Immune cells play an important role in controlling liver tumorigenesis, viral hepatitis, liver fibrosis and contribute to pathogenesis of liver inflammation and injury. Accumulating evidence suggests the effectiveness of natural killer (NK) cells and Kupffer cells (KCs) against viral hepatitis, hepatocellular damage, liver fibrosis, and carcinogenesis. Activation of natural killer cells provides a novel therapeutic strategy to cure liver related diseases. This review discusses the emerging roles of immune cells in liver disorders and it will provide baseline data to scientists to design better therapies for treatment.
Los inmunocitos o células inmunitarias desempeñan un papel importante en el control de la carcinogénesis hepática, la hepatitis vírica, la fibrosis hepática y contribuyen a la patogénesis de la inflamación y la lesión hepáticas. La creciente evidencia sugiere la efectividad de los linfocitos citolíticos naturales (NK, natural killer) y las células de Kupffer (KC, Kupffer cells) frente a la hepatitis vírica, la lesión hepatocelular, la fibrosis hepática y la carcinogénesis. La activación de linfocitos citolíticos naturales ofrece una nueva estrategia terapéutica para curar enfermedades relacionadas con el hígado. Esta revisión trata de las nuevas funciones de los inmunocitos en los trastornos hepáticos y ofrecerá datos básicos a los científicos para diseñar mejores terapias para el tratamiento.
Liver is the metabolic centre of organism controlled by central nervous system. Its anatomic localization and specific tissue structure indicates its defensive role in organism. Approximately 80% of liver cells are hepatocytes. Non-hepatocytes include 40% endothelial cells 20% Kupffer cells, 20% lymphocytes, 20% stellate cells, and biliary cells. Natural killer cells make up 50% of liver population that reside in liver sinusoids. The multitude of cells makes this organ play active role in peripheral immune tolerance of the organism using transforming growth factor-β and haemopoetic cells. The fifth most common cancer in the world is liver cancer, 90% of which is hepatocellular carcinoma (HCC) 1 and the better understanding of liver's immunological processes will provide insight into the role of immune tolerance mechanisms and its contribution in the development of autoimmune diseases and chronic viral infections of liver.
Natural killer cells inhibit liver fibrosis, viral infection and tumor cells growth. The liver immune system is properly equipped with liver immune cells that achieve the critical task of protection against metastatic cells, pathogens, and foreign antigens by coordinating with anti-microbial components (inflammatory cytokines, chemokines, acute phase proteins, complement). Liver plays its role as a buffer between systematic circulation and the contents of gut and about 80% of blood is supplied from gut into liver through portal vein. This blood is rich in harmless environmental antigens, microflora of gut, and dietary elements. Liver must endure immunogenic load by providing immunosurveillance for malignant cells and pathogenic infections.2,3 Innate immune cells of liver such as KCs, monocytes, dendritic cells (DCs), NK cells, natural killer T cells (NKT) cells, and neutrophils produce cytokine and initiate inflammation.4 We will briefly discuss the potential roles of immune cells in the pathogenesis of liver related disorders.
Natural killer (NK) and natural killer T (NKT) cellsThe lymphocytes present in liver are enriched in NK and NKT cells that are key regulators of antitumor defenses, antiviral defenses, and pathogenesis of chronic liver disease. These cells account for 25-40% of total intrahepatic lymphocytes. NK cells have some peculiar functional and phenotypic properties such as specific cytokine profiles, and TRAIL-dependent cytotoxicity.
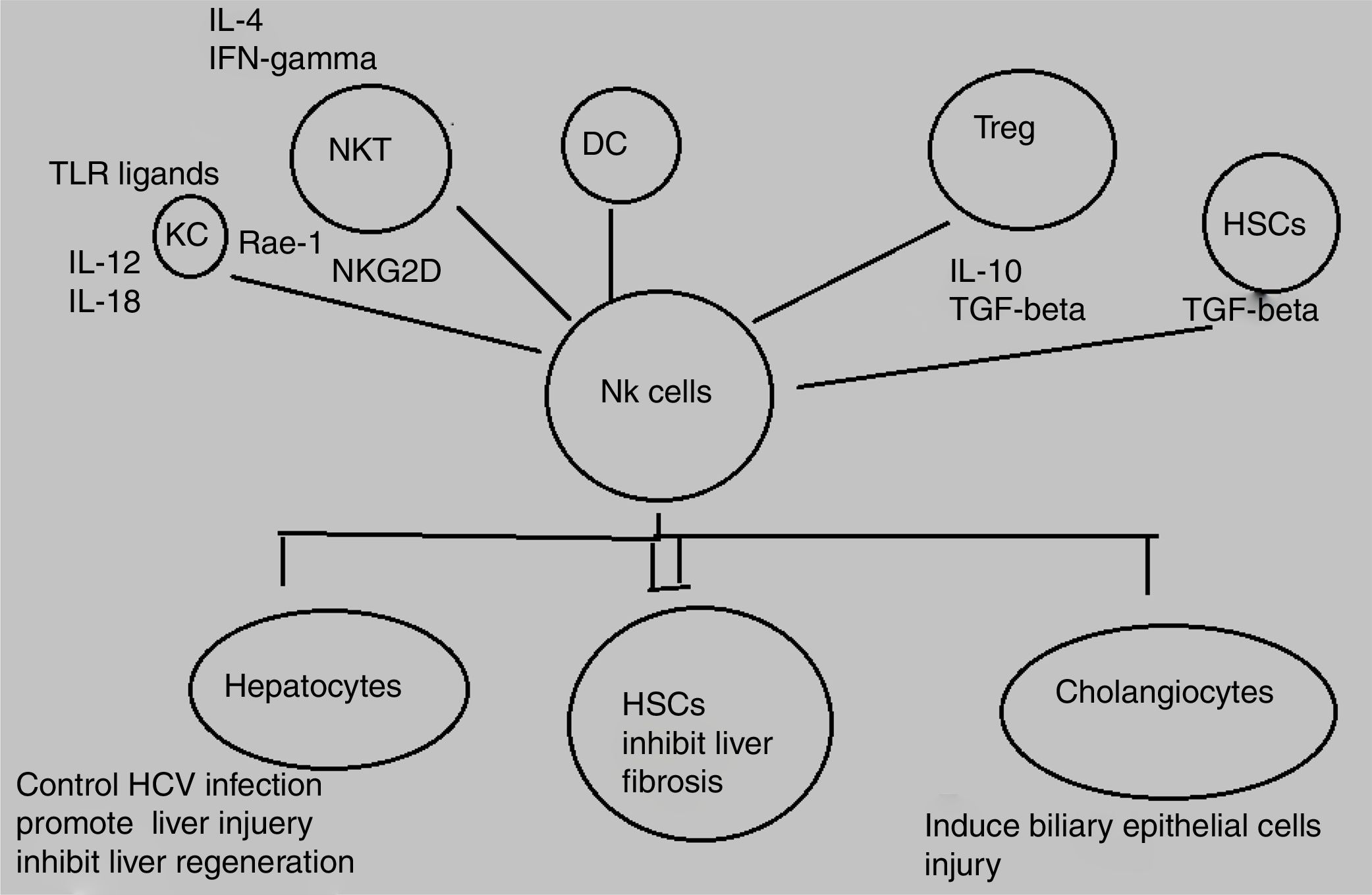
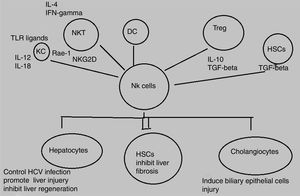
Decline in NK/NKT cells greatly increases tumor metastatsis of liver and enhancement of NK/NKT cells weakens it,5,6 both type of cells produce significant quantity of cytokines that stimulate adaptive immune response and help in removal of food antigens, toxins, and pathogens. The role of NKT cells play dominant role as anti-tumor agent by inhibiting liver fibrosis through suppression of hepatic stellate cells activation.7,8 NK and NKT cells play an important role in pathogenesis of liver inflammation and injury, liver fibrosis and tumorigenesis. Several studies demonstrate that NK and NKT cells control viral hepatitis.9–12 The mechanisms behind this enrichment and peculiar characteristics of liver NK cells are still not completely elucidated however, effects may be attributed to both cross talk between NK cells and other liver cell types as well as high hepatic expression levels of diverse NK cell-recruiting chemokines.13,14 NK cells produce diverse cytokines e.g, interferon-gamma (IFN-γ) and kill target cells (Fig. 1).
Animal model study has proved that immunopathalogy and immunodefense of liver cells is greatly influenced by NK cells which impede tumor cell growth, liver fibrosis, and viral infection and also inhibit liver regeneration but increase hepatocellular damage. Activating NK cells can kill HCV-infected hepatocytes leading to control of HCV infection.14
The opposing signals of stimulatory and inhibitory receptors on NK cells and their association with analogous ligands bound to target cells determines the potential of NK cells to kill target cells.15 Alterations that occur as a result of signal expression of receptors of NK cells and their interaction with ligands present on liver cells contribute to pathogenesis of liver diseases.16 NK cells are activated by cytokines e.g, IFN and interleukins (ILs), IFN-α, IFN- β, IL-12, IL-15, IL-18 and several other related cytokines during acute HCV infection.17 These activated NK cells act as key contributors in prevention of hepatitis C virus (HCV) either by stimulating adaptive immunity or by killing HCV infected liver cells.18,19 IFN-α acts as powerful NK cell activator and cures viral hepatitis by suppressing tumor formation and liver fibrosis. The anti-tumor, anti-fibrotic, and anti-viral effects of IFN-α therapy are stimulated by activation of NK cells. In addition to IFN-α, there are other NK cell activators like IL-12 and IL-18 that have been proved effective against liver carcinogenesis in animal models.20,21
NK cells provide protection against HCV infection because lot of evidences have suggested that selective impairment of NK cell is cause of chronic HCV infection.22 NK cells also increase NK cell ADCC (antibody-dependent cell-mediated cytotoxicity) and target cancers and tumor cells.23 Another study demonstrated that NK cell cytotoxicity against tumor cells is increased by blockade of NK cell inhibitory receptors and monoclonal antibodies such as rituximab, IPH-2101 that block killer-cell immunoglobulin-like receptor to treat hematological cancers, this strategy is currently in phase II clinical trials.24,25 Therefore, blockage of NK cell inhibitory receptors, targeting of NK cells to increase ADCC, and activation of NK cells by cytokines all form the basis of therapeutic strategies against hepatocellular carcinoma (HCC). Adoptive transfer of activated NK cells isolated from donor (cadaveric liver) into recipient (HCC infected individual) is under phase I clinical trial.26
NK cells control liver fibrosis in humans because of cytotoxic nature of NK cells that kill hepatic stellate cells (HSCs) based on FasL, TRAIL, NKG2A, NKG2D, and NKp46.27–29 In case of HCV infection, several studies have shown that the activation of NK cells controls HCV because of reportedly good treatment response associated with higher NK cell cytotoxicity and expression of activating receptors in contrast to, higher expression of inhibitory receptors associated with poor treatment outcome.30,31 The potential of NK cells in the control of hepatitis A virus (HAV) infection has been indicated in one study based on HAV-infected fibroblasts.32 Likewise, another study reported the proinflammatory cytokine production by NK cells in hepatitis E virus (HEV) infected patients as compared to those with resolved HEV infection.33 In HCC patients, circulating and intrahepatic NK cells are functionally impaired and tumor-infiltrating NK cells mediated reduced expression of perforin and granzymes as well as reduced cytotoxic potential was observed in healthy controls as compared to HCC patients.34 Similarly, frequency of NK cells and cytotoxic function is reduced in human peripheral blood of alcoholic liver disease (ALD) patients as compared to healthy controls.35 An increased number of NK cells was observed in the livers of nonalcoholic steatohepatitis (NASH) patients as compared to healthy controls and simple steatosis. The study further elucidated an increase in NKG2D dependent activation of NK cells and increase in expression of NKG2D ligands and apoptotic hepatocytes in NASH patients.36 An autoimmune disorder, primary sclerosing cholangitis (PSC) is also associated with tumor necrosis factor-alpha (TNF-α)-mediated-increased-functionally-impaired NK cell numbers in peripheral blood of patients. Another study revealed two SNPs in NKG2D gene responsible for the development of cholangiocarcinoma.37,38 The number of NK cells, perforin expression, and cytotoxicity are increased in the blood and liver of PBC patients.39
Innate like T cells or NKT cells play an important immunoregulatory role in cancer, infectious diseases, and autoimmune diseases of liver.40,41 Several studies have shown the migration of NKT cells to liver sinusoids in knock-in Cxcr6gfp/+ mice administered with galactosylceramide (αGalCer) injection.42 The activation and enrichment of NKT cells in liver sinusoids reflects the active participation of these cells in the mechanism of controlling the prevention or induction of inflammation in the liver in various immunological responses.43,44 Type 1 NKT cells have been shown to play a pathogenic role in variety of liver disorders such as PBC, con A-induced hepatitis, NAFLD, and ischemia-reperfusion injury (IRI), whereas, type 1 NKT cells play protective role in acute liver injury. Type I NKT cell-dependent inhibition of macrophage inflammatory protein-2, KC and TNF-α production inhibited liver injury as well as neutrophil infiltration in model of acute CCl4-induced fibrosis and mouse model of cholestasis and biliary obstruction.45 From accumulating evidence, it can be inferred that in case of acute liver injury the activation of type 1 NKT cells may be protective whereas, the same cells promote liver injury in chronic conditions.46 Likewise, in another study αGalCer-mediated activation of type1 NKT cells resulted in prevention of liver injury by induction of neutrophil apoptosis through STAT-1-dependent mechanism whereas, this activation can also promote neutrophil infiltration and hepatitis in a STAT-6 dependent manner.47 The role of NKT cells in humans has not been studied properly. However, an increase in the quantity of pro-inflammatory cytokines especially IL-1, IL-6, IL-8, osteopontin (OPN), and TNFα has been observed in the sera and liver biopsies of human liver with alcoholic hepatitis.43 Likewise, another study revealed that patients with alcoholic hepatitis had higher frequencies of IL-22-producing cells and increased IL-17 plasma levels.48,49
In NAFLD, Tim-3/Gal-9 signaling pathway or KCs activates and results in apoptosis of type 1 NKT cells in liver that cause insulin resistance and steatosis.50 Similarly, an increase in number of CD3+CD56+ cells and the expression of CD1d in NASH patients indicates the prominent role of NKT cells in NAFLD.51,52 In the same way, autoimmune hepatitis (AIH), is also characterized by secretion of IL-17 from type I NKT cells. Some recent studies have reflected the association of liver disorders’ severity with high levels of IL-17 in the portal areas of liver biopsies and serum of the patients infected with AIH, NASH, and primary biliary cirrhosis (PBC) as compared to control subjects.53–55 In HBV and HCV infection, NKT cells are reported to control the replication of both viruses during early stages of infection whereas, the cells may also cause liver injury through different mechanisms such as the induction of hepatocyte apoptosis, lysis of hepatocytes, inhibition of hepatocyte proliferation, and production of pro-inflammatory cytokines.56–58Table 1 summarizes the role of NKT cells and NK cells in different lives diseases.
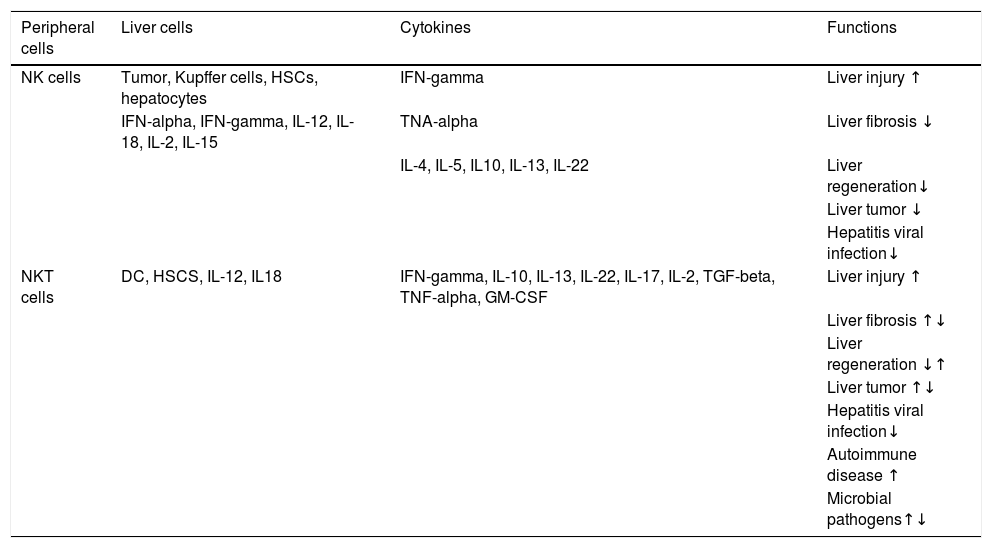
Function of NK and NKT cells in liver diseases.58
| Peripheral cells | Liver cells | Cytokines | Functions |
|---|---|---|---|
| NK cells | Tumor, Kupffer cells, HSCs, hepatocytes | IFN-gamma | Liver injury ↑ |
| IFN-alpha, IFN-gamma, IL-12, IL-18, IL-2, IL-15 | TNA-alpha | Liver fibrosis ↓ | |
| IL-4, IL-5, IL10, IL-13, IL-22 | Liver regeneration↓ | ||
| Liver tumor ↓ | |||
| Hepatitis viral infection↓ | |||
| NKT cells | DC, HSCS, IL-12, IL18 | IFN-gamma, IL-10, IL-13, IL-22, IL-17, IL-2, TGF-beta, TNF-alpha, GM-CSF | Liver injury ↑ |
| Liver fibrosis ↑↓ | |||
| Liver regeneration ↓↑ | |||
| Liver tumor ↑↓ | |||
| Hepatitis viral infection↓ | |||
| Autoimmune disease ↑ | |||
| Microbial pathogens↑↓ |
HSCs play an important role in liver fibrosis.59 The critical step in fibrosis involves two events i.e, activation of HSCs and transdifferentiation into major extracellular matrix-producing cell in fibrotic liver known as myofibroblasts. LPS (lipopolysacchride) like abberant stimuli induces the production of chemokines and cytokines from HSCs in both rodents and humans that leads to regulation of hepatic inflammatory and immune responses via their own gene expression.60,61 Key factors involved in HSC activation belong to platelet-derived growth factor (PDGF) and transforming growth factor beta1 (TGF-beta1) family.62,63 Animal model based study revealed the effective role NK cells in inhibition of liver fibrosis by generating anti-fibrotic cytokine IFN-γ and killing hepatic stellate cells.16
Hepatocyte damage activates HSCs that cause decrease in NK cell inhibition and increase in NK cell stimulation. Increased amount of retinoic acid produced by early activated HSCs increase the expression of RAE-1 that acts as ligand for activation of NK cell receptor NKGD2.64 RAE-1 combines with MICA and stimulate killing of activated HSCs by NK cells.65 This mechanism increases liver fibrosis regression.27 The role of another activating receptor NKp30 has been reported in recent studies.66 Activation of HSCs downregulates MHC-1 resulting in increased killing of NK cells and decreased engagement of inhibitory NK cell receptors.28 Another study based on mice model demonstrated that decreasing the expression of inhibitory Ly49 receptor by siRNA mediated silencing increases HSC killing by NK cells and improves liver fibrosis. Inflammatory cytokines especially IFN-α and IFN-γ can further influence this process.67 IFN-α increases NK-cell mediated HSC killing by increasing the expression of TRAIL receptor on surface of HSCs. NK cell-derived IFN-γ induce HSC apoptosis and cell cycle arrest and produce antifibrotic effects.68,69 Concentration of central regulator in chronic liver disease, transforming growth factor-beta (TGF-β), is increased during chronic liver injury. TGF-β signalling acts as an active participant from early progression of disease to cirrhosis and cancer because of its apoptotic and cytostatic effects in hepatocytes.70 Downregulation of NKG2D and 2B4 surface expression lead to suppression of antifibrotic function of NK cells.71 A recent study has elucidated that activating inhibitory killer immunoglobulin-related receptors iKIR knockdown stimulates NK cells and promotes their antifibrogenic activity in human co-cultures and mice.28 Previously, HSCs were discussed in context of liver fibrosis only whereas, recent studies elucidates its role in liver inflammation via navigation of T lymphocytes into parenchyma, production of inflammatory cytokines, and response towards external signals. Further studies are still required to explore their role in development of hepatitis as well as novel therapeutic target.72,73
The progression as well as onset of hepatocellular carcinoma has been studied in genetically engineered mouse model that showed persistent deregulation of numerous NK cell-related genes in the early stages of the disease. This study suggested the association of early onset of hepatocarcinogenesis with disruption of NK cell-mediated immune surveillance.74 It has been observed that the number of intrahepatic NK cells decreases or impairs in HCC patients specifically during post-surgical recurrence.34 Another study demonstrated that NK cells from peripheral blood mononuclear cells (PBMCs) and tumor infiltrating lymphocytes (TILs) in HCC patients was associated with defective cytokine secretion and cytotoxicity compared to healthy donors whereas, diminished activity of NK cells was observed during the development and invasion of HCC.75,76 A change in distribution of NK sub-populations, reduction in CD56-dim NK subset has also been observed in HCC patients.77,78 Higher number of NK cells associated with high levels of activating and reduced levels of inhibitory NK receptors play an important role in HCC control. Unlike this, some researchers reported that NK cells efficiently killed different cell lines and eliminated metastases and small HCC lesions in vivo.79
Dendritic Cells (DCs)DCs are rare, bone marrow-derived antigen-presenting cells that play a dominant role in the regulation and induction of immune reactivity. Hepatic dendritic cells (HDCs) are localized in portal areas and modulates hepatic immune responses by presenting antigens to lymphocytes. HDCs are classified into myeloid or classical (mHDCs) and plasmacytoid (PDCA-1+; pHDCs) moreover, mHDCs are further classified into DC103+ /CD11b- type 1 (mHDC1) and DC103- /CD11b+ type 2 (mHDC2) cells.80 In healthy livers, HDCs are characterized by high production of kinurenin, IL-10, IL-27, and low capacity to stimulate T-lymphocytes and endocytose antigens leading to a tolerogenic environment in healthy livers.81 According to recent study, murine CD103+ DCs have been reported to provide protection against steatosis progression towards steatohepatitis.82 Altered dendritic cell function leads to immunological changes in hepatic fibrosis. DCs also influence pathogenesis of liver fibrosis. Study based on mouse model demonstrated the role of dendritic cells in altered hepatic immunity during fibrosis and their contribution in regulation of inflammatory milieu within the fibrotic liver. Raised level of inflammatory mediators produced in the fibrotic liver is overturned by decline in dendritic cells. DCs induce T cells, NK cells, and HSCs to trigger proliferation, inflammation and immune responses after liver injury. The immunogenic and proinflammatory impacts of fibrotic dendritic cells were contingent on their secretion of tumor necrosis factor- α. Thus, regulation of DCs may be an effective therapeutic strategy against fibro-inflammatory liver disease.83 DCs play an important role in development of primary biliary cirrhosis 84 and nonalcoholic fatty liver disease because it has been demonstrated that decrease in DCs decrease the severity of NAFDL.85 Likewise, another study suggested indirect involvement of dendritic cells triggering antitumor effects.86 DCs have been proved effective therapeutic agents against cancer.87
Ninomaya et al. demonstrated that immature function and phenotype was exhibited by peripheral blood-derived dendritic cells propagated by hepatocellular carcinoma infected patients compared to normal controls.87 Another study has reported the identification of follicular dendritic cell neoplasm in the liver.88 Several other studies suggest the participation of dendritic cell in hepatic granuloma formation and granulomatous liver disease.89
In patients suffering from HCC, DCs stimulated allogenic T cells at lower pace in allogenic mixed leukocytes reaction as compared to DCs from normal healthy controls and liver cirrhosis. Moreover, decreased amounts of IL-12 and lower levels of HLA DR was expressed by DCs of HCC patients compared to normal controls (p<0.05).The prevalence of immature DCs because of high levels of inflammatory cytokines show defective DCs maturation during hepatocarcinogenesis. These findings suggest that the maturation of DCs can act as effective DC-based immune therapy.87 Another study have suggested the administration of DCs into cancer nodules to control HCC.90
NeutrophilsNeutrophils are most abundant type of white blood cells in human body that have the potential to regulate immune response. Neutrophil recruitment to the liver is mediated by different adhesion molecules during sepsis/endotoxemia and sterile inflammation. Neutrophils migrate to the site of inflammation and mediate hepatocyte injury by producing reactive oxygen species, pro-inflammatory mediators, elastase etc. and after the clearance of inflammation, apoptosis kill neutrophils leading to stimulation of an active program that resolve inflammation.91 Sustained virological response in association with reduced neutrophil level has been observed in interferon treated HCV patients, likewise, in HBV infected transgenic mice, the inhibition of neutrophil elastase led to improvement in liver injury.92 In addition to this, distinct neutrophil subsets have been reported in the peripheral blood of HCC patients. Accumulating evidence suggest that neutrophil dysfunction leads to poor liver cirrhosis outcomes.92–94 Liu ZX et al., reported that neutrophils kill hepatocytes after expressing Fas ligand via an apoptosis-induced mechanism.95 hepatic E-selectin induced infiltration of neutrophils into the liver and its role in pathogenesis of human alcoholic liver disease have been elucidated in a recent study.96 Although, the role of immune cells hasn’t been studied but it is a known fact that leukocytes present in HCC microenvironment regulates tumor growth.97 Another study reported that intratumoral neutrophil-to-CD8+ T cell ratio act as better predictor of outcome.98,99
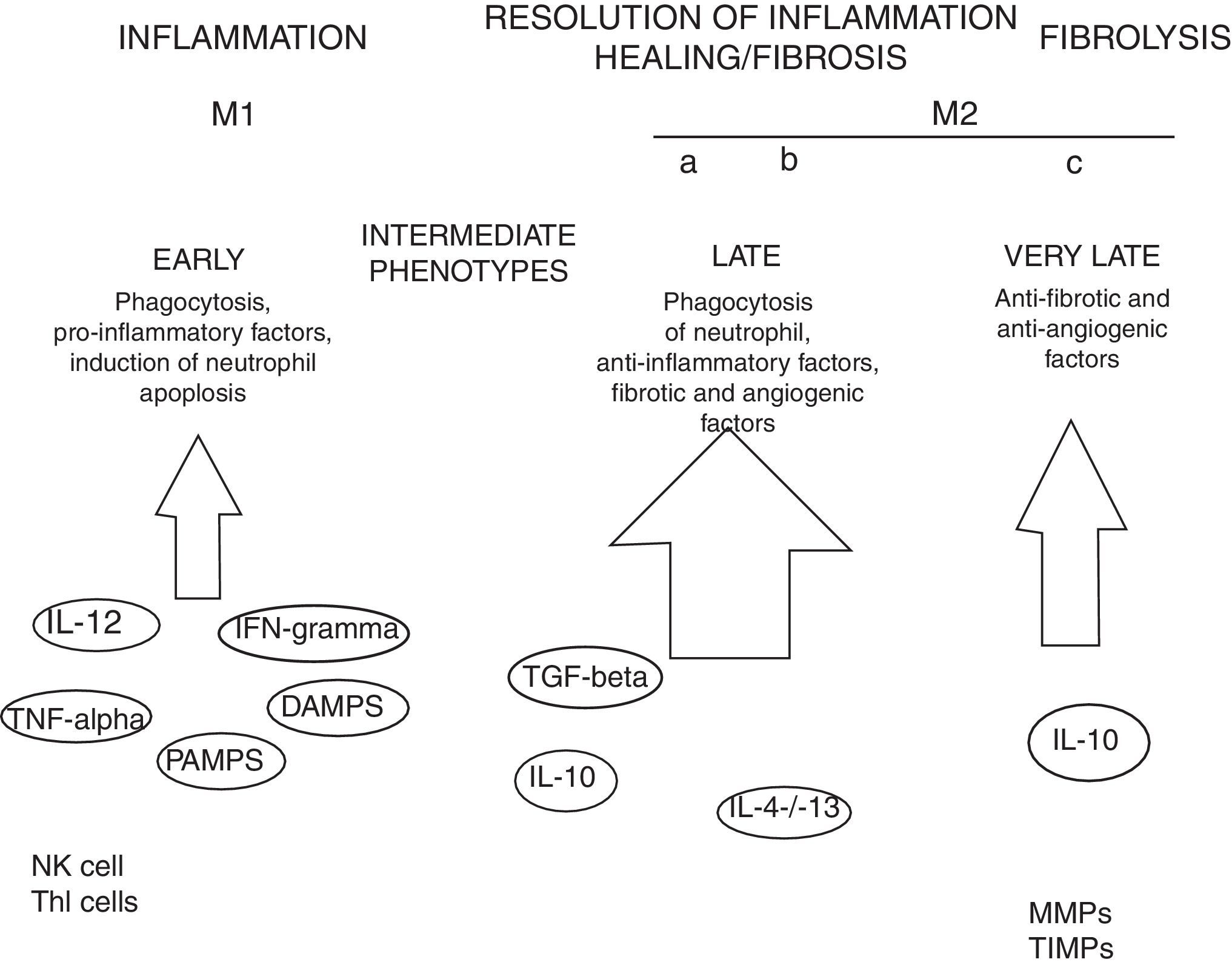
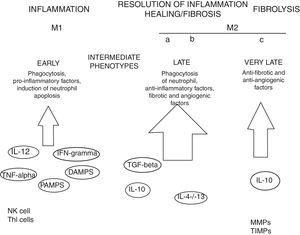
MacrophagesMany experimental models of liver fibrosis demonstrate the role of liver macrophages or kupffer cells in modulating inflammatory response leading to activation of hepatic stellate cells and release of proinflammatory chemokines and cytokines. Macrophages contribute to both fibrosis regression and progression therefore, interactions and differentiation of macrophages with other hepatic cell types in injured liver acts as potential novel target for future therapeutics to combat liver fibrosis (Fig. 2).
Role of macrophages in renal fibrosis: Activation of parenchymal cells that occurs as a result of tissue damage leads to activation of innate immunity. This involves monocytes recruitment and their differentiation into diverse macrophage phenotypes that rely on local tissue environment. Necrotic cells and pathogens secrete factors that trigger activation of immune receptors such as toll-like receptors as a result of which macrophages are polarized towards ‘M1’ proinflammatory macrophage. In contrast to this, phagocytic uptake of inflammatory signals especially apoptotic cells supports polarization of macrophages towards anti-inflammatory or profibrotic ‘M2’ phenotypes. Secretion of proteases by fibrolytic macrophages digests extracellular matrix proteins.99
Kupffer cells are macrophages that reside in the liver. These cells are activated by CD14/TLR4 receptor complex because of elevated intestinal translocation of lipopolysacchrides that may contribute to alchohol-induced liver injury.100 Kupffer cells are necessary for initiating inflammatory responses and sensing tissue injury while infiltrating Ly-6C+ monocyte-derived macrophages that are associated with fibrosis and chronic inflammation. Additionally, proliferation of recruited or local macrophages may lead to their accumulation in injured liver. A recent study has reported the association of M2 macrophages with liver inflammation because of proinflammatory cytokines secreted by M2 macrophages in the liver of HCV infected mice.101
A study published in Nature demonstrates that initial response to liver injury is provided by Kupffer cells which produce cytokines and chemokines such as tumor necrosis factor (TNF) α, CCL2, CCL5, IL-1 β leading to recruitment of other immune cells like monocytes. Dramatic expansion of hepatic macrophages owing to massive influx of monocytes into the liver is observed in both chronic and acute liver injuries.102,103
Several other evidences suggest the involvement of macrophages in the induction and resolution of fibrosis. Slight alterations in the pattern of MMP (matrix metallopeptidase) can significantly influence outcomes with MMP1 and MMP13 exhibiting potent antifibrotic activity and macrophage-derived MMP12 increasing fibrosis regression.104 MMPs promote degradation of extracellular matrix leading to fibrosis regression. MMP9 degrade basement membrane and permits recruited fibroblasts and inflammatory cells to enter injury sites. Secretions of several other factors promote myofibroblast apoptosis and remove cellular debris thus leading to negative regulation of fibrosis. Macrophage-mediated modifications in the extracellular matrix protein can also influence myofibroblasts’ survival and terminate progressive fibrosis.105 Due to expression of Arg-1, marcophages deplete an essential amino acid that is required for proliferation of myofibroblasts and CD4+ T cells thereby helps in down-regulation of profibrotic immune responses.106 All such findings are concomitant with recent evidences that have proved macrophages important component of resolution of fibrosis.
Tumor associated macrophages (TAMs) promote HCC invasion, metastasis, growth, and angiogenesis. Furthermore, TAMs interact with cancer and stromal cells within tumor microenvironment to suppress antitumor immune response. In HCC, CCL2, M-CSF, TGFβ, and VEGF are recruited by TAMs and release number of chemokines, cytokines, and growth factors. In particular, OPN, TNFα, IL-6, and MMPs play an eminent role in metastasis and invasion TGFβ and IL-6 favor tumor growth, whereas, the suppression of antitumor response is promoted by TGFβ and IL-10.107
Liver sinusoidal endothelial cells (LSECs)Liver sinusoidal endpthelial cells are highly specialized endothelial cells that are involved in transport lipoproteins, lipids, and nutrients. These cells represent the interface between blood cells and hepatic stellate cell or hepatocytes. LSCEs act as permeable barrier and possess highest endocytosis capacity of human cells. LSECs maintain low portal pressure by regulating hepatic vascular tone. LSECs inhibit fibrosis development, intrahepatic vasoconstriction and maintain hepatic stellate cell quiescence. LSECs are the key contributors of progression and initiation of chronic liver diseases such as hepatocellular carcinoma, and liver lesions associated with infection and inflammation. LSECs have been found to promote vasoconstriction and angiogenesis because they lose their protective properties after getting capillarized. LSEC injury has been reported in diverse liver diseases specifically non-alcoholic fatty liver disease (NAFLD). LSEC progenitors and or/and LSECs detect alteration in shear stress occuring as a result of surgery, and they interact with inflammatory cells and platelets.108,109
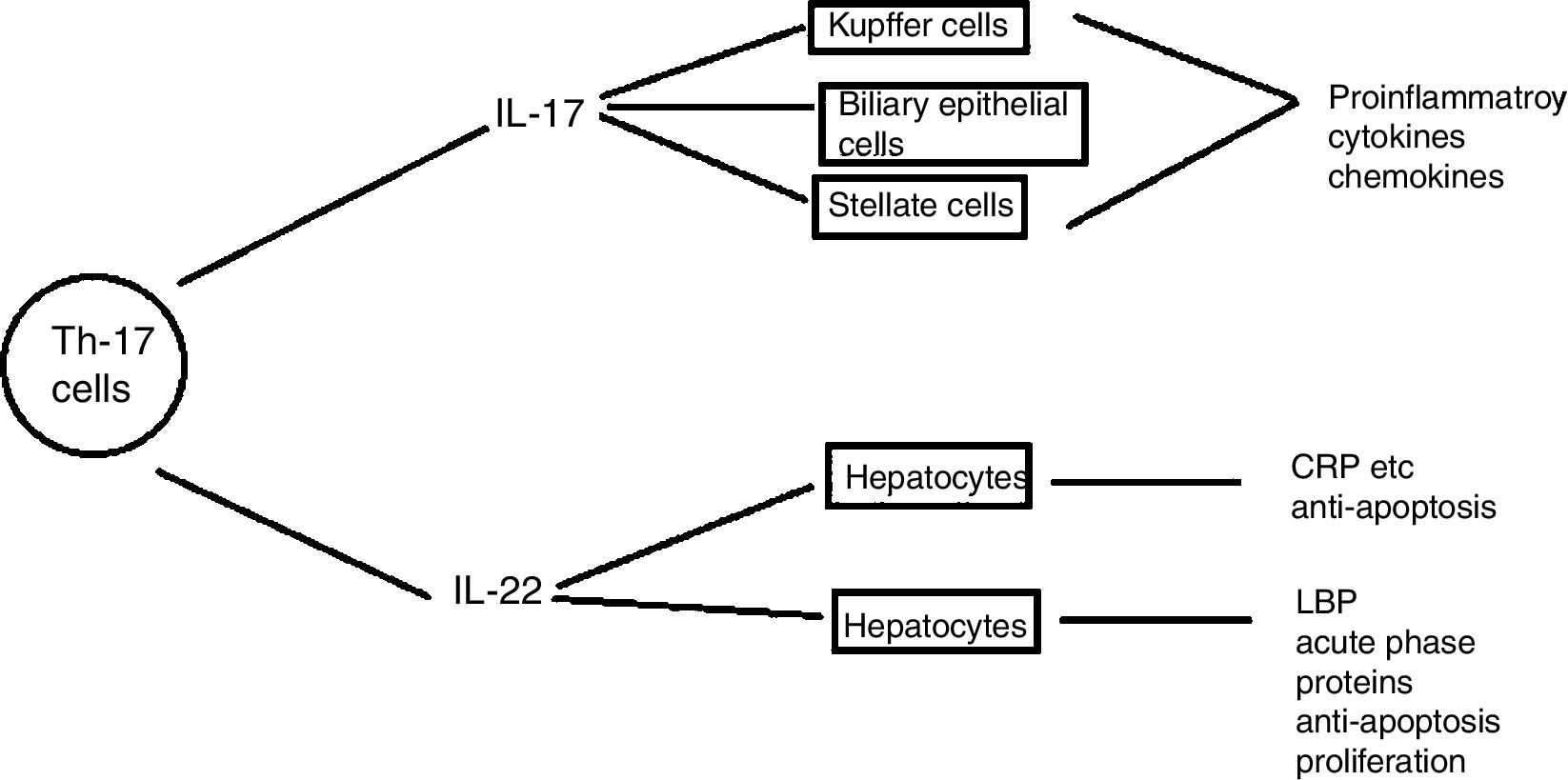
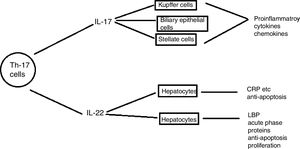
Th17 cellsSubtype of CD4+ T-helper cells known as Th17, which produce IL-17 and IL-22 that triggers host defense against autoimmunity and infections, has recently been discovered. Th17 cells differentiation in response to two cytokines IL-6 and TGF-β that are present in injured liver contributes to hepatic inflammation (Fig. 6).
Role of IL- 17 in the pathogenesis of liver diseases: IL-17 cells produced by Th17 stimulates different types of liver nonparenchymal cells such as Kupffer cells, monocytes/mDC, biliary epithelial cells, stellate cells to produce chemokines and cytokines that ultimately induce liver inflammation. IL -17 activates hepatic stellate cells thereby, promoting liver fibrogenesis. IL -17 can promote hepatocyte survival by producing acute phase proteins such as C -reactive proteins, LPS-binding protein and myeloid dendritic cells also.110
Several other studies have demonstrated the activated Th17 cells and Th17-related cytokines in different liver diseases. However, recruitment of Th17 cells specifically CCR4 or CCR6 promoted by chemokine receptors and chemokines might present novel therapeutic targets meddling with migration or differentiation of TH17 in liver disease.111 Several other evidences have elucidated the involvement of Th17 cells in variety of human liver diseases such as viral hepatitis,112 primary biliary cirrhosis (PBC),113 autoimmune hepatitis,114 non-alcoholic steatohepatitis (NASH), hepatocellular carcinoma (HCC), and induced liver injury.111
The number of Th17 cells has been found higher in HCC tissues than in non-tumor tissues.115 Several other studies suggested that proinflammatory cytokines produced by HCC tumor-activated monocytes stimulate the proliferation of functional Th17 cells within the tumor tissues.116 In short, the role of Th17 cells in HCC is still to be explored because it has been shown to either inhibit tumor growth by stimulation of cytotoxic T-cell response or promote tumor growth by stimulating angiogenesis or inhibit tumor growth. TH17 cells can also promote the growth of HCC by producing IL-22 that is involved on liver tumor cell proliferation.110
Th22 cellsHost immunity against pathogenic invasion is modulated by Th22 cells. A study demonstrated the role of Th22 cells as dominant inducers of tissue inflammation. Blood, fresh HCC (human hepatocellular carcinoma) tissues and adjacent HCC tissues were collected from HCC infected individuals and healthy individuals. Flow cytometry analysis exhibited elevated levels of serum IL-22, Th22 cells, and Th17 cells in HCC infected patients.117
Th22 cells protect host against chronic hepatitis B.118 A study showed an increase in population of Th22/Th17 cells and related cytokines in drug induced liver injury with hepatocellular injury type.119
Other T cellsMany pathological conditions of liver are driven by regulatory T cells (Tregs) and other T cell such as Th1 and Th2. Proliferation of HSCs is increased by Th1 cells via IFN-γ/STAT1 pathway and is attenuated by Tregs. Wen J et al., found decrease in the frequency of Tregs and increased frequency of Th1, Th2 and Th17 in the peripheral blood of biliary atresia patients.120
Cytokines (such as IL-6, IL-22, IL-33, TGF-β, and TNF-α)All nucleated cell types in our body e.g, monocytes/macrophages, fibroblasts, epithelial cells, lymphocytes etc. produce regulatory peptides known as cytokines. Recently published data have demonstrated a very curious relationship between liver repair and injury.121 Some cytokines mediate the liver tissue regeneration after injury whereas, the same mediators also mediate apoptosis and necrosis of liver cells, fibrosis, cholestasis, and hepatic inflammation. An example of this phenomenon is ischemic preconditioning induced protection followed by ischemia-induced injury observed in liver tissues whereas, apoptosis driven ischemia–reperfusion-based liver injury has also been reported.122
A study revealed the correlation of interferon gamma (IFN-gamma), tumor necrosis factor alpha (TNF-alpha), C-reactive protein (CRP), interleukin-1 (IL-1 beta), and interleukin-6 (IL-6) with different hepatic diseases. When serum level of these mediators was investigated in 264 patients infected with chronic liver diseases and 128 individuals in non-cirrhotic stage of chronic hepatic diseases (CHD), a significant increase in serum level of tumor necrosis factor alpha (TNF-alpha), interleukin-1 (IL-1 beta), and interleukin-6 (IL-6) was observed in cirrhotic group of CHD patients. Thus, increased concentration of cytokines in serum is an indicator of cirrhotic liver disease. Therefore, the study suggests that cytokine level increases as a result of liver dysfunction.123,124 Cytokines specifically IL-6 are key factors of liver regeneration.122
According to another study inflammatory chemokines or cytokines are key players of alcoholic liver disease. Kupffer cells lead to generation of TNF-α through TLR4 (Toll like receptor-4) thus, playing a significant role during early stage of alcoholic liver disease.
Immunotherapeutic strategies especially cytokine therapy may serve as a substitute to liver transplantation 125 by restoring normal functioning of liver via regeneration of healthy tissue remnants. TNF- α 126 and IL-22 127 may also form the basis of several novel approaches of treating alcoholic liver disease.
Growth factors (VEGF, PDGF, FGF, and HGF)Vascular endothelial growth factor (VEGF) and platelet derived growth factor (PDGF) trigger hepatic fibrosis and inflammation.128 Abraldes JG et al., indicated a significant increase of VEGF concentration in cirrhotic rats.129 Likewise, another study suggested that VEGF level was elevated in HCC patients.130
Platelet derived growth factor (PDGF) family consists of four members PDGF-A, B, C, and D. PDGF is a potent mitogen to hepatic stellate cells among all polypeptide growth factors.131 Overexpression of PDGF and its receptors has been observed in liver cirrhosis and activity of PDGF was shown to be increased with degree of liver fibrosis.132 Several factors like chemicals, viruses, or mechanical damage to liver cells can induce Kupffer cells to secrete PDGF.133 In response to binding of PDGF with its specific receptors present on membranes of hepatic stellate cells, PDGF activates transcription factors and corresponding signal molecules leading to activation of hepatic stellate cells and downstream target genes.134 PDGF has been found to decrease ECM degradation by inhibiting the activity of collagenase and increase the expression expression of TIMP-1, MMP-2, and MMP-9.135 Isoform PDGF-D is potent enough to activate hepatic stellate cells and induce fibrogenic and mitogenic effects, therefore, plays dominant role in liver fibrosis.136
There are many evidences which highlight the function of fibroblast growth factor in progression of hepatocellular carcinoma (HCC) and metastatis. The expression of FGF2 has been detected in the liver tissues of HCC infected individuals. Likewise, the serum concentration of FGF2 was considerably higher in patients with liver cirrhosis, chronic hepatitis, and HCC compared with healthy individuals.137,138
Hepatocyte growth factor is well known for its morphogenic, motogenic, tumor suppressor, and mitogenic activities.139,140 Endogenous HGF repair injured lungs, liver and kidneys etc. It also exerts protective effects on organs through anti-inflammatory and anti-apoptotic signals. Significant increase in concentration of HGF has been observed during organ diseases and infusion of anti-HGF antibody has been shown to accelerate tissue destruction in mice model. Therefore, endogenous HGF is necessary for disease control while inadequate secretion of HGF cause organ failure.141
Collagen-producing cellsDeposition of collagen causes the disruption of liver and lead to cirrhosis.142 The major collagen producing-cells found in injured liver are portal fibroblasts, myofibroblasts, and activated hepatic cells that are activated by fibrogenic cytokines specifically leptin, angiotensin II,and TGF-β1.143 Hepatic stellate cells (HSCs) are the main matrix-producing cells which play an important role in liver fibrosis. Liver injury activates HSCs which differentiates to fibrogenic myofibroblast-like cells.144 Activated fibroblasts and other cell types of fibroblast lineage like vascular myofibroblasts or portal fibroblasts have been proved as dominant mediators of liver fibrosis.145 Collagen 1 has been reported to promote HCC cell proliferation by regulating integrin β1/FAK signaling.146
ConclusionHepatic immune cells specifically natural killer cells perform many roles after activation, like secretion of cytokines and killing tumor cells and viral-infected cells, and also play important roles in regeneration, liver injury, and fibrosis. The characterization of intrahepatic immune cell functions is offering new opportunities and new therapeutic targets for curbing liver disorders. Although findings of many researchers are promising but further studies are still needed to translate these findings into clinical practice for therapeutics.
Conflict of interestAuthors declare no conflict of interest.
Special thanks to whole crew of Genome Centre for Molecular Based Diagnostics and Research Lahore, Pakistan for their assistance.