Inflammatory fibroid polyp (IFP) is an unusual benign gastrointestinal subepithelial tumor (SET). The endosonographic (EUS) features of IFPs were sporadically reported on imaging tips or small case series study. However, the differential diagnosis and optimal treatment of gastric IFP is still challenging.
We report an unusual case of a large erosioned and prolapsing gastric submucosal lesion, presenting primarily with obstructive symptoms (“ball valve syndrome”) and anemia. On EUS examination, a 50mm SET in the distal antrum was seen, with hypoechoic but heterogeneous echo-pattern, located in the second and third sonographic layers of the gastric wall (deep mucosal and submucosal). The fourth (muscle) layer was intact; no peri-lesional adenopathies were identified. A decision was made to proceed to endoscopic treatment because of the mentioned symptoms. Histopathologic evaluation of the resected specimen with immunohistochemical staining was consistent with the diagnosis of IFP.
IFP rarely reach these large dimensions or cause symptoms. Despite its benign etiology, endoscopic resection was important in both establishing a histologic diagnosis and treatment. EUS was crucial in the differential diagnosis. The literature concerning IFP is also reviewed.
O pólipo fibróide inflamatório (PFI) é uma lesão subepitelial (LSE) gastrointestinal incomum e benigna. As características dos PFIs gástricos na ultrassonografia endoscópica (EUS) foram esporadicamente descritas em relatos de casos clínicos e séries pequenas. No entanto, o diagnóstico diferencial e tratamento são frequentemente um desafio.
Apresentamos um caso invulgar de uma LSE gástrica volumosa, com sintomas obstrutivos face ao seu efeito valvular sobre o piloro (“ball valve syndrome”) e anemia por erosão da mucosa. Na avaliação por EUS observou-se uma LSE hipoecogénica mas heterogénea no antro distal, com 50 mm, e pedi¿culo longo na dependência da segunda e terceira camadas (mucosa profunda e submucosa). Estava mantida a integridade da quarta camada (muscular), e não foram observadas adenopatias peri-lesionais. Optou-se pela exérese endoscópica face à sintomatologia referida. A análise histológica com estudo imuno-histoquímico foi compatível com PFI.
Os PFIs raramente atingem estas dimensões ou causam sintomas. Apesar da sua etiologia benigna, a ressecção endoscópica foi importante para estabelecer um diagnóstico histológico bem como tratamento. Destaca-se a importância da EUS no diagnóstico diferencial desta lesão. A literatura sobre PFIs é revista.
Inflammatory fibroid polyp (IFP) is an unusual benign gastrointestinal subepithelial tumor (SET) with an uncertain origin and natural history. Although it is frequently seen in the stomach, diagnosis is sometimes difficult without surgical or endoscopic resection. The endoscopic ultrasonography (EUS) characteristics of IFPs were sporadically reported on imaging tips or small case series study.1 However, the differential diagnosis and optimal treatment of gastric IFP is still a challenge.
2Case reportA 73-year-old woman with arterial hypertension was admitted for investigation of iron deficiency anemia (serum hemoglobin of 6.9g/dL). On further questioning, the patient acknowledged several months of intermittent postprandial bloating and nausea, but she denied history of weight loss or visible gastrointestinal (GI) bleeding. The clinical examination was unremarkable.
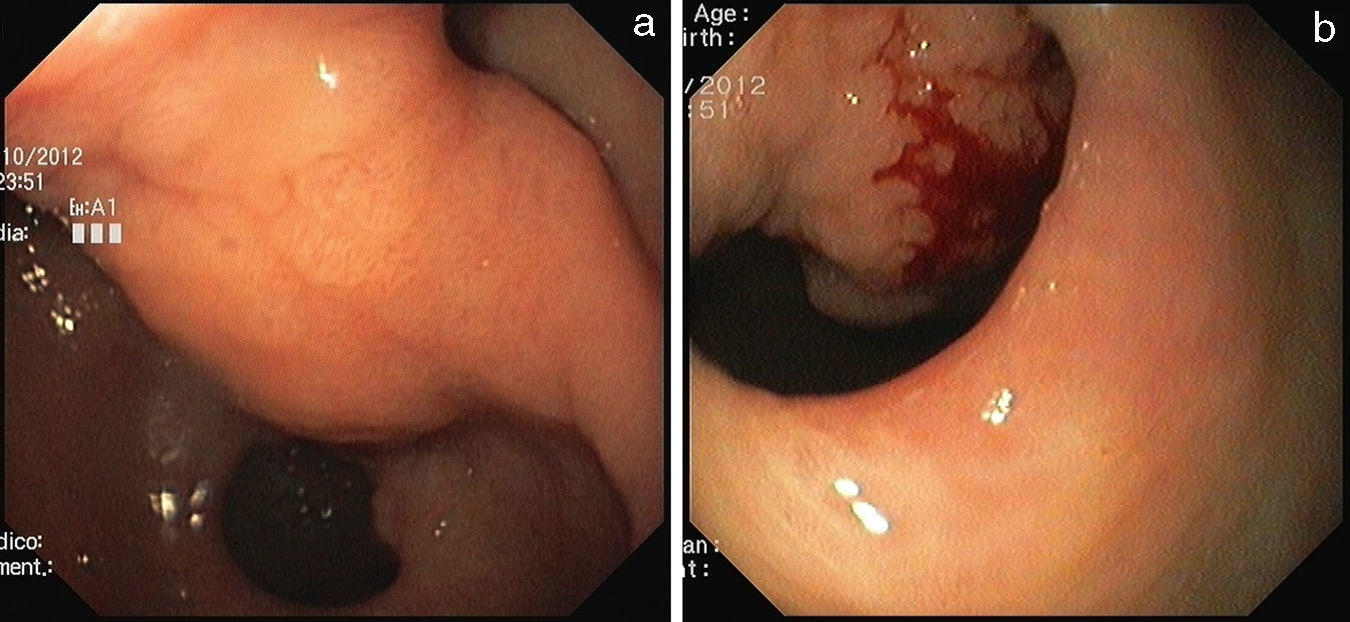
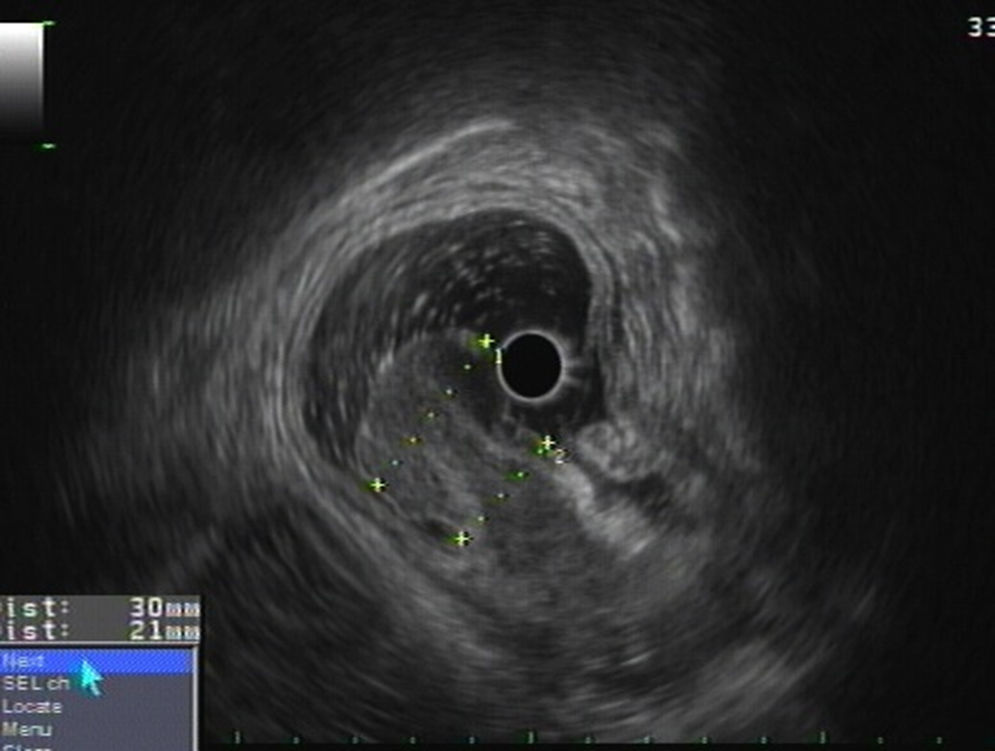
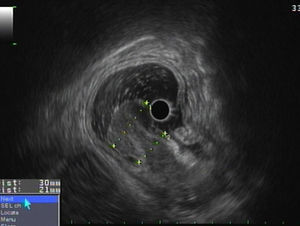
Esophagogastroduodenoscopy revealed a large pedunculated polypoid lesion in the gastric distal antrum, protruding through the pylorus into duodenal bulb, causing intermittent gastric outlet obstruction (Fig. 1a). Mucosal surface was erosioned and easy to bleed (Fig. 1b). For further study, EUS was performed using a GF-UE160 radial echoendoscope (Olympus Medical Systems, Tokyo, Japan). A 50mm polypoid, in most parts hypoechoic, but heterogeneous SET was observed, located in the distal antrum, with involvement of the second and third layer of the gastric wall (the deep mucosal and the submucosal layers) (Fig. 2). The fourth layer (muscle layer) was intact, and no peri-lesional adenopathy was identified. The 20mm-long stalk had no Doppler signal therein. The gastric polyp was suspected to be the cause of anemia and obstructive symptoms, so a decision was made to proceed to endoscopic treatment. Endoscopic snare electrocautery was performed, after a 30-mm detachable snare (Endo-Loop, MAJ-254, Olympus, Tokyo, Japan) was tightened around the base of the stalk. After the procedure, arterial spurting hemorrhage occurred from two small vessels in the scar, which was successfully controlled with a Bipolar Circumactive Probe (BICAP 7Fr, 10″ 20W). Histopathologic evaluation of the resected specimen demonstrated spindle-shaped stromal cells, and a submucosal inflammatory infiltrate with eosinophils (Fig. 3a). Immunohistochemical staining was positive for CD34, and negative for CD117, S100, CK7, desmin and actin, consistent with the diagnosis of IFP (Fig. 3b). The patient was hospitalized for vigilance, and discharged home 1 day after. Endoscopic 3-month follow-up revealed a small sessile polypoid lesion with intact mucosa over the previous site of the polyp. The patient is symptom-free on one-year follow-up.
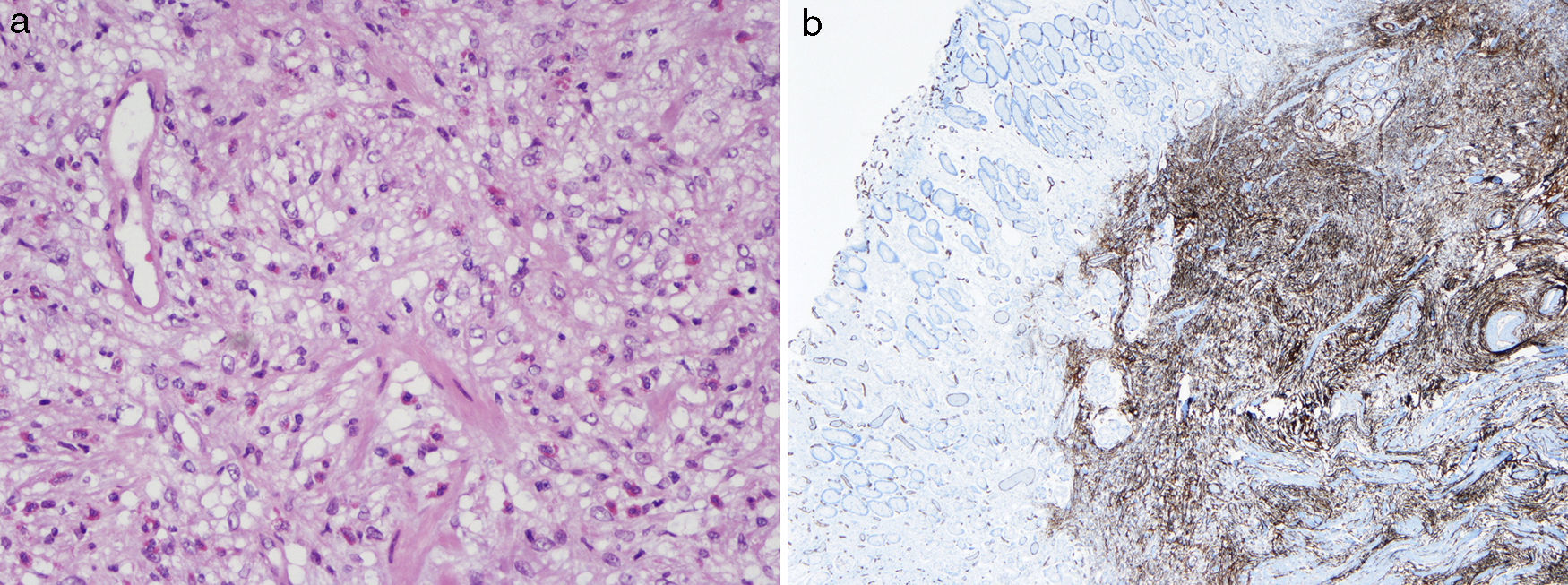
Pathological analysis revealed fibroblast-like spindle cells intermingled with large numbers of mixed inflammatory cells and eosinophil [H&E, original magnification, ×400] (a). Immunohistochemical staining was positive for CD34 and vimentin but negative for CD117, S100, CK7, desmin and actin (b).
IFP is usually solitary, sessile or pedunculated, with an inflammatory basis, initially reported by Vanek, in 1949.2 Helwig and Ranier proposed the term “inflammatory fibroid polyp”, in 1953.3
IFPs can be found in all age groups, but peak incidence is in the sixth and seventh decades.4 They may occur in all parts of the gastrointestinal (GI) tract. The most common site is the gastric antrum (around 70% of cases), followed by the small and large bowel (approx. 25%), gallbladder (approx. 1%), esophagus (approx. 1%), duodenum (approx. 1%), anal canal (approx. 1%), and appendix (<1%).5,6
Gastric IFPs are usually asymptomatic, identified during endoscopy or laparotomy. When symptomatic, the clinical presentation is mostly determined by the size and the anatomic location.7 The current clinical case reports a large gastric IFP with obstructive symptoms due to its effect on the pyloric valve, the so-called “ball valve syndrome” (BVS), and also GI bleeding with severe anemia due to mucosal erosion on the IFP surface. BVS refers to intermittent gastric outlet obstruction due to prolapse of pedunculated polyps through the pylorus into the duodenal bulb. First described by Hobbs and Cohen in 1946, and it has been recognized as a rare but important cause of acute abdomen. Intestinal IFPs may present with intussusception.6
Histologically, IFPs are characterized by vascular and fibroblast proliferation with an eosinophilic inflammatory response. The diagnosis is supported by immunohistochemistry where IFPs usually stain positive for CD34 and vimentin, and sometimes for smooth muscle actin, calponin, CD35 and cyclin-D1.8
Matsushita et al.1,9 described EUS features of 10 patients with gastric IFPs, and compared the EUS images with the resected specimens. IFPs had an indistinct margin, hypoechoic homogeneous lesion, and location within the second and/or third layer with the intact fourth layer. These findings correlated very closely to the histological findings.
In our case, EUS was very suggestive of the diagnosis of IFP before removal, based on endoscopic and EUS features. A decision was made to proceed to endoscopic treatment because of the mentioned symptoms. Also, the size of the polyp and pedunculated morphology made it amenable for endoscopic resection. Although the long and thick stalk appeared to have no Doppler signal therein, a spurting arterial bleeding occurred after endoscopic snare resection. The existence of arterial vessels within the polyp stalk had not been detected by our EUS examination. Maybe, careful Doppler examination with low PRF could have shown the feeding arteries of this vascularized tumor before endoscopic resection.
The treatment of IFP depends on the location and size of the lesion. The current standard treatment strategy for gastric polyps causing obstructive symptoms is complete removal, either endoscopically or surgically, followed by pathological analysis.10 Small, pedunculated, gastric IFPs can be successfully removed by endoscopic polypectomy. Macedo et al.11 have endoscopically treated eight patients with large gastric polyps (35–60mm) and BVS. The procedures were uneventful and all patients had prompt relief after polypectomy.
The natural history of IFP is still unknown. IFPs were described by Vanek, 60 years ago, as “submucosal granuloma with eosinophilic infiltration”.2 They represent polypous proliferations of spindle cells in the mucosa and submucosa, with prominent inflammatory infiltration, and have been regarded as inflammatory and reactive. Recent histopathological studies from Schildhaus et al.12 have shown that IFPs express platelet-derived growth factor receptor alfa (PDGFRA) in more than 90% of cases, and the majority of IFPs harbour activating PDGFRA mutations. Therefore, IFPs represent true benign mesenchymal tumors of the gastrointestinal tract, and although many years thought to be reactive, are now regarded to be true PDGRFA-driven benign neoplasms.13–15 Given their gastrointestinal location, overlapping molecular features, and characteristic CD34 immunoreactivity, IFPs may be confused with other etiologies, namely gastrointestinal stromal tumors (GISTs).12,16,17
In summary, gastric IFP is an unusual benign tumor. We report an unusual case of a prolapsing and erosioned large IFP, presenting primarily with obstructive symptoms with intermittent gastric outlet obstruction (the “Ball valve syndrome”) and anemia by erosion of the mucosa. IFP rarely reach these large dimensions or cause symptoms. Despite its benign etiology, endoscopic resection was important in both establishing a histologic diagnosis and offering treatment. We highlight the value of EUS in the differential diagnosis of this lesion.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.




![Pathological analysis revealed fibroblast-like spindle cells intermingled with large numbers of mixed inflammatory cells and eosinophil [H&E, original magnification, ×400] (a). Immunohistochemical staining was positive for CD34 and vimentin but negative for CD117, S100, CK7, desmin and actin (b). Pathological analysis revealed fibroblast-like spindle cells intermingled with large numbers of mixed inflammatory cells and eosinophil [H&E, original magnification, ×400] (a). Immunohistochemical staining was positive for CD34 and vimentin but negative for CD117, S100, CK7, desmin and actin (b).](https://static.elsevier.es/multimedia/23414545/0000002200000002/v2_201504190202/S2341454514001276/v2_201504190202/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
