pregnancy.
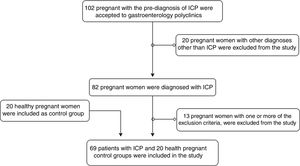
Patients and methodsParticipants of the studyIn 2018, 102 pregnant women were analyzed, who were referred to the gastroenterology outpatient clinic of the hospital with a pre-diagnosis of ICP. Of these patients, 82 pregnant women were diagnosed with ICP, who had a fasting SBA level of >10μmol/L, did not have a hepatobiliary obstruction in abdominal ultrasonography (USG), and had a pruritus. Exclusion criteria were as follows: being under the age of 18, being at less than 12 weeks of gestation and more than 40 weeks and 6 days, having multiple pregnancies of triplets or more, giving birth decision within 48h after admission, and having fetal-lethal anomaly. Thirteen pregnant women who had one or more of these criteria were excluded from the study. Sixty-nine pregnant women fulfilling the criteria were enrolled in the study. Twenty healthy pregnant women who did not have any disease followed-up by the obstetrics outpatient clinic were included in the study as a control group. Age, gestational week, SBA pruritus score, biochemical tests of patients, birth week, and birth weight of infants were recorded. Patient information was accessed from the hospital information processing system and patient files. This is a prospective study and the ethics committee approval was granted by the Clinical Research Ethics Committee of the institution under number 2018/07. Patient selection in the study is shown with a flow chart (Fig. 1).
Pruritus scoreVerbal Rating Scale (VRS) was used to assess the itch intensity. Four different symptom intensities of patients were evaluated through VRS as 0: absent, 1: mild, 2: moderate, 3: severe itching.23 According to this itch scale, which is divided into four categories, the patients were asked verbally for the severity of itching, and the responses received were recorded numerically.
Examination of biochemical parametersFollowing 12-hour fasting for biochemical assays, blood samples taken to dry tubes with gel were delivered to the laboratory within one hour at the latest. 500μl of a serum sample from the first tube, centrifuged for 10min at 1500rpm for serum collection, was reserved for the Eppendorf and stored at −20° for ATX analysis for further studies. Routine biochemistry assays (alanine transaminase, aspartate aminotransferase, total and direct bilirubin, etc.) from serum samples obtained from other tubes were studied with the original kits on Cobas 8000® model biochemistry and hormone modular analyzer series by Roche (Roche Diagnostics, California, US). Serum bile acids were analyzed by colorimetric method.
After the collection of all samples, stored serum samples were thawed at room temperature for ATX analysis. ATX level was quantitatively analyzed under conditions as per the procedure including the recommendations of the manufacturer company with the ELISA (Enzyme-linked immunosorbent assay) method based on the sandwich method using the Human Autotaxin ELISA Kit (Cat no: SG-10887) by SinoGeneClon Biotech Co., Ltd. Results were expressed in pg/mL. In the repeatability study carried out, the intra-assay and inter-assay coefficients of variation (% CV) were <8% and <10%, respectively.
Statistical analysisThe data were analyzed using the Statistical Package for the Social Sciences v22 (SPSS, Inc., Chicago, IL, USA). In the section of descriptive statistics, categorical variables were presented by providing numbers and percentages, and continuous variables were presented with mean±standard deviation (SD) and median (minimum–maximum). Comparative data were shown as mean±standard error. The fitness of continuous variables to normal distribution was evaluated using visual (histogram and probability plots) and analytical methods (Kolmogorov–Smirnov/Shapiro–Wilk tests). In the data that do not conform to the normal distribution as a result of the normality analysis, the Mann–Whitney U test was used for comparative analyses between the two groups, while independent Student's t-test was used in non-compliant cases. Pearson's chi-square (χ2) test was used in comparison analysis for categorical variables between independent groups. Pearson and Spearman correlation analyzes were performed for correlation analysis between categorical and continuous variables. Data were presented with a 95% confidence interval and all tests of significance were two-tailed. p values of <0.05 were considered statistically significant.
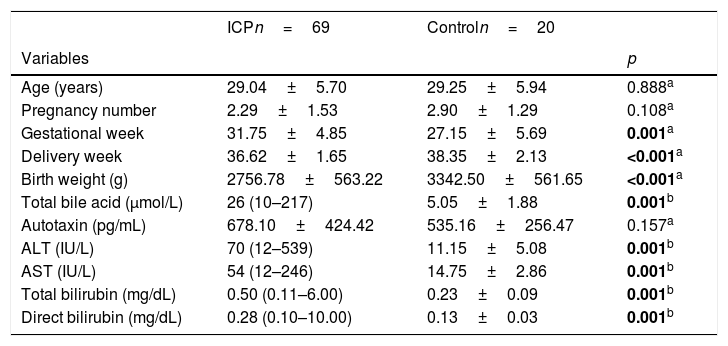
ResultsEighty-nine patients were enrolled in the study, which consists of 69 patients for ICP and 20 for the control group. The mean age of the control group and the ICP group was similar (p=0.888). The mean gestational week at the time of admission of the ICP group (31.75±4.85) and the gestational week of the control group (27.15±5.69) were similar. The mean birth week (36.62±1.65) in pregnant women with ICP was significantly lower than the control group (38.35±2.13) (p<0.001). The mean birth weight (2756.78±563.22) of the infants of pregnant women with ICP was found significantly lower than the control group (p<0.001). There was a statistically significant difference between the groups for total SBA, ALT, AST, total bilirubin, and direct bilirubin (p<0.05). The mean serum ATX concentration was higher in ICP, while the difference between the control groups was not statistically significant (p>0.005). All data have been summarized in Table 1.
Demographic data.
| ICPn=69 | Controln=20 | ||
|---|---|---|---|
| Variables | p | ||
| Age (years) | 29.04±5.70 | 29.25±5.94 | 0.888a |
| Pregnancy number | 2.29±1.53 | 2.90±1.29 | 0.108a |
| Gestational week | 31.75±4.85 | 27.15±5.69 | 0.001a |
| Delivery week | 36.62±1.65 | 38.35±2.13 | <0.001a |
| Birth weight (g) | 2756.78±563.22 | 3342.50±561.65 | <0.001a |
| Total bile acid (μmol/L) | 26 (10–217) | 5.05±1.88 | 0.001b |
| Autotaxin (pg/mL) | 678.10±424.42 | 535.16±256.47 | 0.157a |
| ALT (IU/L) | 70 (12–539) | 11.15±5.08 | 0.001b |
| AST (IU/L) | 54 (12–246) | 14.75±2.86 | 0.001b |
| Total bilirubin (mg/dL) | 0.50 (0.11–6.00) | 0.23±0.09 | 0.001b |
| Direct bilirubin (mg/dL) | 0.28 (0.10–10.00) | 0.13±0.03 | 0.001b |
a Student t test. b Mann–Whitney U test. SD: standard deviation. Values are means±SD. * Median (min–max); ALT, alanine transaminase; AST, aspartate aminotransferase; ICP, intrahepatic cholestasis of pregnancy. a,bp<0.05 was statistically significant.
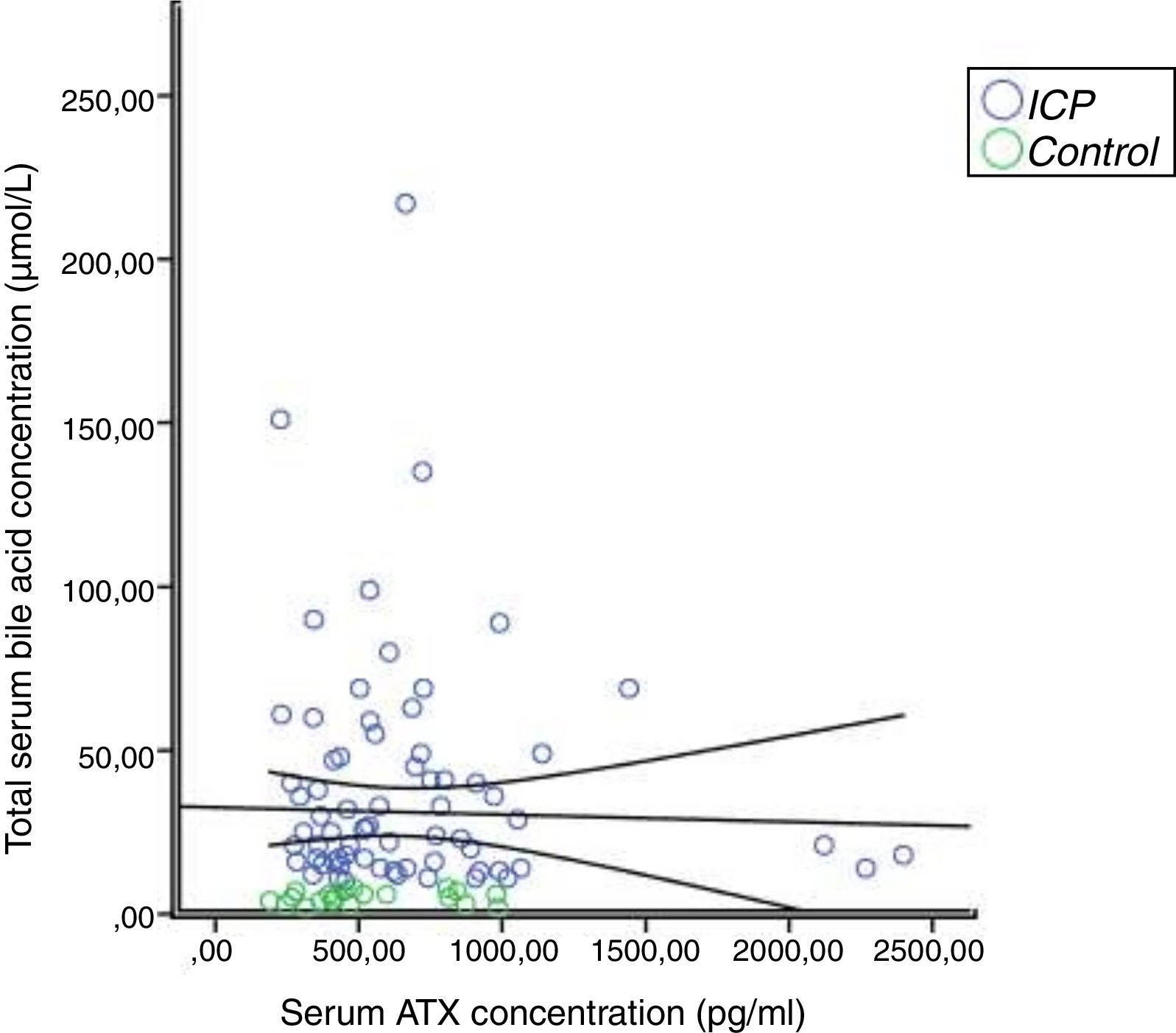
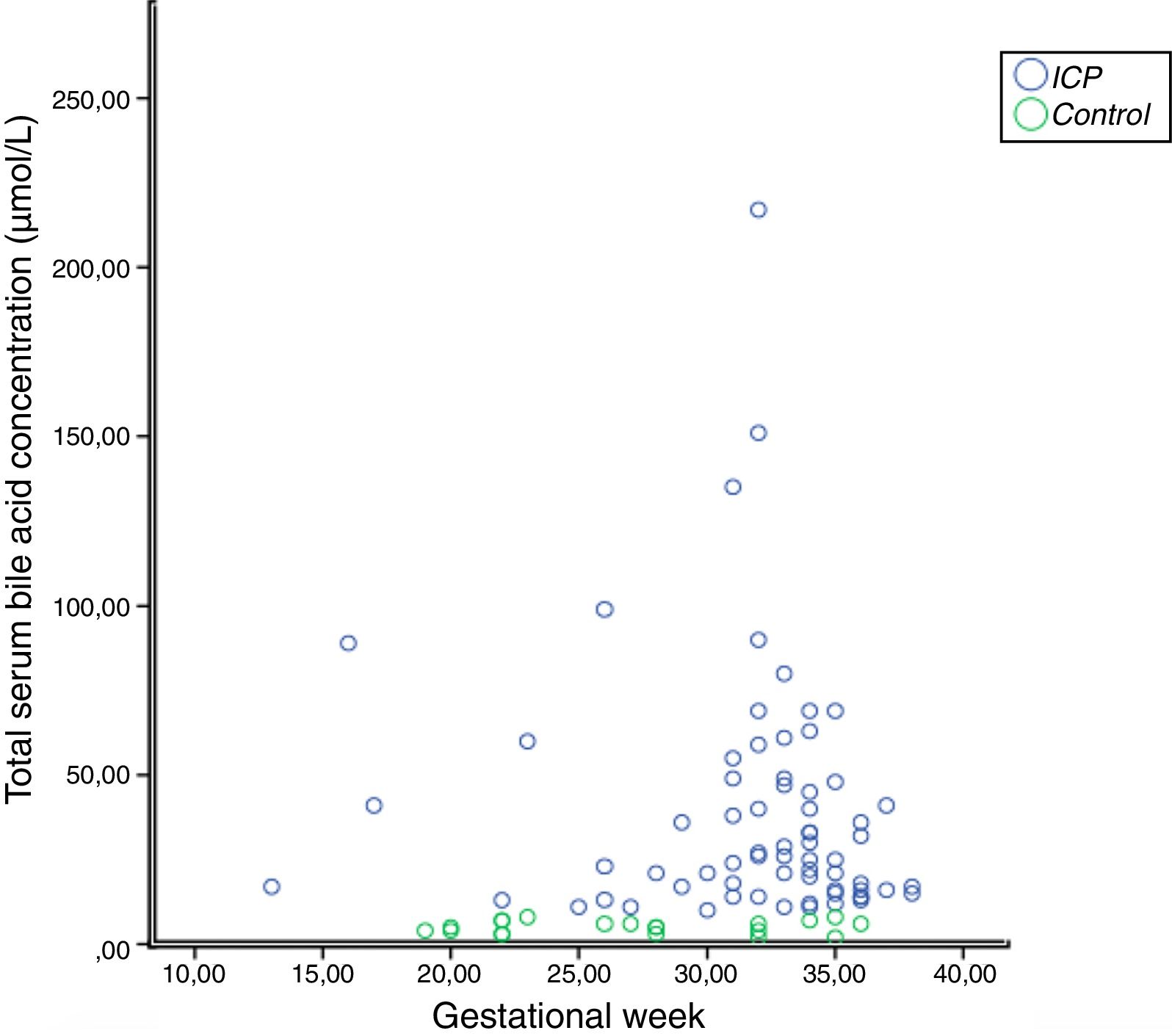
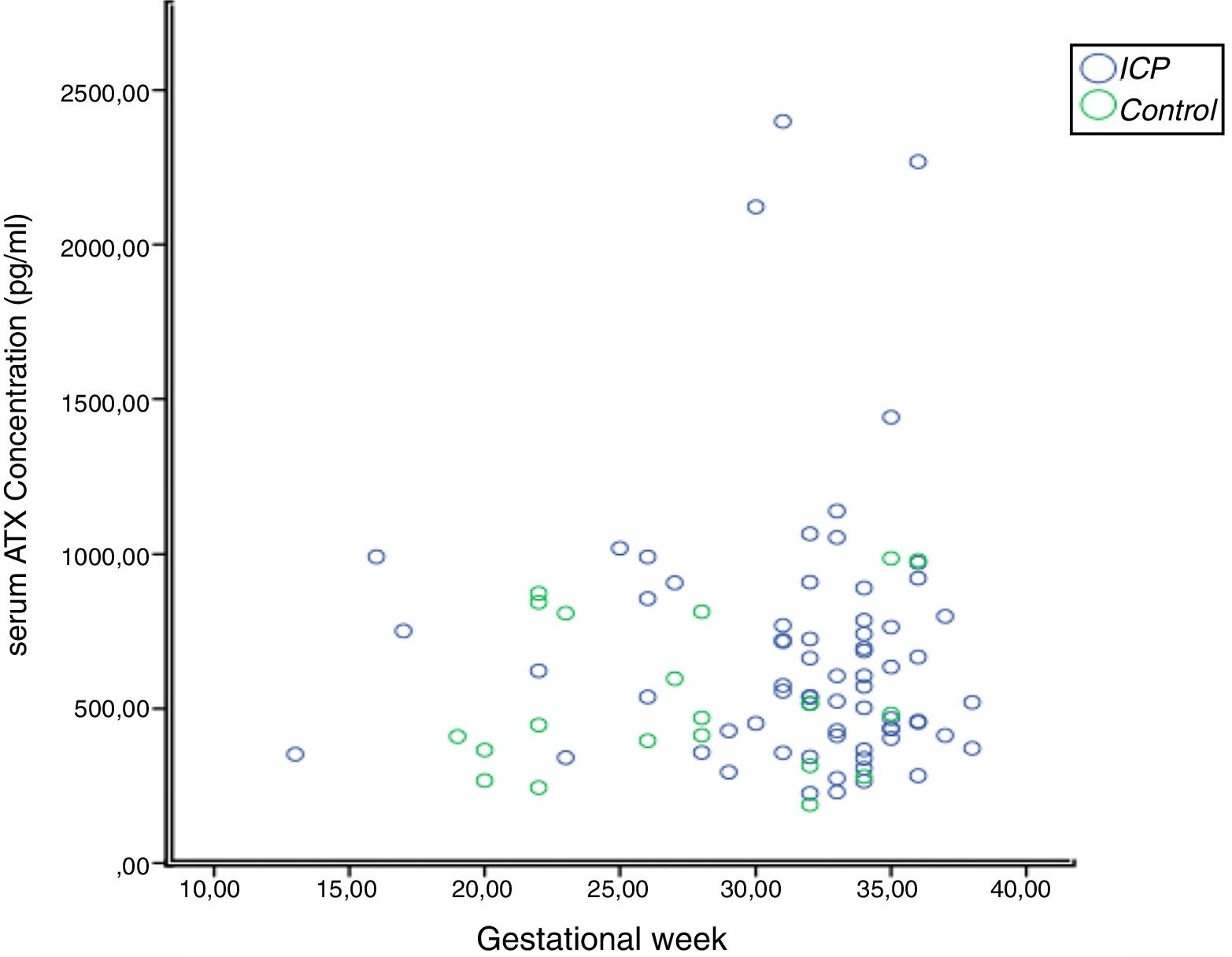
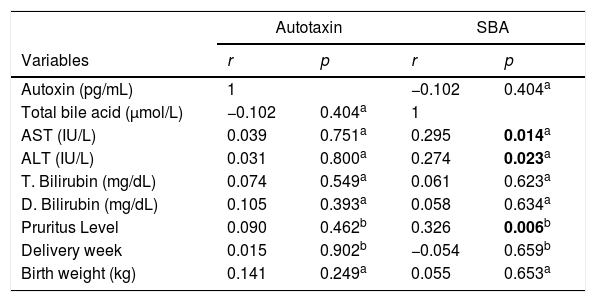
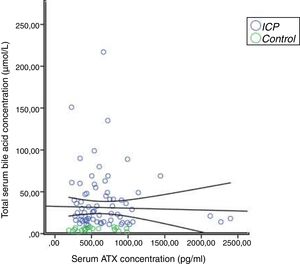
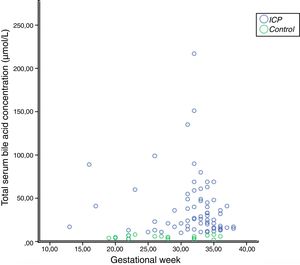
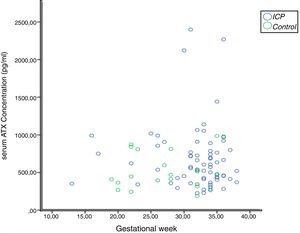
In the correlation study between SBA and ATX in ICP and some biochemical parameters and clinical manifestations, a weak correlation was found out between SBA and AST, ALT, and the level of pruritus (p=0.014 r=0.295, p=0.023 r=0.257, p=0.006 r=0.362, respectively). There was no correlation between ATX and other parameters (Table 2, Fig. 2). Moreover, it was observed that there was no correlation between the gestational week at diagnosis and SBA and ATX levels (Figs. 3 and 4).
Correlation between SBA, ATX, serum parameters and clinical outcomes in ICP.
| Autotaxin | SBA | |||
|---|---|---|---|---|
| Variables | r | p | r | p |
| Autoxin (pg/mL) | 1 | −0.102 | 0.404a | |
| Total bile acid (μmol/L) | −0.102 | 0.404a | 1 | |
| AST (IU/L) | 0.039 | 0.751a | 0.295 | 0.014a |
| ALT (IU/L) | 0.031 | 0.800a | 0.274 | 0.023a |
| T. Bilirubin (mg/dL) | 0.074 | 0.549a | 0.061 | 0.623a |
| D. Bilirubin (mg/dL) | 0.105 | 0.393a | 0.058 | 0.634a |
| Pruritus Level | 0.090 | 0.462b | 0.326 | 0.006b |
| Delivery week | 0.015 | 0.902b | −0.054 | 0.659b |
| Birth weight (kg) | 0.141 | 0.249a | 0.055 | 0.653a |
a Pearson and bSpearman correlation analysis were used. SBA, serum bile acid; ALT, alanine transaminase; AST, aspartate aminotransferase. a,bp<0.05 was statistically significant.
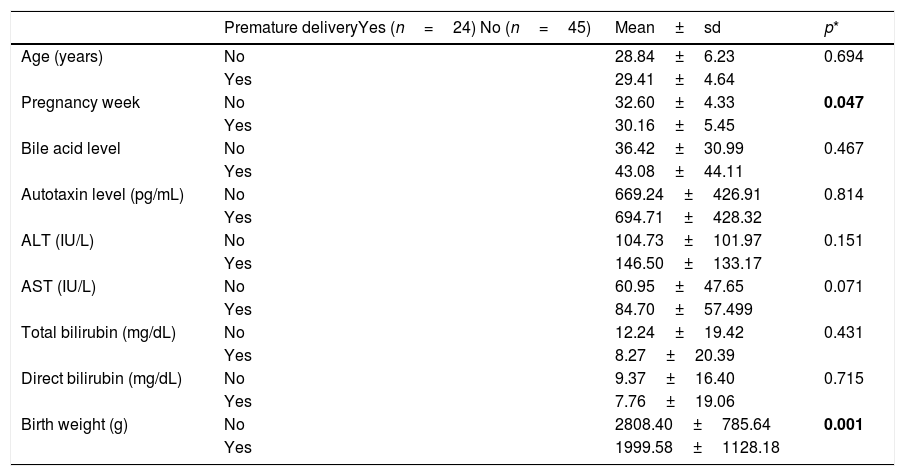
It was found out that 65.2% (n=45) of the patients with ICP had a normal birth and 34.8% (n=24) had a premature birth. Total SBA, ATX concentration, mean ALT and AST were higher at the time of diagnosis in pregnant women with ICP who gave a premature birth, but the difference between them was not statistically significant (p>0.05). The findings are summarized in Table 3.
Comparison of biochemical and clinical results in pregnant women with ICP giving or not giving preterm birth.
| Premature deliveryYes (n=24) No (n=45) | Mean±sd | p* | |
|---|---|---|---|
| Age (years) | No | 28.84±6.23 | 0.694 |
| Yes | 29.41±4.64 | ||
| Pregnancy week | No | 32.60±4.33 | 0.047 |
| Yes | 30.16±5.45 | ||
| Bile acid level | No | 36.42±30.99 | 0.467 |
| Yes | 43.08±44.11 | ||
| Autotaxin level (pg/mL) | No | 669.24±426.91 | 0.814 |
| Yes | 694.71±428.32 | ||
| ALT (IU/L) | No | 104.73±101.97 | 0.151 |
| Yes | 146.50±133.17 | ||
| AST (IU/L) | No | 60.95±47.65 | 0.071 |
| Yes | 84.70±57.499 | ||
| Total bilirubin (mg/dL) | No | 12.24±19.42 | 0.431 |
| Yes | 8.27±20.39 | ||
| Direct bilirubin (mg/dL) | No | 9.37±16.40 | 0.715 |
| Yes | 7.76±19.06 | ||
| Birth weight (g) | No | 2808.40±785.64 | 0.001 |
| Yes | 1999.58±1128.18 |
ATX is a lysophospholipase D required for angiogenesis and neuronal development in the embryogenesis process.24 High levels of ATX mRNA have been reported in the human placenta 25 and it has been suggested that this enzyme is expressed from syncytiotrophoblasts by immunohistochemical analysis.16 Besides, lysophospholipase D levels have been reported to increase during pregnancy and show a positive correlation with gestational age.15
ATX has been the subject of research in other cholestatic liver diseases, as well as in patients with ICP, to explain the etiopathogenesis of pruritus. In a study involving patients with primary biliary cholangitis (PBC), it was stated that ATX and its product LPA might be one of the causes of cholestatic pruritus.26 Another study indicated that SBA and ATX activation significantly increased in PBC, a correlation was found between fasting SBA and ATX levels and there was a decrease in these levels in correlation with the ileal bile acid transporter (IBAT) inhibitor.27 In a study conducted by Kremer et al., ATX level was found to be high in non-pregnancy-related cholestatic pruritus and had not any relationship with other itchy diseases; also, it was claimed that ATX was involved in cholestasis related itching.28 This allowed us to think more about the origin of ATX in cholestatic pruritus, and to question the level of ATX synthesis outside the placenta, especially in the liver, and its contribution to itching.17
Although there are some studies trying to explain the relationship between ATX and pregnancy-related diseases,14,18–22 these are not yet sufficient to eliminate contradictions. Here, patients with preeclampsia can be given as an example. In one study18 it was found that ATX levels were higher in preeclamptic patients compared to the control group, while other studies indicated the exact opposite results, in which the serum ATX levels decrease in patients with PE; also, there may be a decrease in trophoblast-associated ATX expression due to placental pathology in early-onset preeclampsia.14,19
Just as with PE, there are contradictory cases in terms of ATX in patients with ICP. Upon examination of few studies in the literature.20–22 Kremer et al. claimed that there was a strong correlation between ATX and ICP, and there might be a relationship between ATX level and preterm births.20 In a later study by the same author, it was reported that though the elevated serum ATX level in ICP, there was no difference in ATX mRNA levels in placental tissue between healthy pregnancies and patients with ICP.21 However, in a study by Macias et al., it was reported that ATX mRNA expression was higher in the placenta of patients with ICP.22
In our study, although the mean serum ATX level in patients with ICP was higher than in the control pregnant women, no statistically significant difference was found between them. It was noteworthy that while the serum ATX levels increased as the gestational week progressed, the difference in serum ATX level was not statistically significant in our study, although the gestational week was lower than the ICP group. Additionally, in general, although we found the number of the preterm birth and low birth weight babies significantly higher in patients with ICP, we could not find a relationship between them and the ATX level.
Despite claiming that ATX (and relevant products) and LPA may be a mediator for itching,20 in our study, there was no relation between ATX level and pruritus intensity. A weak correlation was detected between SBA level and pruritus intensity. According to this knowledge, we think that there is a need for future studies investigating the relationship between the pruritis in ICP and LPA.
There were some limiting factors in this study. The study population was relatively small; therefore, it was not able to examine the effect of UDCA on serum bile acid and serum ATX levels.
In conclusion, in the current study, ATX was not found to be correlated with biochemical and clinical endpoints in patients with ICP and in this point, the current study contributed to the literature. Noting the physiological role of ATX in the continuation of pregnancy, we indicate the need for larger studies on this subject.
Author's distributionAll authors were involved in the study concept and design, analysis and interpretation of the data, and the drafting and critical revision of the manuscript for important intellectual content. Study concept, design and drafting: SC; Data acquisition: KI, MB, CK, ZA; Statistical analysis and interpretation of the data: NE, SC; Critical review and editing: NE, HS. SC is the article guarantor. All authors read and approved the final manuscript.
Availability of data and materialsPatient's data was obtained from hospital data system.
Ethical approvalClinical Research Ethics Committee of Kanuni Sultan Süleyman Training and Research Hospital under number 2018/07.
FundingThe authors declared that this study has received no financial support.
Conflicts of interestThe authors declared no conflicts of interest.
We thank the patients and Department of Gastroenterology.
S.C. participated in the study design, acquisition of the data, data analysis and interpretation of the data and wrote the manuscript. N.E. participated in the data analysis and interpretation of the data and wrote the manuscript. M.B., K.I., C.K. and Z.A. performed the measurements and acquisition of the data.