C-reactive protein (CRP) and Bedside Index for Severity in Acute Pancreatitis (BISAP) have been used in early risk assessment of patients with AP.
ObjectivesWe evaluated prognostic accuracy of CRP at 24hours after hospital admission (CRP24) for in-hospital mortality (IM) in AP individually and with BISAP.
Materials and MethodsThis retrospective cohort study included 134 patients with AP from a Portuguese hospital in 2009–2010. Prognostic accuracy assessment used area under receiver–operating characteristic curve (AUC), continuous net reclassification improvement (NRI), and integrated discrimination improvement (IDI).
ResultsThirteen percent of patients had severe AP, 26% developed pancreatic necrosis, and 7% died during index hospital stay. AUCs for CRP24 and BISAP individually were 0.80 (95% confidence interval (CI) 0.65–0.95) and 0.77 (95% CI 0.59–0.95), respectively. No patients with CRP24 <60mg/l died (P=0.027; negative predictive value 100% (95% CI 92.3–100%)). AUC for BISAP plus CRP24 was 0.81 (95% CI 0.65–0.97). Change in NRInonevents (42.4%; 95% CI, 24.9–59.9%) resulted in positive overall NRI (31.3%; 95% CI, −36.4% to 98.9%), but IDInonevents was negligible (0.004; 95% CI, −0.007 to 0.014).
ConclusionsCRP24 revealed good prognostic accuracy for IM in AP; its main role may be the selection of lowest risk patients.
A proteína-C reativa (CRP) e o Bedside Index for Severity in Acute Pancreatitis (BISAP) têm sido usados na avaliação de risco precoce de doentes com pancreatite aguda (AP).
ObjectivosNós avaliámos o valor prognóstico da CRP às 24 horas após a admissão hospitalar (CRP24) na mortalidade intrahospitalar (IM) na AP, individualmente e com o BISAP.
Materiais e MétodosEste estudo coorte retrospetivo incluiu 134 doentes com AP de um hospital português em 2009–2010. A acuidade prognóstica foi avaliada usando a área debaixo da receiver-operating characteristic curve (AUC), o continuous net reclassification improvement (NRI), e o integrated discrimination improvement (IDI).
ResultadosTreze por cento dos doentes tiveram AP grave, 26% desenvolveram necrose pancreática, e 7% morreram durante a hospitalização índice. As AUCs da CRP24 e do BISAP individualmente foram 0,80 (intervalo de confiança (IC) 95%, 0,65–0,95) e 0,77 (IC 95%, 0,59–0,95), respectivamente. Nenhum doente com CRP24 <60mg/l morreu (P=0,027; valor predictivo negativo 100% (IC 95%, 92,3–100%)). A AUC para o BISAP mais a CRP24 foi 0,81 (IC 95%, 0,65–0,97). A mudança no NRI-não-eventos (42,4%; IC 95%, 24,9–59,9%) resultou num NRI-total positivo (31,3%; IC 95%, −36,4 a 98,9%), mas num IDI-não-eventos negligenciável (0,004; IC 95%, −0,007 a 0,014).
ConclusõesA CRP24 revelou um valor prognóstico bom para a mortalidade intrahospitalar na AP; o seu papel principal poderá ser a selecção dos doentes de menor risco.
The incidence of acute pancreatitis (AP) seems to be increasing in several countries.1,2 Most cases of disease follow a course without complications, but about 20% of patients develop severe AP (SAP).3 The overall mortality is 2.1–7.8%, but SAP leads to prolonged hospitalization and higher mortality.4,5
The early risk assessment of patients with AP by accurate and reliable methods is necessary to improve clinical outcomes and reduce costs of treatment. Several tools for the early risk assessment of these patients have been developed; they include single biochemical markers, imaging methods, and complex scoring systems.6
C-reactive protein (CRP) is an acute phase reactant that has been largely used in the early risk assessment of patients with AP. Among single biochemical markers, CRP remains the most useful in predicting SAP, especially due to its accuracy and accessibility.6,7 Despite these advantages, its prognostic accuracy has been validated for measurements no sooner than 36hours after hospital admission.8,9 In this regard, some other tools for earlier risk assessment of patients with AP, as Bedside Index for Severity in AP (BISAP), might be more useful.10
BISAP has been validated as an accurate and reliable scoring system for early identification of patients with AP at risk for in-hospital mortality (IM).10–12 It comprises the assessment of 5 clinical variables in the first 24hours after hospital admission: Blood urea nitrogen greater than 25mg/dl; presence of impaired mental status on clinical evaluation; presence of systemic inflammatory response syndrome; age greater than 60 years; presence of pleural effusion on clinical or radiological evaluation. The index final score is calculated by adding one point per each variable present (out of 5 points). According to this scoring system, IM increases progressively with its final score, being 5–20% for a final score equal or greater than 3 points.10
In this study, we hypothesized that CRP measured at 24hours after hospital admission (CRP24) may have relevant prognostic value for IM in AP and this may add to the one from BISAP. Accordingly, we aimed to determine CRP24 individual prognostic accuracy for IM in AP and identify a clinically significant cutoff point; additionally, we sought to quantify the combined prognostic accuracy for IM in AP of CRP24 and BISAP.
2Materials and MethodsThe reporting of this study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.13 The local Health Research Ethics Board approved this study and the requirement for individual informed consent was waived.
2.1Design, setting, participants, and data collectionThis was a single center retrospective cohort study which included initially all patients consecutively admitted to the emergency department of a Portuguese general district hospital (Hospital Professor Doutor Fernando Fonseca, Amadora) with AP between January 2009 and December 2010. Of this set, patients with incomplete data on CRP24, BISAP score, or in-hospital final vital status were excluded.
Patients were identified from the hospital's diagnostics dedicated computerized database. Demographic and clinical data were collected by 3 investigators from a variety of sources including patients’ charts (manual and electronic), laboratory reports, and radiology reports.
2.2Operational definitionsThe diagnosis of AP was made if 2 of the following 3 features were present: (i) Abdominal pain suggestive of AP; (ii) serum amylase and/or lipase activity at least 3 times greater than the upper limit of normal; and (iii) characteristic findings of AP on transabdominal ultrasonography or on contrast-enhanced computed tomography (CECT).14 Despite these criteria, all patients in whom the minimum 3-fold hyperamylasemia was proved to be from other cause, rather than AP, were excluded.
Patients were classified as SAP if organ failure was present for more than 48hours. In accordance to the Marshall Scoring System, organ failure includes at least one of the following features: (i) Respiratory failure, defined as PO2/FiO2 ≤300mmHg; (ii) renal failure, defined as serum creatinine level ≥1.9mg/dl; and (iii) shock, defined as systolic blood pressure <90mmHg and unresponsive to fluid therapy.14
As a local complication, PNec was diagnosed by CECT when there was lack of enhancement of pancreatic parenchyma after contrast infusion.14
For each patient, all CRP determinations obtained during the first 24hours after hospital admission were collected and only the greatest level of those was considered for analysis. These CRP determinations were obtained using the standard technique at the hospital's laboratory (Dimension Vista System, Flex reagent cartridge, Siemens Healthcare Diagnostics Products, Germany) and expressed in mg/l.
BISAP score was determined according to what has been previously published.10
2.3Primary exposures and primary outcomePrimary exposures were CRP level and BISAP score in the first 24hours after hospital admission. Primary outcome was IM, which referred to death occurring from AP or its complications (inflammatory, septic, morphological or others) during index hospital stay.
2.4Statistical analysisCategorical variables were presented as frequencies and percentages and continuous variables as median and inter-quartile range (percentile 25–percentile 75) or range (minimum–maximum). Missing data were not replaced or estimated.
Univariable analysis used nonparametric tests (Chi-square, Fisher's Exact, Mann–Whitney U) due to the existence of outliers and skewed distributions. Logistic regression models were used to evaluate the prognostic accuracy of CRP24 and BISAP for IM with odds ratios (ORs) being calculated after cross-validation. Discriminative ability was assessed by the area under the receiver–operating characteristic curve (AUC)15 and comparison between AUCs was made using the method described by DeLong et al.16 To compensate for possible shortcomings of AUC analysis in quantifying the added value of a biomarker to an existing test, continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI) parameters were also calculated.17,18
All statistical tests were two-sided and P <0.05 was considered statistically significant. All analyses were performed with IBM SPSS Statistics, Version 19.0 (IBM Corp., North Castle, New York, USA) and Stata/IC 12.0 (Statacorp. LP, College Station, TX, USA).
3Results3.1Patients’ characteristicsA total of 299 patients were initially included. Of this set, only 134 patients (44.8%) had complete data on CRP24, BISAP, and in-hospital final vital status and were subsequently considered.
Median age was 64 years (range, 13–96 years), with 80 men (59.7%). Biliary lithiasis was the most frequent identifiable cause of AP with 59 patients (44.0%). Of a total of 70 patients (52.2%) with complete data on organ failure parameters, 9 (12.9%) developed organ failure for more than 48hours and were classified as having SAP. CECT was performed in 91 patients (67.9%) and in 24 of those (26.4%) pancreatic necrosis was found. Median day for CECT performance was the fourth day of hospital stay (range, 1–26 days). From the 70 patients with Marshall score evaluation, 51 (72.9%) underwent CECT; from the 9 patients with SAP, 4 (44.4%) were diagnosed with pancreatic necrosis. Median length of hospital stay was 9 days (range, 1–80 days). Nine patients (6.7%) died during index hospital stay. Median time to death was 15 days (range, 1–60 days).
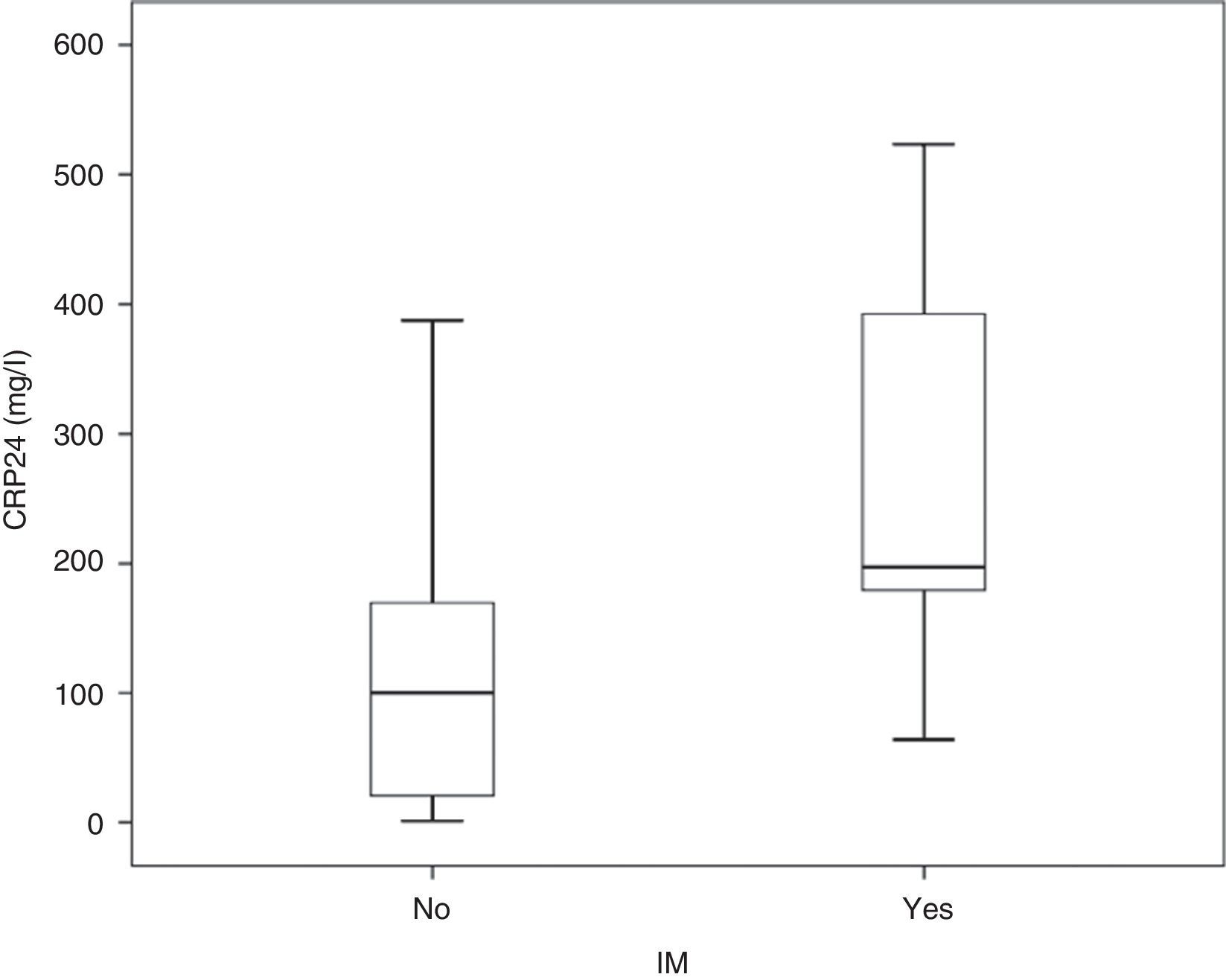
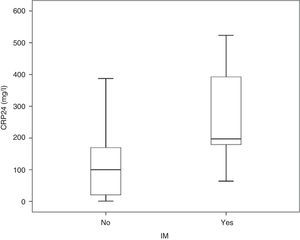
3.2CRP24 prognostic accuracy for IMMedian overall CRP24 level was 104.4 (inter-quartile range, 29.2–191.2mg/l) and its distribution by IM status is shown in Fig. 1. Median CRP24 level was higher in patients who died during index hospital stay (197.2 vs. 100.2, p=0.003). CRP24 univariable OR for IM was 1.11 (95% CI, 1.04–1.17) and corresponding AUC was 0.80 (95% CI, 0.65–0.95). Analysis of the coordinate points of the receiver–operating characteristic curve showed that, from the 46 patients with a CRP24 level lower than 60mg/l, none died (p=0.027). Additionally, from the 9 patients with SAP, only one (11.1%) had a CRP24 level lower than 60mg/l. This CRP24 cutoff point yielded the following sensitivity, specificity, negative predictive value, and positive predictive value for IM: 100% (95% CI, 66.4–100%), 36.8% (95% CI, 28.4–45.9%), 100% (95% CI, 92.3–100%), and 10.0% (95% CI, 4.8–18.5%), respectively.
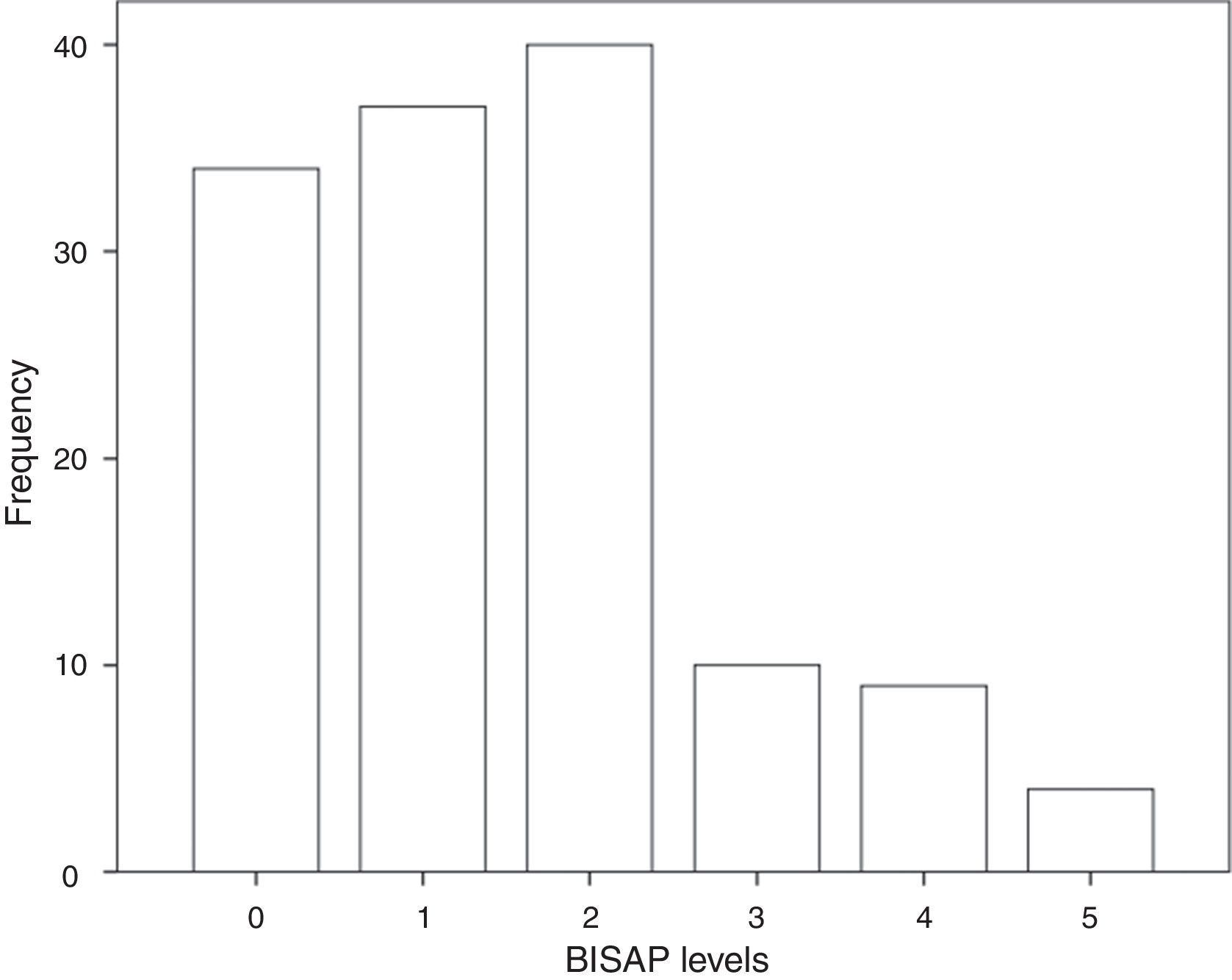
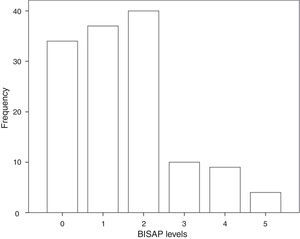
3.3BISAP prognostic accuracy for IMThe distribution of patients by BISAP categories is shown in Fig. 2. Twenty-three patients (17%) had a BISAP score equal or greater than 3. BISAP univariable OR for IM was 2.27 (95% CI, 1.37–3.77) and corresponding AUC was 0.77 (95% CI, 0.59–0.95).
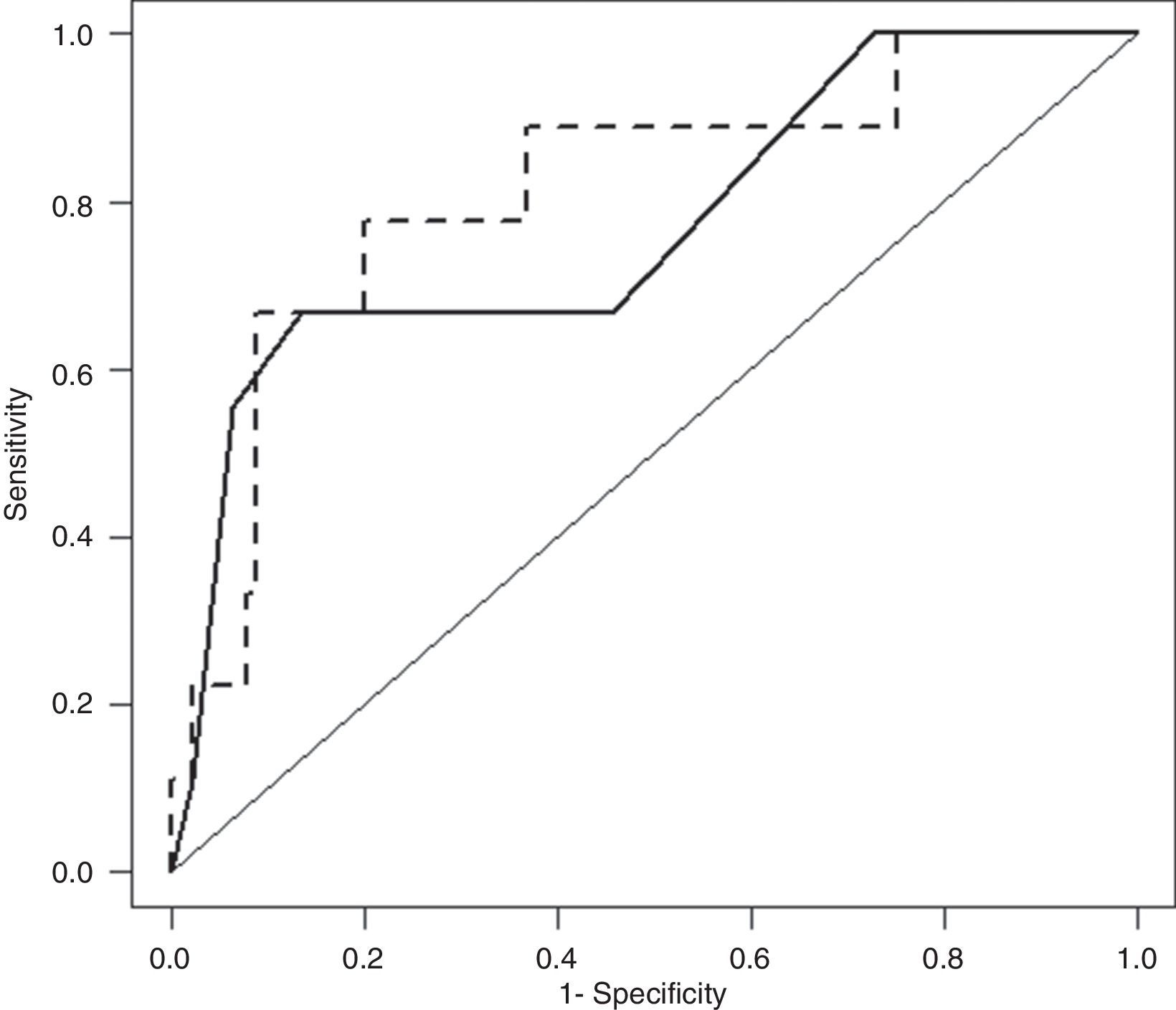
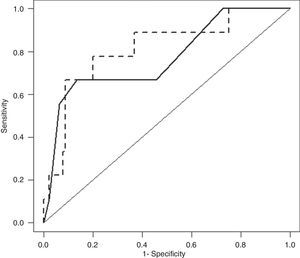
3.4BISAP plus CRP24 model prognostic accuracy for IMAfter cross-validation, BISAP plus CRP24 model ORs for IM were 1.77 (95% CI, 1.05–3.00) for BISAP and 1.09 (95% CI, 1.02–1.16) for CRP24; corresponding AUC was 0.81 (95% CI, 0.65–0.97). AUCs of BISAP alone and BISAP plus CRP24 model were not significantly different (Fig. 3: P=0.62).
Receiver–operating characteristic curve (ROC) of Bedside Index for Severity in Acute Pancreatitis (BISAP; full line) and BISAP plus C-reactive protein at 24hours after hospital admission (CRP24; dashed line) prognostic accuracy for in-hospital mortality (IM). BISAP area under ROC (AUC) of 0.77 (95% CI, 0.59–0.95) and BISAP plus CRP24 AUC of 0.81 (95% CI, 0.65–0.97).
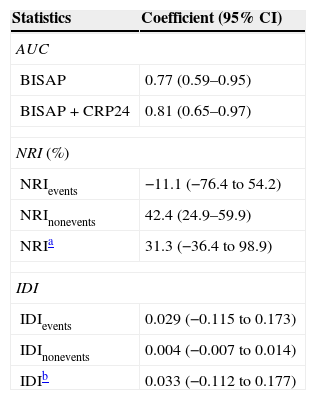
Statistics of BISAP prognostic accuracy for IM improvement with the addition of CRP24 are summarized in Table 1. The overall reclassification of patients (NRI) was mainly due to the reclassification of survivors (non-events), as absolute NRInonevents was greater than NRIevents. In fact, the NRInonevents showed a significant change (42.4%; 95% CI, 24.9–59.9%) which resulted in a positive overall NRI (31.3%; 95% CI, −36.4 to 98.9%), but the IDInonevents was negligible, with a 95% CI straddling zero (0.004; 95% CI, −0.007 to 0.014). This means that the addition of CRP24 to BISAP reduced the calculated risk of IM in about 42% of patients who survived, albeit the overall effect was not statistically significant.
Statistics of BISAP's prognostic accuracy for in-hospital mortality in acute pancreatitis improvement with the addition of CRP24.
| Statistics | Coefficient (95% CI) |
|---|---|
| AUC | |
| BISAP | 0.77 (0.59–0.95) |
| BISAP+CRP24 | 0.81 (0.65–0.97) |
| NRI (%) | |
| NRIevents | −11.1 (−76.4 to 54.2) |
| NRInonevents | 42.4 (24.9–59.9) |
| NRIa | 31.3 (−36.4 to 98.9) |
| IDI | |
| IDIevents | 0.029 (−0.115 to 0.173) |
| IDInonevents | 0.004 (−0.007 to 0.014) |
| IDIb | 0.033 (−0.112 to 0.177) |
CI, confidence interval; AUC, area under receiver–operating curve; BISAP, Bedside Index for Severity in Acute Pancreatitis; CRP24, C-reactive protein at 24hours after hospital admission; NRI, continuous net reclassification improvement; IDI, integrated discrimination improvement; Events, patients that died; Nonevents, patients that survived.
In this study, we found that CRP24 may be a useful biochemical marker for the early risk assessment of patients with AP.
CPR24 showed good individual prognostic accuracy for IM in AP. Moreover, the evaluation of a possible cutoff point with clinical relevance revealed that 60mg/l warranted a negative predictive value of 100%, meaning that patients with AP and a CRP24 level lower than that cutoff point were not at risk of dying from AP or its complications during the following hospital stay. The low prevalence of IM in this population-based study may have precluded the determination of a cutoff point with a high positive predictive value.19
Adding CPR24 to BISAP may have represented an improvement in prognostic accuracy for IM in AP; in this regard, the real gain may have been due to the reclassification of patients at low risk to die from AP or its complications during index hospital stay. Despite these observed tendencies, statistical significance was not achieved.
Overall, this study emphasizes the role of CRP24 in the early risk assessment of patients with AP individually and in combination with BISAP. These results underline the possible advantages of combining simple and accessible tools for clinicians to perform a more reliable early risk assessment of patients with AP, as it has been outlined elsewhere20; this may be done not only by selecting patients at high risk for complications, but also by identifying those within the low risk subgroup that most probably will not develop complications. This approach may help specific institutional policies on AP by improving efficiency in patient management and resources utilization.
There were some methodological limitations to this study. First, this was a retrospective analysis of prospectively collected data from a single center and may have been prone to selection bias and limited generalization. Second, missing CRP24 determinations, which made the final sample size smaller, might have impacted negatively the final prognostic accuracy results for the BISAP plus CRP24 model. The methodological strengths of this study were mainly the community based and consecutive admission of patients and the additional use of NRI and IDI to assess the prognostic performance of BISAP plus CRP24 model. In the future, prospective and properly powered studies should address CRP24 prognostic accuracy in AP and the possibility of combining it with BISAP or other early risk assessment tools available for patients with AP.
In conclusion, there seems to be a role for CRP24 in the early risk assessment of patients with AP, especially by identifying lowest risk patients.
Conflicts of interestNone declared.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.