To evaluate the impact of magnetic resonance enterography (MRE) diagnosis on clinical decision-making regarding treatment choice and maintenance of treatment over time in patients with inflammatory bowel disease (IBD).
MethodsA cohort of patients who underwent MRE for IBD assessment between 2011 and 2014 was analyzed. From clinical records, we retrospectively retrieved their demographic data and clinical data on their IBD at the time of MRE, the results of MRE and the patient's clinical course. Medical management decisions made during the three months following MRE and at the 15-month follow-up were assessed.
ResultsIn total, 474 MREs were reviewed. In the first three-month period, MRE results led to changes in the medical management of 266 patients (56.1%). Of those, maintenance therapy was altered in 140 patients (68.3%) (90.7% step-up and 9.3% top-down strategy), 65 (24.4%) were prescribed a course of steroids and 61 (22.9%) underwent surgery. MRE confirmed a CD diagnosis in 14/41 patients (34.1%) previously diagnosed with indeterminate colitis or ulcerative colitis and in 4/18 patients (22.2%) with suspected IBD. At the 15-month follow-up, treatment remained unchanged in 289 patients (65.8%).
ConclusionsThese results suggest that MRE is a diagnostic tool that provides valid information for the clinical-decision making process for patients with CD.
Evaluar el impacto del diagnóstico de la enterografía por resonancia magnética (ERM) en la toma de decisiones clínicas con respecto a la elección del tratamiento y el mantenimiento del mismo a lo largo del tiempo en pacientes con enfermedad inflamatoria intestinal (EII).
MétodosSe analizó una cohorte de pacientes que se sometieron a ERM para la evaluación de EII entre 2011 y 2014. De los registros clínicos recuperamos retrospectivamente sus datos demográficos y datos clínicos sobre su EII en el momento de la ERM, los resultados de la ERM y la evolución clínica del paciente. Se evaluaron las decisiones de manejo médico tomadas durante los 3 meses posteriores a la ERM y a los 15 meses de seguimiento.
ResultadosSe revisaron 474 ERM. En el primer período de 3 meses, los resultados de la ERM llevaron a cambios en el manejo médico en 266 pacientes (56,1%). De ellos, se modificó el tratamiento de mantenimiento en 140 (68,3%) pacientes (se escaló en el 90,7% y top-down en el 9,3%), 65 (24,4%) recibieron un curso de esteroides y 61 (22,9%) se sometieron a cirugía. La ERM confirmó un diagnóstico de enfermedad de Crohn (EC) en 14/41 pacientes (34,1%) diagnosticados previamente con colitis indeterminada o colitis ulcerosa y en 4/18 pacientes (22,2%) con sospecha de EII. A los 15 meses de seguimiento, el tratamiento se mantuvo sin cambios en 289 (65,8%) pacientes.
ConclusionesEstos resultados sugieren que la ERM es una herramienta de diagnóstico que proporciona información válida para el proceso de toma de decisiones clínicas para pacientes con EC.
Inflammatory bowel disease (IBD) is a chronic inflammatory condition of the gastrointestinal tract affecting up to 0.5% of the population in Western countries.1,2 Crohn's disease (CD) and ulcerative colitis (UC) are the two main subtypes of IBD: CD is a relapsing-remitting chronic inflammatory disorder involving more frequently the ileum and manifesting as a discontinuous transmural inflammation, while UC usually consists of diffuse mucosal inflammation and ulceration mainly affecting the colon.3–6 The prevalence of IBD is high in Europe with 505 and 322 reported cases per 100,000 for UC and CD, respectively, followed by North America (286 and 319 reported cases per 100,000 for UC and CD, respectively).7 The main symptoms of IBD include abdominal pain, ulceration, diarrhea, bleeding, anemia and weight loss.8 IBD is thought to be a result of an uncontrolled inflammatory response to some components of the human microbiota in genetically susceptible individuals, although its exact cause is still not known.6–9
Treatment of IBD involves the use of corticosteroids, antibiotics, immunomodulators, 5-aminosalicylates (5-ASA) and biologic agents, frequently used in step-up or top-down strategies.10,11 However, it is important to accurately identify the type and severity of IBD along with any underlying complications in order to initiate appropriate treatment in the affected individuals. Although endoscopy, of both upper and lower gastrointestinal tract, is essential for the differential diagnosis between CD and UC, ileocolonoscopy can only assess a limited portion of the small bowel and may not be sufficient for the diagnosis of CD.12,13 Therefore, patients with IBD need to undergo additional imaging studies to confirm their diagnosis in order to initiate appropriate treatment.
Imaging techniques provide information about the disease activity by examining areas which are not accessible to endoscopy and identifying any underlying complications such as the formation of abscess and fistulae.5 Computed tomography enterography (CTE) and magnetic resonance enterography (MRE) have recently emerged as effective techniques for the diagnosis of patients with CD.14,15 Although both CTE and MRE have similar sensitivity and accuracy in the diagnosis of small bowel disease,16–21 MRE has an advantage over CTE as it does not involve the use of ionizing radiations, and has been used in clinical practice for the follow-up of patients with CD.9,22–24 Furthermore, it is a cost-effective alternative to CTE that allows effective identification of unsuspected cases of small bowel inflammation.9,25,26 MRE has become a routine imaging test to evaluate the small bowel in patients with established or suspected CD, and its use is recommended by the Society of Abdominal Radiology, the European Society of Gastrointestinal and Abdominal Radiology (ESGAR), the European Society of Paediatric Radiology (ESPR) and the European Crohn's and Colitis Organization (ECCO).14,15,27 As well as being an effective imaging technique for the diagnosis of IBD, MRE can also help physicians in decision-making about pharmacological or surgical treatment of patients with IBD.14 We hypothesized that MRE may determine the clinical management of a high proportion of patients with confirmed CD, indeterminate colitis (IC) or with clinical suspicion of IBD.
Therefore, the primary aim of this study was to determine the impact of MRE testing on the follow-up clinical decision-making of patients with IBD. In addition, the usefulness of MRE in confirming the diagnosis of IBD in patients with IC or clinical suspicion of IBD was also assessed.
Materials and methodsStudy design and patientsThis retrospective observational study included patients with an established or suspected diagnosis of IBD who underwent MRE testing for IBD assessment at three hospitals in Spain between January 2011 and August 2014. The indication of MRE examination was established as follows: (a) confirmed CD, where patients had a previous established diagnosis of CD confirmed by endoscopy and/or histology of >6 months; (b) IC or UC suspected to be CD, where patients were diagnosed by endoscopy and/or histology ≥6 months earlier with no previous evidence of involvement of small bowel; (c) suspected IBD, where patients had clinical suspicion of having IBD (symptoms such as diarrhea, weight loss, anemia, and/or increased inflammation reactants) but not confirmed by routine endoscopic examinations.
Baseline data collected from clinical records included the demographic characteristics of patients included in the study, data related to their diagnosis and extent of IBD (involvement of upper gastrointestinal tract [L4]) according to the Montreal Classification),28 treatments and surgeries before MRE, and the reason for requesting MRE.
MRE results were reviewed and categorized as: (1) findings of inflammation and/or complications suggesting active IBD; (2) morphological changes in the intestinal anatomy suggestive of CD but without acute inflammation (irregularities or thickening of the bowel, without contrast enhancement); and (3) normal (no pathological findings). Medical records of patients at 3 months and 15 months after MRE testing to assess changes, if any, in the management of IBD from the initial medical decision were reviewed and categorized as: (A) no change in treatment or (B) change in treatment by isolated use of corticosteroids (no change in maintenance therapy-B1) or change in maintenance treatment by step-up (B2.1) or top-down therapy (B2.2) or/and surgery (B2.3). The Harvey Bradshaw Index (HBI)29 was assessed at the time of the initial medical decision and after 15 months of follow-up.
Study outcomesThe main study outcome was to determine the proportion of patients in whom results of MRE led to a change in the treatment regimen, and those in whom the treatment decision was maintained over time. Secondary outcomes included identifying patients in whom MRE effectively diagnosed CD in suspected IBD cases as well as those whose diagnosis changed from IC or UC to CD.
The study was approved by the Clinical Research and Ethics Committee of the University Hospital of Canarias (Spain). This was a retrospective study and individual informed consent was not requested from each patient included in the study. Patient records and data collected were handled confidentially in an encrypted database and only researchers involved in the study could access it.
Statistical analysesStatistical analyses were performed using SPSS statistical software, version 23.0. Continuous variables were expressed as means with standard deviation (SD) and 95% confidence intervals (CI). Categorical variables were expressed as proportions and percentages. The cumulative probabilities of outcome events occurring were calculated using the Kaplan–Meier estimator.
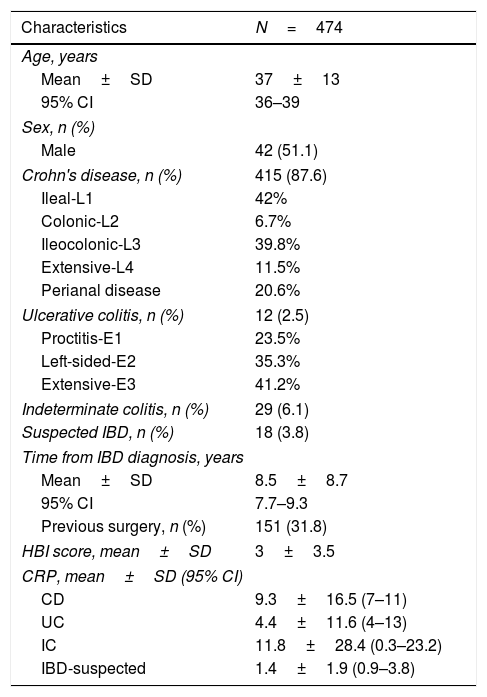
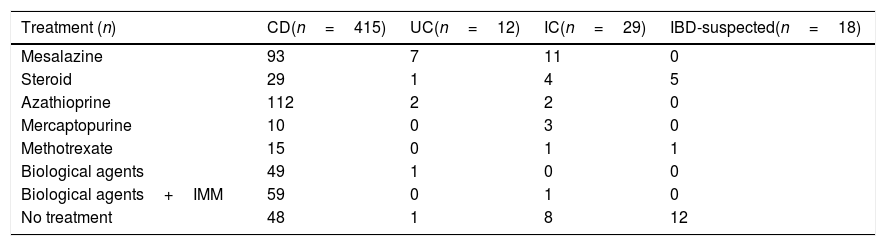
ResultsPatientsResults of MREs performed in 474 patients (51.1% males, mean age 37±13 years) at the Hospital Universitario de Canarias (n=110), Hospital Universitario Nuestra Señora de Candelaria (n=165), and Hospital de Galdakao (n=199) were reviewed. Baseline demographic and clinical characteristics are presented in Table 1 and treatments received at baseline in Table 2. Most patients with CD at baseline were treated with azathioprine (n=112; 27%) or mesalazine (n=93; 22.4%).
Baseline demographic and clinical characteristics of inflammatory bowel disease patients evaluated with magnetic resonance enterography.
| Characteristics | N=474 |
|---|---|
| Age, years | |
| Mean±SD | 37±13 |
| 95% CI | 36–39 |
| Sex, n (%) | |
| Male | 42 (51.1) |
| Crohn's disease, n (%) | 415 (87.6) |
| Ileal-L1 | 42% |
| Colonic-L2 | 6.7% |
| Ileocolonic-L3 | 39.8% |
| Extensive-L4 | 11.5% |
| Perianal disease | 20.6% |
| Ulcerative colitis, n (%) | 12 (2.5) |
| Proctitis-E1 | 23.5% |
| Left-sided-E2 | 35.3% |
| Extensive-E3 | 41.2% |
| Indeterminate colitis, n (%) | 29 (6.1) |
| Suspected IBD, n (%) | 18 (3.8) |
| Time from IBD diagnosis, years | |
| Mean±SD | 8.5±8.7 |
| 95% CI | 7.7–9.3 |
| Previous surgery, n (%) | 151 (31.8) |
| HBI score, mean±SD | 3±3.5 |
| CRP, mean±SD (95% CI) | |
| CD | 9.3±16.5 (7–11) |
| UC | 4.4±11.6 (4–13) |
| IC | 11.8±28.4 (0.3–23.2) |
| IBD-suspected | 1.4±1.9 (0.9–3.8) |
Abbreviations: CD, Crohn's disease; CI, confidence interval; CRP, C-reactive protein; HBI, Harvey–Bradshaw Index; IBD, inflammatory bowel disease; IC, indeterminate colitis; SD, standard deviation; UC, ulcerative colitis.
Treatment administered at baseline to patients in the study (n=474).
| Treatment (n) | CD(n=415) | UC(n=12) | IC(n=29) | IBD-suspected(n=18) |
|---|---|---|---|---|
| Mesalazine | 93 | 7 | 11 | 0 |
| Steroid | 29 | 1 | 4 | 5 |
| Azathioprine | 112 | 2 | 2 | 0 |
| Mercaptopurine | 10 | 0 | 3 | 0 |
| Methotrexate | 15 | 0 | 1 | 1 |
| Biological agents | 49 | 1 | 0 | 0 |
| Biological agents+IMM | 59 | 0 | 1 | 0 |
| No treatment | 48 | 1 | 8 | 12 |
Abbreviations: CD, Crohn's disease; IBD, inflammatory bowel disease; IC, indeterminate colitis; IMM, immunomodulator; UC, ulcerative colitis.
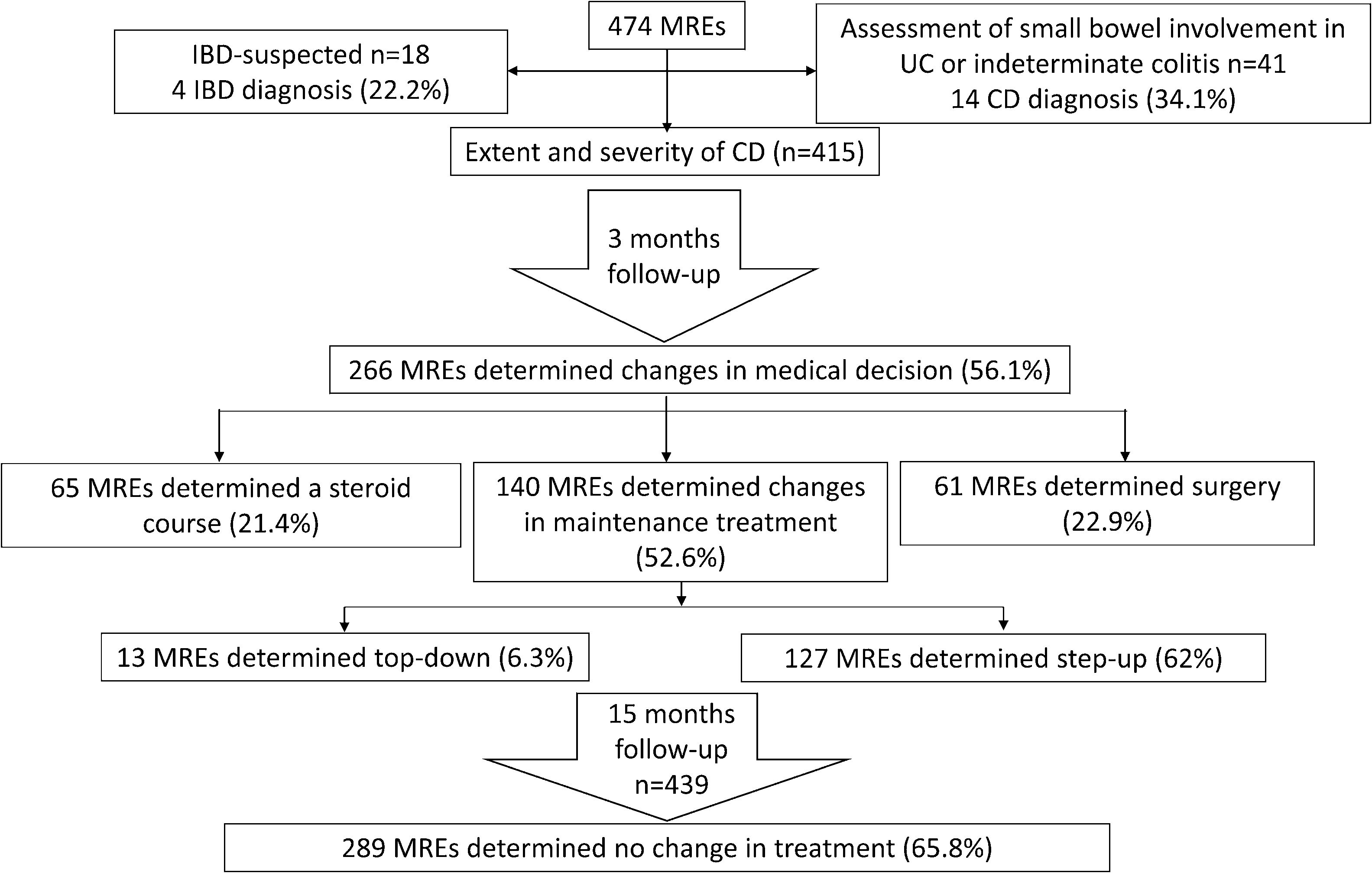
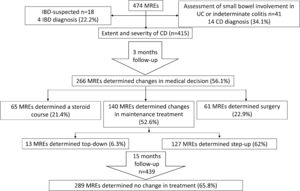
Of the 474 patients included in the initial assessment, MRE confirmed CD in 83 (17.5%) patients, indicated inflammatory activity in 326 (68.7%) patients, and identified 65 (13.7%) patients with small bowel involvement without active inflammation. Overall, MRE allowed for the diagnosis of the severity and extent of CD in 415 (87.5%) patients (Fig. 1).
The results of MRE in patients with a pre-MRE diagnosis of CD were: normal in 81 (19.5%), findings of CD in 146, and active IBD in 188. Among patients with a pre-MRE diagnosis of UC (12 patients), normal MRE results were seen in seven (58%), findings of CD without active inflammation in one (8.3%), and active CD in four (34%). For patients with pre-MRE diagnosis of IC (29 patients), the results of MRE were normal in 20 (69%), findings of CD in two (6.8%), and active CD in seven (24.2%). Lastly, in patients with suspected IBD, the results of MRE were normal in 14 (77.7%), findings of CD in one (5.5%), and active IBD in three (16.8%). Thus, MRE confirmed the diagnosis of CD in 14 out of 41 (34.1%) patients with IC or UC, and in four out of 18 (22.2%) patients with suspected IBD (Fig. 1). Overall, MRE determined small bowel involvement of CD in 18 (30.5%) patients with suspected IBD or a previous diagnosis of UC or IC.
Management of IBD after MREAmong 474 MRE-assessed patients, 208 (43.9%) MRE results led to no change in the medical management of IBD while 266 (56.1%) MRE results influenced the treatment strategy: 65 (24.4%) patients received a steroid course, 140 (52.6%) underwent a change in their maintenance treatment, and 61 (22.9%) patients underwent surgery (Fig. 1). Among the 140 (29.5%) patients who underwent a change in maintenance treatment, this modification corresponded to a step-up strategy in 127 (90.7%) patients and to a top-down treatment in 13 (9.3%) patients. Step-up therapy included adding treatment with immunomodulators in 52 (40.3%) cases, anti-tumor necrosis factor (anti-TNF) agents in 23 (18.1%) cases, anti-TNF escalation in eight (6.2%) cases, addition of immunomodulators to anti-TNF agents in nine (7.1%) cases, changing the anti-TNF agent in five (3.93%) cases, and mesalazine treatment in 30 (23.6%) cases. Changes to top-down therapy included withdrawal of immunomodulators in seven (53.8%) cases, withdrawal of anti-TNF in three (23.1%) cases and anti-TNF de-escalation in three (23.1%) cases.
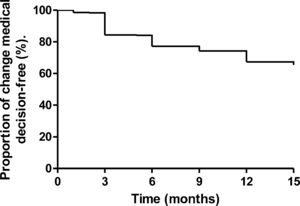
Fifteen-month follow-upOverall, 439 patients were followed-up 15 months after MRE testing, of which 289 (65.8%) patients (264 with CD, 6 with UC, and 19 with IC) showed no change in the treatment strategy during the follow-up, six (1.37%) underwent surgery, and treatment course was changed in 144 (32.8%) patients (115 underwent step-up therapy and 29 top-down therapy; Fig. 1). A Kaplan–Meier curve for maintenance of clinical decision over time is shown in Fig. 2. The mean (±SD) HBI score at the 15-month follow-up was 1.5±2, indicating persistence of remission.
DiscussionThe results of this study showed that MRE was useful in confirming the diagnosis of CD in approximately 30.5% of patients with suspected IBD or a previous diagnosis of UC or IC. MRE also led to a change in medical management in 56.1% of cases at 3 months which were maintained in 65.8% of cases at 15-month follow-up.
Our results are in line with several studies showing the usefulness of MRE in the diagnosis and monitoring of CD. A prospective study in Norway that assessed a cohort of 237 patients with CD at a 20-year follow-up after diagnosis showed that almost 68% of them had imaging features of CD; MRE detected CD in approximately half of these patients.30 Based on the MRE findings, disease location and behavior was recategorized in eight patients (8.3%).30 In addition, MRE found that 42.7% of patients had small bowel CD.30 This is higher than our finding of 30.5% of patients with small bowel involvement detected using MRE; our patient population were assessed earlier in their disease course and were younger (mean [±SD] disease duration 8.5±8.7 years and mean [±SD] age 37±13 years versus mean disease duration [±SD] of 20.1±1.4 years and mean [±SD] age of 49.6±12.0 years in the Norwegian study). However, our diagnosis results were similar to those of Mendoza et al.,31 who used MRE in 150 patients with suspected or diagnosed CD. Their MRE findings confirmed the diagnosis of IBD in eight of 24 (33.3%) clinically suspected patients.31 We confirmed the diagnosis of IBD in 34.1% patients with IC or UC, and the diagnosis of IBD in 22.2% of IBD-suspected cases.
Although many studies have assessed the value of MRE in the diagnosis of IBD, there are limited data regarding its usefulness in medical decisions in routine clinical practice. Ha et al.32 found that MRE provided effective information for evaluating and taking medical decisions, particularly in CD patients with obstructive symptoms. A number of retrospective studies have reported changes to medical management for at least 50% of patients on the basis of MRE results.31,33,34 Rajabi et al.34 reported that abnormalities detected by MRE lead to a change in patient management for 57.6% (38/66) of MRE's conducted. Messaris et al. found in their retrospective study of 120 CD patients that 31% had no change in medical therapy after MRE and 53% had additional medical management for active inflammation.33 Mendoza et al.31 used MRE imaging results to change the clinical decision-making in 55.3% of 150 patients with known or suspected CD, initiating immunosuppressants or biologic agents or switching biologic agent (step-up strategy) in 38% of patients, a change to monotherapy (top-down) in 2% of patients, and referral for surgery in 10% of patients. Our study results were similar: we optimized the medical decision in 56.1% of cases. When changes to maintenance therapy were made in our study, 90.7% of patients had their treatment intensified (starting treatment with a biological agent, starting IMMs, or intensification of biologic agent) and 9.3% had treatment de-escalated (suspension of IMM or biologic agent, indication of monotherapy, biologic agent de-intensification). Unlike previous studies, the present study also reviewed if the initial treatment decision after MRE was maintained over time in patients with IBD. We found that, after a follow-up period of 15 months, the initial medical decision was maintained in up to 65.8% of cases. This observation indicates that the treatment regimens were appropriately chosen in the majority of cases in clinical practice based on the information provided by MRE. A top-down strategy was also possible in some cases which allowed for a reduction in the cost and adverse events associated with treatments.
Although the study was conducted in a large patient population, it has several limitations. First, since this was a retrospective observational study, there is the potential for introduction of selection bias. Second, decisions about treatment changes within 3 months of MRE may not have been made solely on the basis of MRE results, but may have been influenced by other factors such as tolerability or patient preference. In addition, treatment decisions were made by several physicians at the three participating hospitals, and these physicians may not have applied a consistent approach to management decisions based on MRE findings. Third, the study had a limited follow-up period of 15 months and we cannot ensure the persistence of the clinical decision making only due to MRE findings.
ConclusionMRE can be a useful tool in the management of patients with IBD as it allows effective diagnosis of CD and assessment of disease severity and extent and supports appropriate decision-making regarding the medical management of these patients.
Authors’ contributionLR and AHC have contributed equally to this work. LR and AHC: study concept, design, statistical analysis, interpretation of data and wrote the manuscript. IR-L, MCP, LCD, AE, IAA, MV, AH, NHA-B, GER, YR, CT, LD-F, DE, MA, MSG and JAL: were involved in acquisition of data. IR-L, JLC and EQ: critical revision of the manuscript. The authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
FundingThis research has not received specific aid from public sector agencies, commercial sector or non-profit entities.
Conflict of interestLR has received educational and travel grants and speaker fees from Abbvie, Dr. Falk Pharma Ferring, Janssen, MSD, Pfizer, Shire Pharmaceuticals and Takeda.
AHC has received educational and travel grants and speaker fees from Abbvie, Ferring, Janssen, Kern Pharma Pfizer, Takeda and Tillots Pharma.
IR-L has received educational and travel grants and speaker fees from Abbvie, Dr. Falk Pharma Ferring, Janssen, MSD, Otsuka, Pfizer, Shire Pharmaceuticals, Takeda and Tillotts Pharma. MCP has received educational and travel grants and speaker fees from Abbvie, Faes Farma, Ferring, MSD, Pfizer and Takeda.
EQ is an advisor to Sysmex España SL.
All other authors declare no conflict of interest relating to this study.
The author wishes to thank Dr. Ana Moreno Cerro, who provided medical writing assistance on behalf of Springer Healthcare Communications, and Nishad Parkar, PhD, of Springer Healthcare Communications, who performed a technical edition of the manuscript. This medical writing assistance was funded by an unrestricted grant from Kern Pharma.