Nonalcoholic steatohepatitis (NASH) is the most aggressive form of nonalcoholic fatty liver disease (NAFLD) and involves the risk of progression to more advanced stages of liver disease. Non-invasive methods are needed to identify patients with NASH.
ObjectiveTo evaluate the diagnostic performance of the determination of serum levels of cytokeratin-18 (CK-18) as a non-invasive marker of NASH in the Chilean population.
MethodsSerum CK-18 levels were determined in a group of 41 patients with biopsy-proven NAFLD. NASH diagnosis was based on Brunt's criteria (histological parameters and ballooning), and the NAFLD activity score (NAS) and the presence of fibrosis were determined. The correlation between the NAFLD activity score (NAS) and CK-18 was evaluated with Spearman's rank correlation coefficient. A ROC curve was produced to assess the diagnostic value of CK-18 for NASH. The NAFLD fibrosis score (NFS) (to predict fibrosis and NASH) was compared to CK-18 with simple linear regression. Data were expressed in median [25–75th percentile] and evaluated with the Wilcoxon rank test.
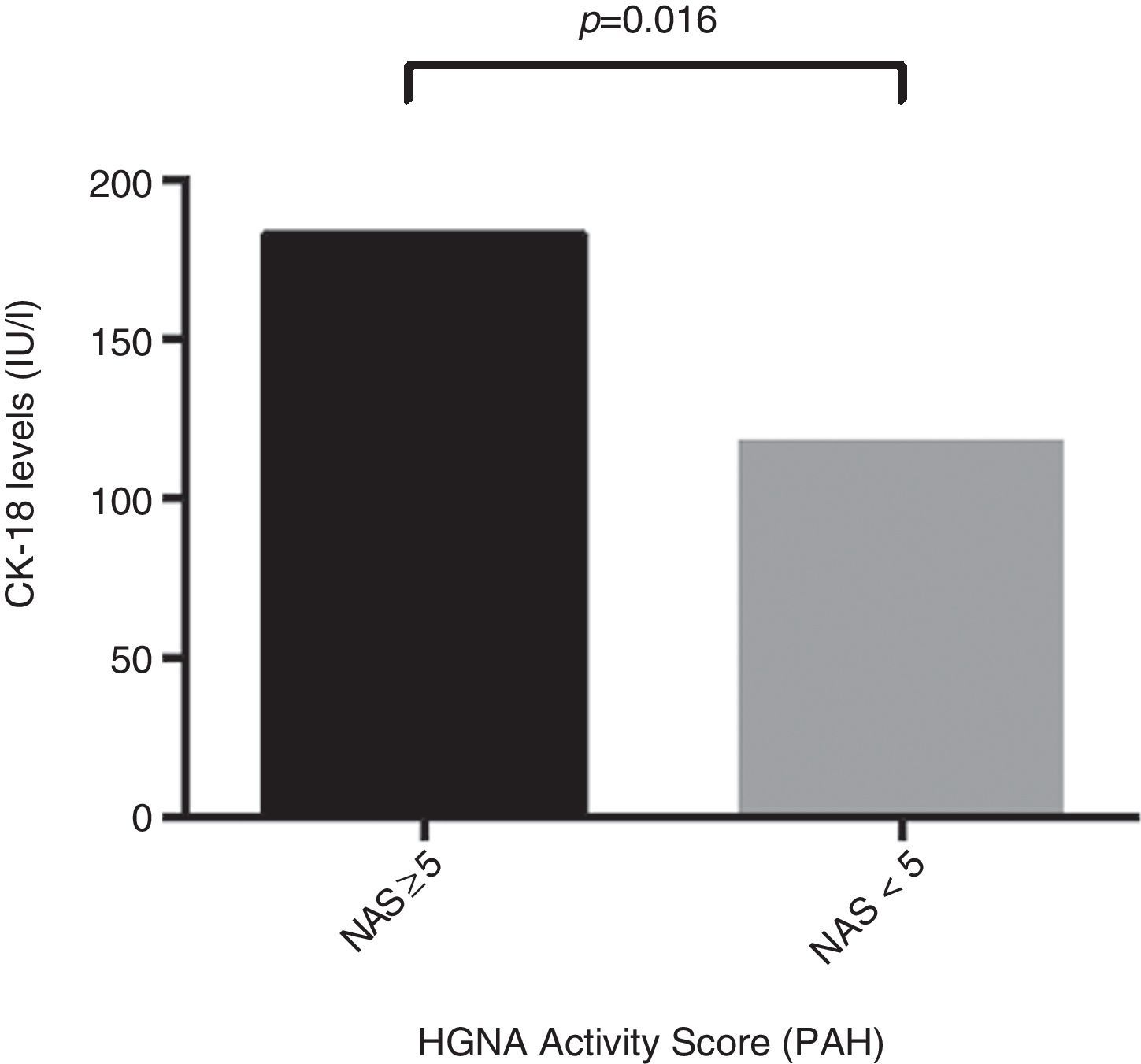
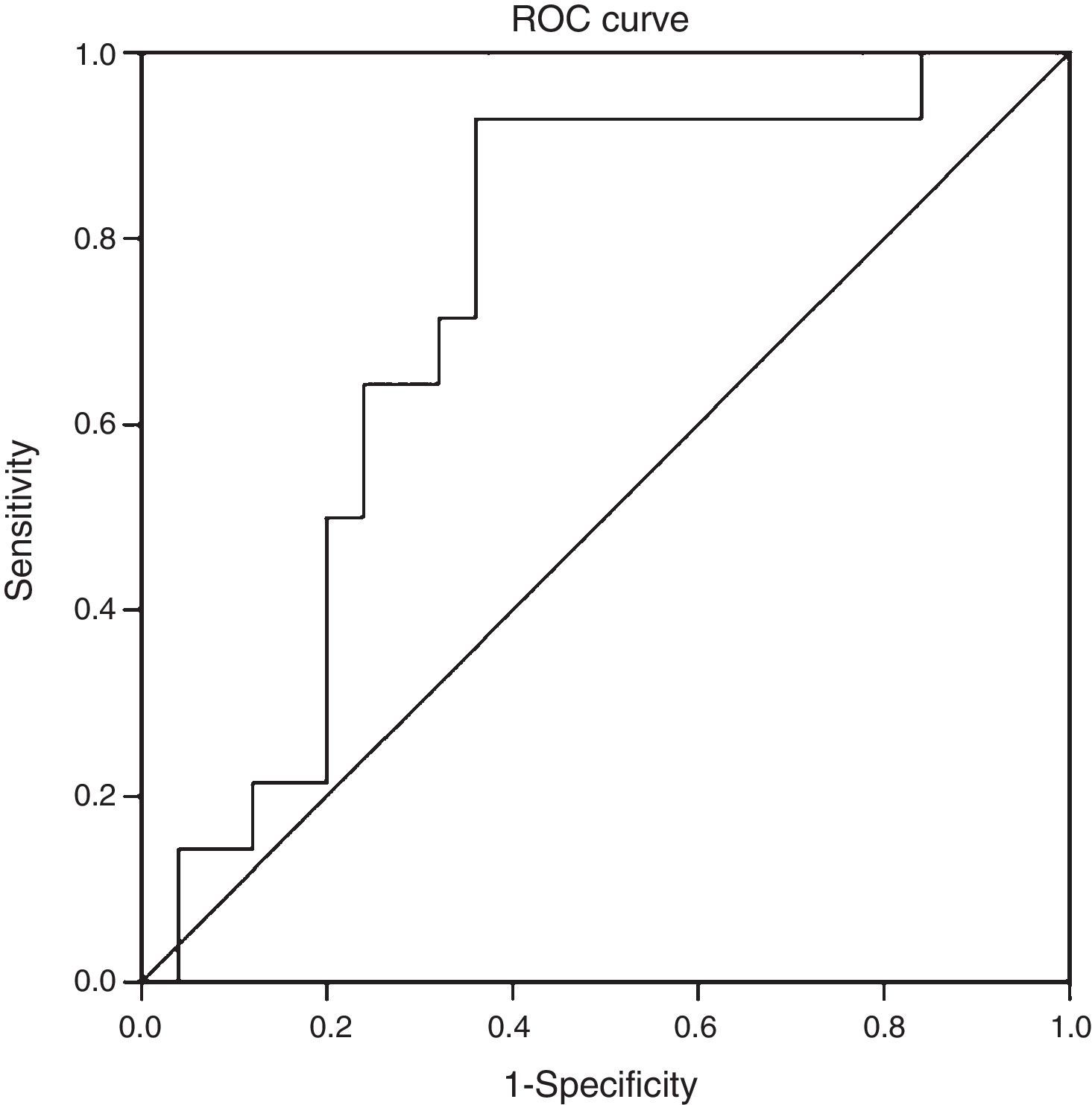
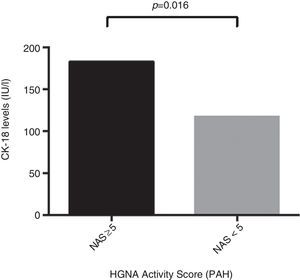
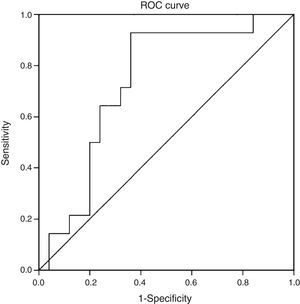
ResultsThe mean age of the study group (23% male) was 50.4±11.1 years. 34.2% were diagnosed with NASH (NAS≥5). CK-18 levels were significantly higher in patients with NASH versus those without NASH (183.6IU/l [97.4–734.4] vs. 117.2IU/l [83.8–954.8], p=0.016). CK-18 levels were a good predictor of NASH on biopsy with an area under the curve (AUC) of 0.732 (95% CI, 0.572–0.897). A CK-18 cut-off of 130.5IU/l had a sensitivity of 92.9%, specificity of 63%, positive predictive value of 56.5% and negative predictive value of 94.4%, and was able to correctly classify 73.2% of patients with NASH. NFS identified advanced liver fibrosis (AUC 0.739, 95% CI, 0.56–0.91), but was of limited value to identify NASH (AUC 0.413, 95% CI, 0.21–0.61).
ConclusionCK-18 is a good non-invasive marker for NASH. Although NFS was found to be an accurate marker of advanced liver fibrosis, it was not of value to identify NASH. In patients with NAFLD, CK-18 and NFS could be useful in predicting NASH and liver fibrosis, respectively.
La esteatohepatitis no alcohólica (EHNA) es la forma más agresiva de hígado graso no alcohólico (HGNA) e involucra el riesgo de progresión a etapas más avanzadas de enfermedad hepática. Se requieren métodos no invasivos para identificar a pacientes con EHNA.
ObjetivoEvaluar el rendimiento diagnóstico de la determinación de los niveles séricos de citoqueratina-18 como marcador no invasivo de EHNA en población chilena.
MétodosSe determinaron los niveles séricos de CK-18 en un grupo de 41 pacientes con HGNA-probado por biopsia. El diagnóstico de EHNA se basó en los criterios histológicos recomendados (presencia de balonamiento) y se calculó el puntaje de actividad del HGNA (PAH) y grado de fibrosis. Mediante correlación de Spearman se evaluó la asociación entre CK-18 y PAH. Se confeccionó una curva ROC para evaluar la capacidad de CK-18 como test diagnóstico para EHNA. Además, se evaluó el rendimiento del puntaje de fibrosis en hígado graso no alcohólico (NFS) para pesquisa de fibrosis y EHNA y se lo comparó con CK-18 por regresión lineal simple. Los datos son expresados en medianas [percentil 25-75] y evaluados con test de rangos de Wilcoxon.
ResultadosLa edad promedio del grupo estudiado (23% hombres) fue de 50,4±11,1 años. Un 34,2% fue diagnosticado con EHNA (PAH≥5). Los niveles de CK-18 fueron mayores en los pacientes con EHNA versus los sin EHNA (183,6 UI/l [97,4-734,4] vs. 117,2 UI/l [83,8-954,8], p=0,016). Los niveles de CK-18 fueron buenos predictores de la presencia de EHNA en la biopsia con un área bajo la curva (AUC) de 0,732 (IC95% 0,572-0,897). Un punto de corte de 130,5 UI/l de CK-18 exhibió una sensibilidad de 92,9% y una especificidad de 63%, con un VPP de 56,5% y un VPN 94,4%, y clasificó correctamente al 73,2% de los pacientes con EHNA. El NFS tuvo un buen rendimiento para diagnóstico de fibrosis avanzada (AUC 0,739, IC95% 0,56–0,91), pero limitado para identificar EHNA (AUC 0,413, IC95% 0,21-0,61).
ConclusiónLa determinación de CK-18 es un buen marcador no invasivo de EHNA. Si bien, NFS tiene un buen rendimiento en la identificación de pacientes con fibrosis avanzada, no fue de utilidad para diagnosticar EHNA. En pacientes con HGNA, la determinación de CK-18 y NFS es útil en la pesquisa de EHNA y fibrosis hepática respectivamente.
Non-alcoholic fatty liver disease (NAFLD) is a clinical and pathological entity characterised by steatosis in at least 5% of hepatocytes. It may or may not be associated with inflammatory changes and fibrosis.1 Histological elements of inflammation and ballooning of hepatocytes with or without fibrosis identify so-called non-alcoholic steatohepatitis (NASH), which is considered a more aggressive form of the disease and the significance of which lies in its potential progression to hepatic cirrhosis or hepatocellular carcinoma.2
The overall prevalence of NAFLD has been estimated at 25.24% (95% CI: 22.10–28.65) of the population.3–5 In Chile, a study with abdominal ultrasound reported a prevalence of NAFLD of 23% in the population studied.6
Serum aminotransferase levels have traditionally been used as indicators of disease seriousness. However, it should be borne in mind that these levels may fluctuate over time and that they are seen to be elevated in only 50% of patients with NASH. This means that there may be significant liver disease even with normal aminotransferase levels.7 For this reason, liver biopsy remains the gold standard for diagnosing NAFLD and for distinguishing isolated steatosis from NASH and more advanced forms of liver damage.8 This procedure has a significant cost and a clinical risk that render its rational use advisable.9 Currently, non-invasive biochemical and imaging models (transient elastography, magnetic resonance elastography, shear wave elastography, etc.) are being developed and validated to identify patients with more advanced liver disease. However, most of these tools identify fibrosis10,11 and not inflammation, and therefore do not distinguish patients with NASH from patients with simple steatosis.
At present, there is a need to validate a simple, inexpensive diagnostic method that distinguishes between simple steatosis and NASH. Experimental and clinical studies have suggested that hepatocyte apoptosis could play a central role in liver damage and disease progression in NAFLD.12 Indeed, it has been observed both in patients with NASH and in animal models of NASH that apoptosis is the predominant cell death process in hepatocytes.13 The process of apoptosis activates effector caspases (mainly caspase-3). This is associated with cleavage of different substrates, including cytokeratin-18 (CK-18), the most important intermediate filament protein in the liver.14 CK-18 has been evaluated in different populations as a potential marker of hepatocellular damage that distinguishes between patients with isolated steatosis and NASH.15
The objective of this study was to evaluate the diagnostic usefulness and clinically validate serum levels of the serum CK-18 fragment as a non-invasive marker of NASH in a Chilean population.
Patients and methodsStudy populationPatients with a diagnosis of NAFLD demonstrated by biopsy between January 2010 and November 2013, at Hospital Clínico Universidad Católica de Chile, were selected. These patients were contacted within 3 months after confirmation of their histological diagnosis and asked to grant their informed consent for measurement of their serum CK-18, following approval by the institution's ethics committee.
The inclusion criteria were persistent elevation of plasma levels of aminotransferases (aspartate aminotransferase and alanine aminotransferase), an imaging study consistent with fatty liver and biopsy consistent with a diagnosis of NAFLD. Patients with hepatitis A, B, C, D or E virus infection, autoimmune liver disease, Wilson's disease, α1-antitrypsin deficiency, a history of blood transfusions or use of alcohol or hepatotoxins were excluded. In addition, a clinical evaluation (of a history of hypertension, diabetes mellitus type 2 [DMT2] or smoking habit, as well as anthropometrics) and a biochemical evaluation (liver tests, albumin, blood glucose, blood insulin, lipid profile, platelets and aminotransferases) were performed. Clinical and biochemical variables were used to calculate the NAFLD fibrosis score (NFS) as recommended.11
Histological analysisPatients who underwent liver biopsy fasted for more than 8h. The procedure was performed under sedation and local anaesthesia and guided by ultrasound using the Menghini technique.
The samples were processed in formalin and stained with haematoxylin–eosin and Sirius Red. The histological evaluation was performed by an expert pathologist blinded to the clinical data. The diagnosis of NASH was based on the criteria of Brunt et al.,16 and each patient's NAFLD activity score (NAS)17 and degree of fibrosis were calculated. Subsequently, the population studied was classified according to a NAS< or ≥5, as non-NAFLD or NAFLD, respectively. Each sample was further classified according to degree of fibrosis (0: no fibrosis, 1: periportal or perisinusoidal fibrosis, 2: portal/periportal and perisinusoidal fibrosis, 3: bridging fibrosis, 4: cirrhosis).
Measurement of CK-18 levelsThe patients selected underwent venipuncture, and the sample was processed to obtain plasma, which was stored at −80°C. The samples were subsequently used for quantitative determination of CK-18 levels using the M30 Apoptosense commercial ELISA kit (PEVIVA; VLVbio: Bromma, Sweden). All measurements were made in duplicate.
Statistical analysisThe data were expressed as averages (95% confidence interval [CI]), medians (with interquartile ranges) or percentages (%), as appropriate. Ordinal and continuous variables were evaluated using Student's t test and the Wilcoxon rank test, depending on normality tests. Spearman's coefficient of correlation was used to evaluate the association between CK-18 and NAS. Sensitivity and specificity were calculated as a diagnostic test for different cut-off points. The area under the curve (AUC) was evaluated and a ROC curve was prepared to evaluate the capacity of CK-18 as a diagnostic test for NASH, according to a NAS≥5, using the Youden index to identify an optimal cut-off point for ROC curve analysis. At the same time, the NAFLD fibrosis score (NFS)10 was determined, and its diagnostic performance was evaluated for fibrosis in histology and for a NAS≥5 using ROC curves. Similarly, NFS was compared to CK-18 levels using simple linear regression. A p value<0.05 was considered statistically significant. All analyses were performed with IBM SPSS Statistics, Version 20.
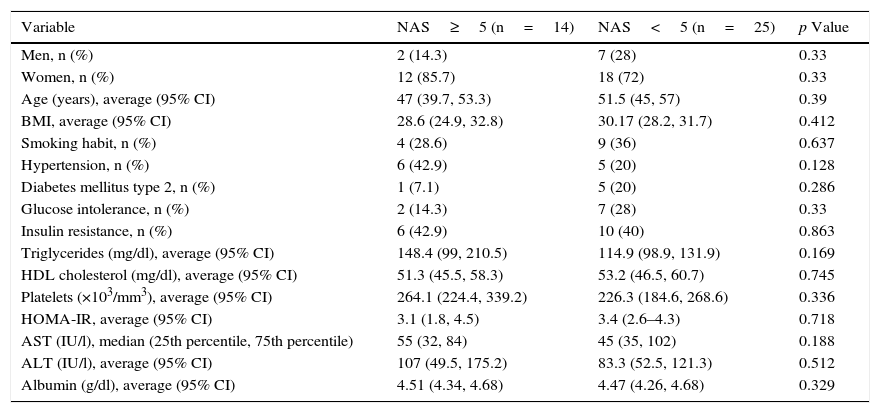
ResultsA total of 41 patients with a diagnosis of NAFLD demonstrated by biopsy, who agreed to participate in the study and signed an informed consent form, were recruited. The main demographic, clinical and laboratory characteristics of the population studied are presented in Table 1. The average age was 50.4±11.1 years, 23% were men and 34.2% were diagnosed with NASH (NAS≥5). The population's characteristics, including age, body mass index, smoking habit, hypertension, DMT2, glucose intolerance and insulin resistance (IR), as well as triglyceride, HDL cholesterol, platelet, HOMA-IR, AST and ALT levels, did not differ significantly between the two groups (NAS≥5 and NAS<5) (Table 1).
Demographic and clinical characteristics of the population studied.
| Variable | NAS≥5 (n=14) | NAS<5 (n=25) | p Value |
|---|---|---|---|
| Men, n (%) | 2 (14.3) | 7 (28) | 0.33 |
| Women, n (%) | 12 (85.7) | 18 (72) | 0.33 |
| Age (years), average (95% CI) | 47 (39.7, 53.3) | 51.5 (45, 57) | 0.39 |
| BMI, average (95% CI) | 28.6 (24.9, 32.8) | 30.17 (28.2, 31.7) | 0.412 |
| Smoking habit, n (%) | 4 (28.6) | 9 (36) | 0.637 |
| Hypertension, n (%) | 6 (42.9) | 5 (20) | 0.128 |
| Diabetes mellitus type 2, n (%) | 1 (7.1) | 5 (20) | 0.286 |
| Glucose intolerance, n (%) | 2 (14.3) | 7 (28) | 0.33 |
| Insulin resistance, n (%) | 6 (42.9) | 10 (40) | 0.863 |
| Triglycerides (mg/dl), average (95% CI) | 148.4 (99, 210.5) | 114.9 (98.9, 131.9) | 0.169 |
| HDL cholesterol (mg/dl), average (95% CI) | 51.3 (45.5, 58.3) | 53.2 (46.5, 60.7) | 0.745 |
| Platelets (×103/mm3), average (95% CI) | 264.1 (224.4, 339.2) | 226.3 (184.6, 268.6) | 0.336 |
| HOMA-IR, average (95% CI) | 3.1 (1.8, 4.5) | 3.4 (2.6–4.3) | 0.718 |
| AST (IU/l), median (25th percentile, 75th percentile) | 55 (32, 84) | 45 (35, 102) | 0.188 |
| ALT (IU/l), average (95% CI) | 107 (49.5, 175.2) | 83.3 (52.5, 121.3) | 0.512 |
| Albumin (g/dl), average (95% CI) | 4.51 (4.34, 4.68) | 4.47 (4.26, 4.68) | 0.329 |
Median (25th percentile, 75th percentile): AST, CK-18.
Percentage, i.e. n (%): sex, smoking habit, hypertension, diabetes mellitus type 2, glucose intolerance, insulin resistance.
Average (95% CI): age, body mass index (BMI), triglycerides, HDL cholesterol, platelets, HOMA-IR, ALT, albumin.
Regarding CK-18 levels in the population studied, a minimum value of 83.8IU/l and a maximum value of 385.2IU/l were found, respectively. In addition, patients with NAS≥5 were found to have significantly higher CK-18 levels compared to patients with NAS<5 (183.6IU/l [132.300] vs. 117.2IU/l [103.2, 164.5]; p=0.016) (Fig. 1). Moreover, a significant correlation was found between an increase in CK-18 and NAS (R2=0.376, p=0.018). From this, it may be inferred that the risk of having NASH on liver biopsy increases as CK-18 levels increase.
When we evaluated the diagnostic performance of serum CK-18 levels as a predictor of NASH on biopsy (determined by a NAS≥5), we found an AUC of 0.732 (95% CI: 0.572–0.897) (Fig. 2). When a cut-off point for CK-18 of 130.5IU/l was considered a benchmark, the test had a sensitivity of 92.9%, a specificity of 62.9%, a positive predictive value of 56.5% and a negative predictive value of 94.4%. Thus it had a Youden index of 0.6 and managed to properly classify 73.2% of patients with NASH in histology.
Regarding assessment of fibrosis, patients with advanced fibrosis (n=10, 24.39%) had a median CK-18 of 128IU/l, while patients without advanced fibrosis (n=31, 75.61%) had a median CK-18 of 132.7IU/l (p=0.499). Although an inverse relationship between degree of fibrosis and CK-18 levels could be seen, with a Spearman's coefficient of −0.79, this trend did not achieve statistical significance (p=0.632), with an AUC for advanced fibrosis of 0.426 (95% CI: 0.213–0.638).
Using the values obtained for NFS in our group of patients with NAFLD, we found that 36.6% were deemed at low risk, 29.3% were indeterminate and 34.2% were deemed at high risk of having significant fibrosis; NFS had an AUC for detection of advanced fibrosis in histology of 0.739 (95% CI 0.563, 0.915). This was consistent with that reported in the literature.11 When we evaluated the diagnostic performance of NFS for NAS≥5, we found that the AUC was 0.413 (95% CI 0.21–0.61). This means that it does not represent a good diagnostic test to distinguish inflammation in NAFLD. Similarly, NFS values correlated poorly with CK-18 levels in simple linear regression (R2=−0.01, p=0.553).
DiscussionThe prevalence of the disease NAFLD has increased at the same time as the obesity and diabetes pandemic. It is associated with potential complications, both hepatic (such as cirrhosis and hepatocellular carcinoma) and extrahepatic (such as DMT2, cardiovascular diseases and cancer).18 The most significant risk factors associated with the development of the disease include obesity, DMT2 and metabolic syndrome.4 Patients with DMT2 have a higher prevalence of NAFLD and of advanced fibrosis. Consequently, they represent a special risk group.19,20 In addition, some studies have suggested that ethnic differences determine the prevalence of NASH in different populations, NASH being more common in Latinos and less prevalent in African Americans.21 The above may be related to a different genetic susceptibility to the development of visceral adiposity and DMT2 and specifically to the development of IR.22
The data on the natural history of NAFLD are limited, and studying them is complex due to a need for long-term follow-up, selection bias in patients and the diagnostic modalities used in the different studies. Using analysis of paired biopsies separated by significant intervals, it has been estimated that 10–20% of patients with NASH could develop cirrhosis in a decade.3,4 In addition, patients with NASH exhibit an increased risk of developing hepatocellular carcinoma.3 However, it should be noted that a recent study conducted by McPherson et al. demonstrated, using paired biopsies an average of 6 years apart, that patients with isolated steatosis may progress both to NASH and to clinically significant fibrosis. Consequently, this condition does not seem to be benign in all cases, as previously believed.23 Moreover, several studies have determined fibrosis, not NASH, to be the most precise predictor of mortality in patients with NAFLD.24,25 Given the long natural history of the disease, it is difficult to demonstrate that NASH influences mortality resulting from hepatic disease. However, this may be so, since cirrhosis and hepatocellular carcinoma develop more commonly in patients with NASH. This may explain the increase in mortality due to NAFLD-associated hepatic disease.4,26
In the context described above, having non-invasive markers such as CK-18 could allow individuals with NASH, and therefore at higher risk of progression, to be identified and further diagnostic actions and intensive therapeutic efforts to be focused on them. In this regard, it could be proposed that patients with NASH markers (such as high CK-18 levels) and other risk factors for progression, such as DMT2 and obesity, should be added to multidisciplinary metabolic disease management programmes.
The main finding of our study was that measurement of plasma CK-18 levels in the Chilean population is a reliable marker of NAFLD on biopsy, with an AUC of 0.732 (95% CI 0.572–0.897) and, when a cut-off point of 130.5IU/l was established, according to the Youden index, the test has a sensitivity of 92.9% and a specificity of 63%. Other groups had previously reported the use of CK-18 as a predictor of NASH, with an AUROC of 0.71–0.93.27–30 The use of plasma measurement of CK-18 has consistently been validated by multi-centre studies and a meta-analysis that showed a combined AUC of 0.82 (95% CI; 0.76–0.88).31,32 Feldstein et al. reported that for every 50IU/l that CK-18 increases, the likelihood of having NASH increases by 30% (odds ratio: 1.74, 95% CI: 1.31–2.31).31
The limitations of our study included reference bias, since it was conducted in a tertiary hospital, and its results may not apply to the general population of patients with NAFLD. In addition, given the characteristics of the study, it is not possible to establish a prognosis for higher CK-18 values in the course of the disease compared to lower values. Further studies are therefore required to validate it. The relationship between CK-18 levels and the clinical course of NAFLD, progression and regression of NASH was reported in a recent study.33 Another matter which would be interesting to investigate is the potential effect of insulin sensitisers on CK-18 measurement.
Interestingly, in our series of patients, CK-18 levels were not correlated with advanced fibrosis. This situation has been reported previously in the literature.34 These observations could have been related to sample size, or to the fact that most patients were in initial stages of the disease. By contrast, NFS had a suitable diagnostic performance for advanced fibrosis in histology, similar to that reported in the literature.11 However, its usefulness in distinguishing between simple steatosis and inflammation in histology was limited, suggesting that its usefulness in initial stages of the disease with no fibrosis would also be limited.
In conclusion, CK-18 is a non-invasive marker that is simple to determine since it requires only a plasma sample. CK-18 is a good non-invasive marker of NASH and superior to NFS. Moreover, in this study, NFS was superior in identifying patients with advanced fibrosis. CK-18 in combination with NFS could be useful non-invasive markers in identifying NASH and hepatic fibrosis in patients with NAFLD.
Authors/contributorsJuan Pablo Arab and Cristián Hernández-Rocha contributed equally to the article (shared primary authorship).
Conflicts of interestThe authors declare that they have no conflicts of interest.
This study was partly funded by the Chilean National Fund for Scientific and Technological Development (FONDECYT) (#1150327 to MA) and the Chilean National Commission for Scientific and Technological Research (CONICYT) (grant PFB 12/2007, Basal Centre for Excellence in Science and Technology [to MA]) of the Government of Chile.
Please cite this article as: Arab JP, Hernández-Rocha C, Morales C, Vargas JI, Solís N, Pizarro M, et al. Fragmento sérico de citoqueratina-18 como marcador no invasivo de esteatohepatitis no alcohólica en población chilena. Gastroenterol Hepatol. 2017;40:388–394.