Granulocyte and monocyte apheresis (GMA) is a potential therapeutic option when combined with various drugs for treatment of ulcerative colitis (UC). In this study, we analyze the efficacy and safety of GMA combined with vedolizumab (VDZ) during induction in patients with moderate–severe UC and incomplete response to steroids.
Patients and methodsSingle-center retrospective review of patients receiving GMA+VDZ. Data on the disease and previous treatments were collected. Clinical response was classified as no response, response without remission, and remission. Available data on biochemical and endoscopic response were included. Adverse events (AEs) were recorded.
ResultsThe study population comprised 6 patients with UC who had received GMA+VDZ during induction after failure of an anti-TNF agent. The median number of GMA sessions was 5 (IQR 4–5; 3–10). All the patients received VDZ 300mg iv at 0, 2, and 6 weeks, and 5 (83%) received an additional dose at week 10. During maintenance, all the patients continued VDZ iv every 8 weeks. The median follow-up was 57.6 months (IQR: 39–74). Four of the 6 patients achieved clinical remission after GMA+VDZ and continued in deep remission until the end of follow-up. A median, non-significant decrease of 1378μg/g (IQR: 924–5778μg/g) was observed for calprotectin and 42.2mg/l (IQR: 15.3–113.5) for CRP vs. baseline. No patient underwent colectomy. No treatment-related AEs were observed.
ConclusionsGMA+VDZ during induction can be effective and safe in selected patients with moderate–severe UC and partial response to steroids.
La granulocitoaféresis (GMA) supone una potencial vía terapéutica al combinarse con distintos fármacos para tratar la colitis ulcerosa (CU). Este trabajo analiza la eficacia y la seguridad de la GMA combinada con vedolizumab (VDZ) durante la inducción en pacientes con CU moderada-grave y respuesta incompleta a corticoides.
Pacientes y métodosSe realizó un estudio retrospectivo unicéntrico en pacientes tratados con VDZ+GMA. Se recopilaron datos sobre la enfermedad y los tratamientos previos. La respuesta clínica se clasificó como ausencia de respuesta, respuesta sin remisión y remisión. Se incluyeron los datos disponibles de respuesta bioquímica y endoscópica. Se documentaron los eventos adversos (EA).
ResultadosSe incluyeron 6 pacientes con CU que habían recibido GMA+VDZ durante la inducción tras fracaso a anti-TNF. La mediana de sesiones administradas de GMA fue 5 (RIQ 4-5; 3-10). Los pacientes recibieron VDZ 300mg iv a las 0, 2 y 6 semanas, y en 5 (83%) se dio una dosis extra la semana 10. Durante el mantenimiento, todos recibieron VDZ iv cada 8 semanas. La mediana de seguimiento fue de 57,6 meses (RIQ: 39-74). De los 6 pacientes, 4 alcanzaron remisión clínica tras GMA+VDZ y continuaron en remisión hasta el final del seguimiento. Se objetivó un descenso mediano, no significativo, de 1.378μg/g en la calprotectina (RIQ: 924-5.778μg/g) y de 42,2mg/dl en la PCR (RIQ: 15,3-113,5). Ningún paciente fue colectomizado. No se observaron EA relacionados con el tratamiento.
ConclusionesLa combinación GMA+VDZ durante la inducción puede ser eficaz y segura en pacientes seleccionados con CU moderada-grave con respuesta parcial a corticoides.
Steroid therapy is usually effective for controlling inflammation in ulcerative colitis (UC), although the response is partial in many cases.1,2 Therapeutic options after incomplete response to steroids in patients with moderate–severe UC are limited, with infliximab and cyclosporine administered as second-line agents. New treatments have been proposed as potentially useful in this setting, albeit based on limited evidence.3–5
Vedolizumab (VDZ) is a monoclonal antibody against integrin α4β7 that has proven effective in moderate to severe UC.6,7 Onset of its anti-inflammatory effect is often slow.3 Therefore, it is usually combined with steroids during induction. In the induction phase, better clinical remission rates have been described for VDZ in anti-TNF naıve UC patients (39.3%) compared with anti-TNF exposed patients (18.5%).8 Regarding maintenance, Stallmach et al. reported the results in this same UC cohort. Clinical remission at Week 54 was significantly more frequent in anti-TNF naive than in anti-TNF experienced patients (55% vs 18%, respectively).9 Therefore, therapeutic options in anti-TNF experienced patients are commonly sought.
New indications in granulocyte and monocyte apheresis (GMA) have paved the way for nonpharmacologic treatment of UC. Based on selective adsorption, GMA avoids migration of activated monocytes and granulocytes to the gastrointestinal wall. In addition to reducing the synthesis of proinflammatory factors, it could prevent the inflammatory cascade.10,11 GMA has been combined with various drugs in patients with inflammatory bowel disease,12–16 and several authors have suggested combining it with VDZ in this setting.17–21
The objective of our study was to retrospectively analyze, in a pilot study, the efficacy and safety of combining GMA and VDZ during the induction phase in patients with moderate–severe UC and incomplete response to steroids.
Patients and methodsWe performed a retrospective pilot study in the Inflammatory Bowel Disease (IBD) Unit of Hospital Universitario La Paz, Madrid. We included all patients who received GMA+VDZ from January 2015 to December 2019 for induction of remission in the setting of a severe flare-up of UC and partial response to steroids.
The inclusion criteria were as follows: age≥18 years, diagnosis of UC according to the ECCO criteria,21 no/partial response to steroids, indication of GMA+VDZ for treatment of IBD, and follow-up for a minimum of 12 months. We excluded patients participating in clinical trials.
Patients received VDZ as induction therapy with the standard regimen (300mg iv at weeks 0, 2, and 6). An additional dose could be administered in week 10 at the physician's discretion. Patients subsequently received a standard maintenance dose of VDZ 300mg iv every 8 weeks.
Given the lack of commercially available alternatives for UC during the study period, we proposed VDZ; apheresis was added as bridge therapy, owing to the slow onset of action of VDZ. The use of GMA plus VDZ is not an established treatment for UC, so the decision to combine GMA plus VDZ and the number of GMA sessions was made on a case-by case basis according to the individual characteristics of the patient and the clinical situation.
All the patients were treated using the Adacolumn® system (JIMRO, Takasaki, Japan) at weekly sessions. The rate of perfusion was 30ml/min up to a total of 1800ml per session. GMA was performed using a column containing 220g of cellulose acetate beads in physiological saline.
Variables analyzedWe collected the patients’ baseline characteristics, the extent of the disease, previous medication, and relevant disease history. As for GMA, we recorded the regimen and number of sessions and evaluated the response after induction until the time of the analysis.
The clinical response was evaluated after a review of the clinical history. Response was classified as no remission, response (without remission), and remission. To assess the response to corticosteroids, the Ho index was used.22 A score of 2–3 was considered a partial response to corticosteroids (and therefore a patient susceptible to receiving GMA+VDZ). When available, data on the biochemical response (fecal calprotectin and C-reactive protein [CRP]) after induction and during maintenance were included, as was the endoscopic response (Mayo score).23 Adverse events (AEs) were recorded.
Statistical analysisDescriptive statistics of the sample were examined, using frequencies and percentages for categorical variables and means and SD for continuous variables. Non-normally distributed variables were expressed as median and interquartile range (IQR). The variables that followed a normal distribution were checked using the Shapiro–Wilk test. The Student T test was used to assess differences in continuous variables, which followed a normal distribution. Statistical significance was set at p<0.05. The analyses were performed using Stata, Version 15.
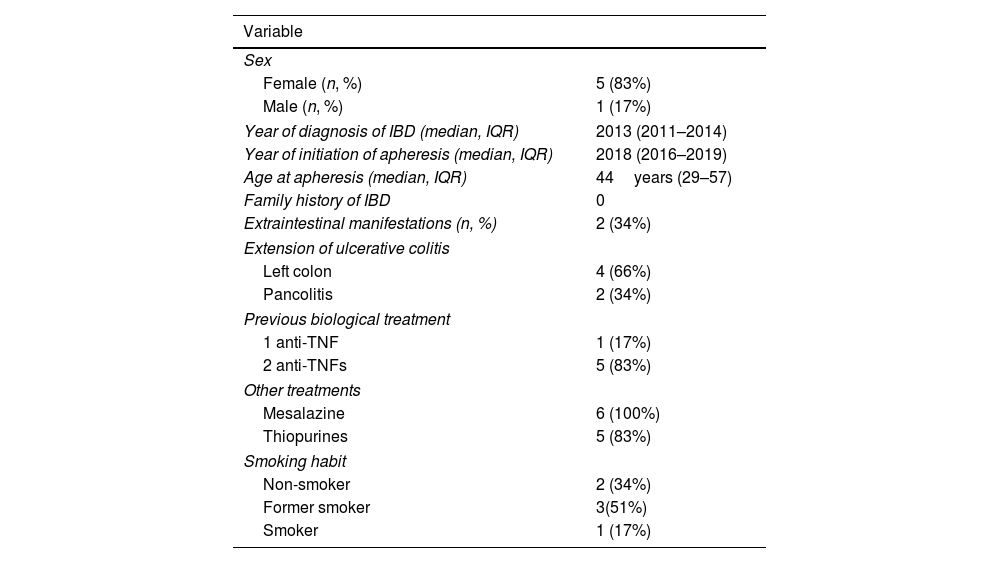
ResultsThe study population comprised 6 patients who received GMA+VDZ during induction. The baseline characteristics can be seen in Table 1. Median age at initiation of GMA was 44 years (IQR: 29–57), and 5 patients (83%) were women. Median time since diagnosis of IBD at initiation of GMA+VDZ was 5.85 years (IQR: 4–6).
Baseline characteristics.
| Variable | |
|---|---|
| Sex | |
| Female (n, %) | 5 (83%) |
| Male (n, %) | 1 (17%) |
| Year of diagnosis of IBD (median, IQR) | 2013 (2011–2014) |
| Year of initiation of apheresis (median, IQR) | 2018 (2016–2019) |
| Age at apheresis (median, IQR) | 44years (29–57) |
| Family history of IBD | 0 |
| Extraintestinal manifestations (n, %) | 2 (34%) |
| Extension of ulcerative colitis | |
| Left colon | 4 (66%) |
| Pancolitis | 2 (34%) |
| Previous biological treatment | |
| 1 anti-TNF | 1 (17%) |
| 2 anti-TNFs | 5 (83%) |
| Other treatments | |
| Mesalazine | 6 (100%) |
| Thiopurines | 5 (83%) |
| Smoking habit | |
| Non-smoker | 2 (34%) |
| Former smoker | 3(51%) |
| Smoker | 1 (17%) |
IQR: interquartile range; IBD: inflammatory bowel disease; TNF: tumor necrosis factor.
All patients had been diagnosed with moderate–severe UC with a partial Mayo index prior to the start of treatment median 6 (IQR: 6–8). Likewise, all patients had received treatment with corticosteroids, with a partial response (median decrease in partial Mayo index 2 points (IQR: 1–5) 3–5 days after the start of treatment). Three (50%) had been admitted to hospital and were receiving iv steroids. Second-line agents were administered because their disease was refractory to steroids (1 infliximab and 2 cyclosporine) identifying in these patients Ho index values after 7 days of corticosteroid treatment of between 4 and 7 points. The other 3 patients were taking oral systemic steroids after having started the regimen at the full dose (1mg/kg prednisone, maximum 60mg/d). Disease affected the left colon in 4 patients (66.7%) and was extensive in 2 (33.3%).
In all patients, at least 1 anti-TNF agent had failed. In 5 cases (83%), 2 anti-TNF agents had failed, and in 1 patient, only 1 anti-TNF agent was suspended owing to extraintestinal neoplasm. All had received immunosuppressive treatment with thiopurines, which they suspended before receiving GMA+VDZ. Of these, 4 (66.6%) did so because of AEs, and the remainder because of lack of efficacy. All had received oral and topical mesalazine.
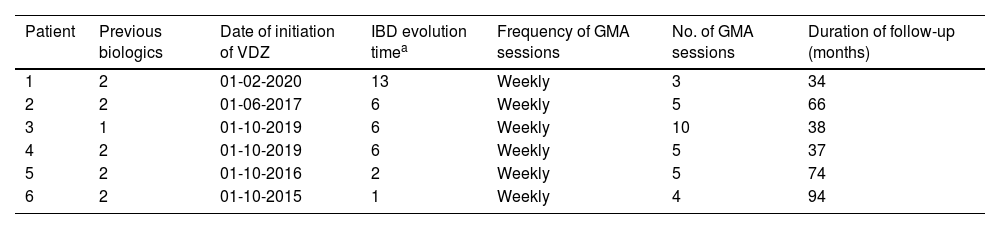
The median number of GMA sessions was 5 (IQR 4–5; range 3–10) (Table 2). Patients received VDZ at the standard regimen with 300mg iv at weeks 0, 2, and 6; an additional dose was administered to 5 patients (83%) at week 10.
Treatment received and duration of follow-up.
| Patient | Previous biologics | Date of initiation of VDZ | IBD evolution timea | Frequency of GMA sessions | No. of GMA sessions | Duration of follow-up (months) |
|---|---|---|---|---|---|---|
| 1 | 2 | 01-02-2020 | 13 | Weekly | 3 | 34 |
| 2 | 2 | 01-06-2017 | 6 | Weekly | 5 | 66 |
| 3 | 1 | 01-10-2019 | 6 | Weekly | 10 | 38 |
| 4 | 2 | 01-10-2019 | 6 | Weekly | 5 | 37 |
| 5 | 2 | 01-10-2016 | 2 | Weekly | 5 | 74 |
| 6 | 2 | 01-10-2015 | 1 | Weekly | 4 | 94 |
IBD: inflammatory bowel disease; VDZ: vedolizumab; GMA: granulocyte and monocyte apheresis.
Response after induction was measured at a median time of 8 weeks (IQR: 6–8.4) after the start of the combined GMA+VDZ treatment, no patient began treatment with apheresis prior to biological treatment. Four patients (66.7%) reached clinical remission after GMA+VDZ and remained in remission at the end of follow-up. Median follow-up was 57.6 months (IQR: 39–74). A response after induction was recorded in 2 patients (34.6%), although this was without remission. Therefore, treatment was suspended (at 10 months in 1 patient and 18 months in the other).
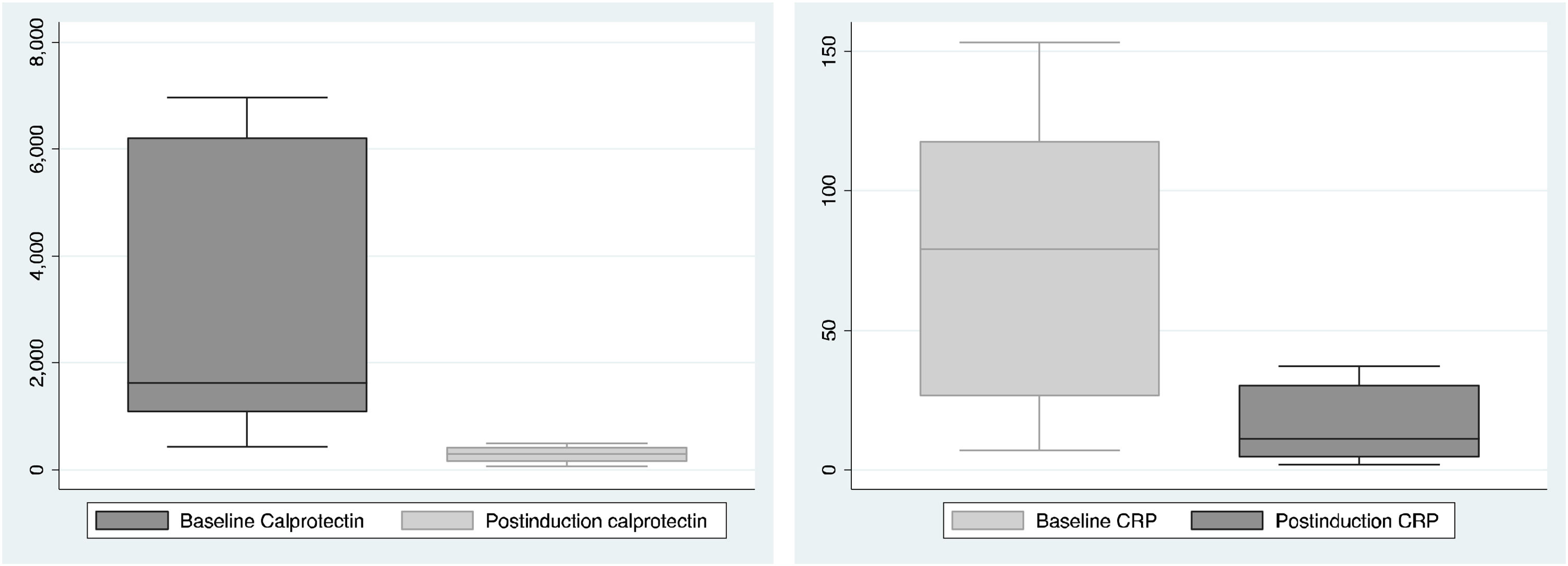
The biochemical activity was assessed at baseline and after completing the induction (8 weeks after starting vedolizumab), the results are shown in Fig. 1. Compared with baseline, we observed a median decrease in fecal calprotectin of 1617μg/g (IQR: 1070–6210μg/g) to 293μg/g (IQR: 145.15–432) (p=0.09) and a decrease in CRP of 79.2mg/l (IQR: 26.2–118) to 10.9mg/l (IQR:4.5–30.4) (p=0.06). These differences were not statistically significant.
Four patients underwent endoscopy before treatment and at 1 year after initiation. A median improvement of 1 point (IQR: 0–3) was observed, and 2 patients (33%) were in endoscopic remission (Mayo score=0). No patients underwent colectomy. No AEs associated with GMA+VDZ were observed during follow-up.
DiscussionThe analysis of this case series shows the combination of GMA+VDZ to be safe and efficacious when administered during induction to patients with moderate–severe UC and incomplete response to steroids. We opted for this combination because of anti-TNF failure in patients with partial response to steroids.
VDZ was prescribed as a second- and third-line biologic in 17% and 83% of cases, respectively. Four patients achieved clinical remission after receiving GMA+VDZ, and 2 achieved endoscopic remission. The 4 patients remained in deep remission after a median follow-up of 57.6 months after induction. We recorded decreases in both fecal calprotectin and CRP. The differences were not significant, probably because of the small sample size.
Treatment of UC has changed radically in the last few years. Anti-TNF monoclonal antibodies and, more recently, anti-integrin antibodies have proven highly efficacious in this disease.4,6 However, flare-ups continue to be associated with considerable morbidity, as well as mortality close to 1% and a colectomy rate of 30% during the first admission.24,25 It is necessary to optimize treatment of UC, especially in patients whose other lines had failed).
The efficacy of GMA+VDZ in UC has been reported in a case series and various case reports.17,18,20,21 Rodríguez-Lago et al. reported a benefit in 8 patients who received GMA after a loss of response to VDZ.20 Partial Mayo score decreased after 1 and 6 months (p=.01 and .06, respectively). Three patients (38%) showed steroid-free clinical remission and five (63%) withdrew VDZ. Sáez-González et al.17 reported a benefit in a patient with extensive UC who had received adalimumab and infliximab without achieving remission. Given the absence of response to VDZ, the drug was combined with GMA, and the patient achieved clinical remission. In a similar case reported by Scrivo et al.,18 a patient with ulcerative proctitis received VDZ after failure of infliximab and adalimumab. GMA was started 4 months after initiation of VDZ. After 5 sessions, the patient achieved clinical remission and mucosal healing. Nakamura et al.21 described another case on the concomitant use of VDZ and GMA for the initial induction of UC. A 20-year-old man with refractory UC and ineffective previous VDZ monotherapy received GMA+VDZ and achieved clinical remission.
GMA is well tolerated by patients with IBD and improves how they view their treatment.26 GMA+VDZ aims to complement both mechanisms of action and thus curb migration of leukocytes to inflamed tissue. Both treatments could contribute to the increase in some peripheral lymphocyte subpopulations (especially Treg) observed during the first weeks of treatment with GMA+VDZ.27,28 A potential interaction between both treatments has been postulated, owing mainly to an increase in minimum levels of the drug in blood or reduced antidrug antibodies29—although no direct evidence is available. GMA induces significant changes in the profile of cytokines expressed in the colonic mucosa, potentially boosting the effect of VDZ.18 This area should be researched in future studies.
Our study is subject to a series of limitations. The retrospective design, small sample, and variations in the apheresis regimens could limit the robustness of our conclusions. Notwithstanding, our data support the potential of GMA+VDZ in patients with moderate–severe UC and inadequate response to steroids.
In conclusion, in this preliminary set of data GMA+VDZ during induction could be efficacious and safe in selected patients with moderate–severe UC and a partial response to steroids. Combination of VDZ with GMA may offset its slow onset of therapeutic effect and deserves more research in prospective studies with a larger sample size.
- -
Granulocyte and monocyte apheresis (GMA) is a potential therapeutic option when combined with various drugs for treatment of ulcerative colitis (UC).
- -
We analyze the efficacy and safety of GMA combined with vedolizumab (VDZ) during induction in 6 patients with moderate–severe UC and incomplete response to steroids.
- -
GMA+VDZ during induction can be effective and safe in selected patients with moderate–severe UC and partial response to steroids, thus influencing clinical practice.
Suárez Ferrer C, Martin-Arranz E, Martín-Arranz MD have made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
Ethical considerationsWritten informed consent was obtained from all patients. The study was conducted with strict adherence to the Helsinki Declaration.
Financial supportWriting assistance was provided by Aldacyte. The company did not have an influence on the study design or interpretation of the results.
Conflict of interest- •
Suárez Ferrer C has received funding for training or has collaborated with Abbvie, Takeda, Jannsen, MSD, Tillots Pharma, Pfizer.
- •
Martin-Arranz E has received financial support for traveling and educational activities or has received fees as a speaker or consultant from Janssen, Ferring, MSD, AbbVie, and Takeda.
- •
Martín-Arranz MD has received fees as a speaker, consultant an advisory member for o has received research funding from MSD, AbbVie, Hospira, Pfizer, Takeda, Janssen, Shire Pharmaceuticals, Tillotts Pharma, Faes Pharma. These companies make medical treatments for inflammatory bowel disease.