An 85-year-old male developed constipation after orthopaedic surgery and was treated with oral laxatives and enemas. After starting the medication he complained of anal pain and rectal bleeding. The primary care physician diagnosed an anal fissure and prescribed anal ointments.
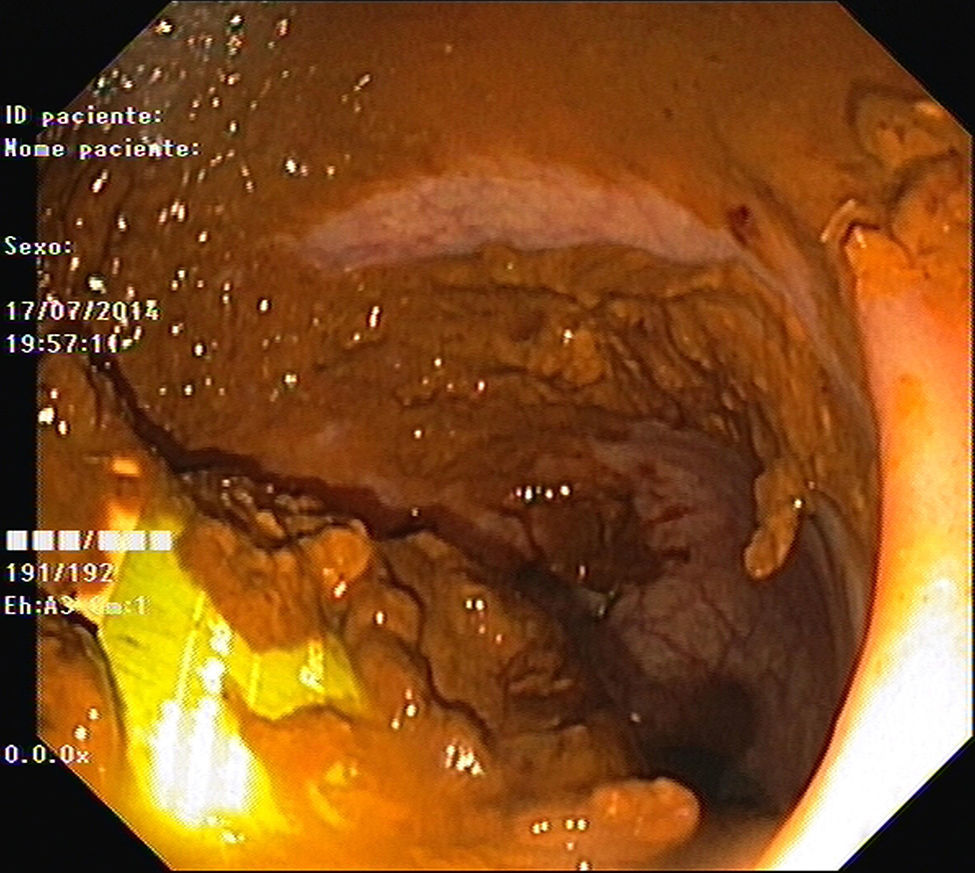
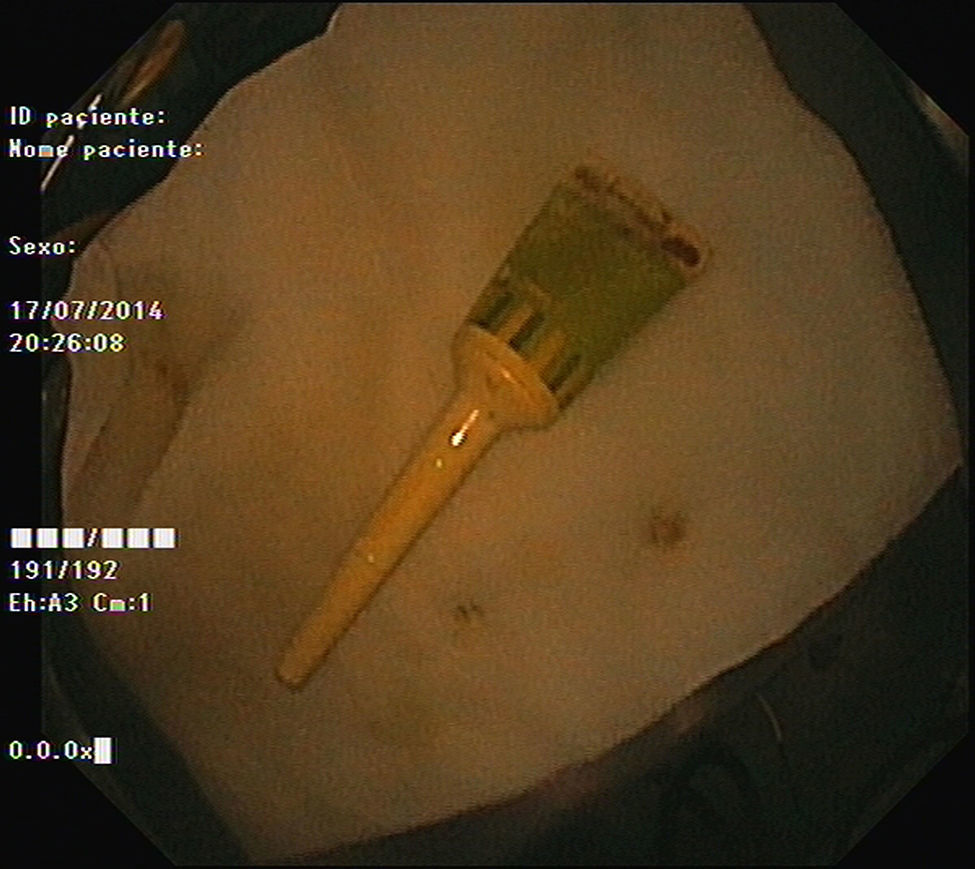
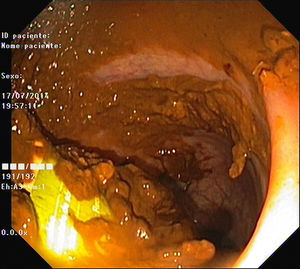
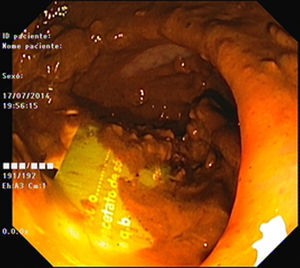
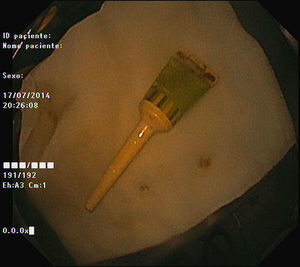
As pain became more severe he came to our hospital for observation. When asked, the patient admitted a single use of rectal microenema one month before and digital rectal examination revealed a foreign body within the rectal ampulla. Endoscopic examination of the rectum identified a microenema device (Figs. 1 and 2), which was removed with an anoscopy and a foreign body forceps with addition of a Valsalva manouver by the patient (Fig. 3). No sedation was needed because of the distal localization of the object and good tolerability to the procedure. No complications were registered and the patient was rapidly symptoms free.
2DiscussionMost rectal foreign bodies are introduced consciously by persons seeking sexual stimulation.1,2 In our case the patient incorrectly applied the microenema and did not give that information to the physicians as he believed he had used it properly. We point out the fact that the foreign body remained in the rectum for a month before it was brought to attention to health care providers.
Most objects can be removed transanally.3 In clinically stable patients without evidence of perforation or peritonitis, the rectal foreign body can be removed in the emergency department.3,4 The management depends on the texture, size, shape and location of the object.4 If the foreign body can be easily palpated, it is possible to be extracted transanally using one of many clamps and instruments, with or without direct visualization through a rigid or flexible endoscope.3–5 Open surgery should be reserved only for patients with perforation, peritonitis or impaction of the foreign body.4,5
Conflicts of interestThe authors have no conflicts of interest to declare.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.