Since liver fibrosis index (LFI) was developed by Fujimoto et al., real-time elastography (RTE) has become a promising non-invasive technique to assess fibrosis in chronic hepatitis C (CHC). The aims of this study were to compare the diagnostic performance of RTE versus laboratory tests to predict advanced fibrosis (METAVIR scoring system: F≥3) in patients with CHC, using liver biopsy (LB) as the reference standard; and to evaluated the impact of patient anthropometric features on RTE histogram acquisition.
MethodsThis prospective study included 37 patients with CHC scheduled for LB. Aspartate aminotransferase (AST)/alanine aminotransferase (AST) ratio, AST/platelet ratio index (APRI), and Fibrosis-4 index (FIB-4) were calculated from recent (≤6 months) laboratory data. RTE was performed by two independent operators blind to each other’ findings and to LB results, using Hitachi HI-VISION Avius ultrasound system. According to Hitachi RTE software, liver elasticity was evaluated through the LFI. Percutaneous ultrasound-assisted LB was performed in the same day of RTE. All LB specimens were analyzed by an expert pathologist blind to RTE results. Hepatic fibrosis was staged according to METAVIR scoring system. The diagnostic performance of the LFI, AST/ALT ratio, APRI and FIB-4 for predicting advanced fibrosis was assessed using area under receiver-operating characteristic curve (AUROC), sensitivity, specificity, positive-predictive and negative-predictive (NPV) values.
ResultsThirty-seven LB were performed without complications. The distribution according to METAVIR scoring system was F0–1 in 13 patients (35%), F2 in 13 (35%), F3 in 9 (25%) and F4 in 2 (5%). Thirty-seven RTE procedures were performed. Histogram acquisition was successfully achieved in 32 patients (86%). Abdominal wall thickness ≥23mm was associated with no histogram acquisition (p=0.018). Using the optimal cut-off value of 2.38, the AUROC for the LFI was 0.73. The AUROC for the AST/ALT ratio, APRI and FIB-4 were 0.62, 0.79, and 0.82, respectively.
ConclusionsThe LFI calculated by RTE showed a very good diagnostic performance to predict advanced fibrosis in CHC, with remarkable sensitivity and NPV (both 100%).
Após o desenvolvimento do liver fibrosis index (LFI) por Fujimoto K. et al., a elastografia em tempo real tornou-se uma técnica não invasiva promissora na avaliação do grau de fibrose na hepatite C crónica. Os objetivos deste estudo foram comparar a acuidade diagnóstica da elastografia em tempo real com a de testes laboratoriais para predizer fibrose avançada (sistema de estadiamento METAVIR: F≥3) em doentes com hepatite C crónica, utilizando a biópsia hepática como goldstandard; e avaliar o impacto das características antropométricas do doente na aquisição de histograma.
MétodosEste estudo prospetivo incluiu 37 doentes com hepatite C crónica referenciados para biópsia hepática. A razão aspartato aminotransferase (AST)/alanina aminotransferase (AST) (AST/ALT ratio), o índice da razão AST/plaquetas (APRI) e o índice Fibrosis-4 (FIB-4) foram calculados a partir de dados laboratoriais recentes (≤6 meses). O procedimento de elastografia em tempo real foi realizado por dois operadores independentes, cegos entre si e para o resultado da biópsia hepática, utilizando o ecógrafo Hitachi HI-VISION Avius. De acordo com o software de elastografia em tempo real da Hitachi, a elasticidade hepática foi avaliada através do LFI. No mesmo dia da elastografia em tempo real, realizou-se biópsia hepática percutânea após marcação ecográfica do local de punção. Todas as biópsias hepáticas foram analisadas por um anatomopatologista cego para o resultado da elastografia em tempo real. Para estadiamento da fibrose hepática foi utilizada a classificação METAVIR. A acuidade diagnóstica do LFI, AST/ALT ratio, APRI e FIB-4 para predizer fibrose avançada foi avaliada com base nos valores da área abaixo curva recebedora das características dos operadores (AUROC), sensibilidade, especificidade e valores preditivos positivo e negativo.
ResultadosForam realizadas 37 biópsias hepáticas, sem complicações. A distribuição segundo o sistema de estadiamento METAVIR foi F0-1 em 13 doentes (35%), F2 em 13 (35%), F3 em 9 (25%) e F4 em 2 (5%). Foram realizados 37 procedimentos de elastografia em tempo real. A aquisição de histograma foi conseguida em 32 doentes (86%). A espessura da parede abdominal ≥23mm foi um factor independente associado a não aquisição de histograma (p=0,018). Utilizando o cut-off óptimo de 2,38, o valor da AUROC para o LFI foi 0,73. Os valores da AUROC para o AST/ALT ratio, APRI e FIB-4 foram 0,62, 0,79 e 0,82, respectivamente.
ConclusõesO LFI determinado por elastografia em tempo real demonstrou uma performance diagnóstica muito boa para predizer fibrose avançada (F≥3) na hepatite C crónica, com sensibilidade e valor preditivo negativo excepcionais (ambos 100%).
In patients with chronic hepatitis C (CHC), prognosis, surveillance and treatment management are driven largely by the extent of fibrosis.1,2 Percutaneous liver biopsy (LB) has been traditionally considered the gold standard method for the assessment of liver fibrosis in CHC.3 However, it is an invasive and costly procedure associated with patient discomfort and, in rare instances, with serious complications.4 Moreover, LB accuracy is limited by sampling errors and significant intra- and interpathologist variability.5,6 Therefore, in the past few years, research has been focused on the development and evaluation of non-invasive methods for the assessment of liver fibrosis, such as laboratory tests and non-invasive imaging techniques. Laboratory markers, such as the aspartate aminotransferase (AST)/alanine aminotransferase (AST) ratio, AST/platelet ratio index (APRI), and Fibrosis-4 index (FIB-4), have been reported to be useful to predict liver fibrosis. Also, various ultrasound-based techniques have emerged, such as transient elastography, acoustic radiation force impulse (ARFI) imaging, shearwave elastography and real time elastography (RTE).7–37
RTE is a novel non-invasive ultrasound method for measuring tissue elasticity. It uses ultrasound equipment with an embedded elastography module and can be performed during routine ultrasound examination of the liver. During conventional b-mode ultrasonography, RTE can measure the tissue strain response to mechanically induced deformation, providing qualitative (colorimetric) and quantitative readouts (scores). Hitachi medical systems have recently developed a new RTE method that does not require external stress, but instead, liver compression is achieved from rhythmic pulsations of the abdominal aorta and the heart.22–24
Published for the first time in 2010 by Fujimoto et al.,25 the Liver Fibrosis index (LFI) is the most advanced RTE quantitative score to evaluate liver fibrosis and is automatically calculated by an image analysis software. Since then a limited number of studies, almost exclusively outside of Europe, have assessed the diagnostic performance of RTE using the LFI to predict fibrosis in CHC.26–29 According to their results, the LFI has shown a very good diagnostic accuracy for assessing liver fibrosis, particularly to predict advanced fibrosis (METAVIR scoring system F≥3) (area under receiver–operator characteristic curve – AUROC – 0.80–0.84).26–28 This is of great interest because in patients with CHC, the diagnosis of advanced fibrosis is an important indication for antiviral treatment. As with other ultrasound techniques, RTE imaging acquisition appears to be limited by patient anthropometric features.29–32 However, to date, no studies have determined their impact on RTE histogram acquisition.
The aims of this study were, first, to compare the diagnostic performance of RTE (LFI) versus laboratory tests (AST/ALT ratio, APRI and FIB-4) to predict advanced fibrosis (F≥3) in patients with CHC, using LB as the reference standard; and, second, to evaluated the impact of patient anthropometric features, such as body mass index (BMI), waist circumference and abdominal wall thickness, on RTE histogram acquisition.
2MethodsThis was a single-centre prospective study. From November 2012 to October 2014 (24 months), a total of 37 consecutive patients with CHC scheduled for LB were enrolled, after informed consent. Exclusion criteria comprised the following: (i) co-infection with hepatitis B virus or HIV, (ii) history of autoimmune hepatitis or primary biliary cirrhosis and (iii) history of alcohol abuse (≥40mg/day). For each patient demographic data (gender and age) and anthropometric parameters (weight, height, BMI, waist circumference and abdominal wall thickness determined by ultrasound) were recorded. Obesity, overweight and increased waist circumference were defined according to WHO criteria (BMI≥30kg/m2, BMI≥25kg/m2 and waist circumference ≥88cm in men and ≥102mm in women, respectively).35,36
2.1Laboratory tests of liver fibrosisBlood tests (including platelet count, ALT and AST levels) were drawn within 6 months of the RTE date. Based on their results, the AST/ALT ratio, APRI and FIB-4 were calculated for each patient as follows:
AST/ALT ratio=AST level (UI/L)/ALT level (UI/L);
APRI=[AST level (UI/L)/AST laboratory upper limit of normal (UI/L)]/Platelet count (109/L);
FIB-4=[Age (years)×AST level (UI/L)]/[Platelet count (109/L)×√ALT level (UI/L)].
RTE was performed using Hitachi HI-VISION Avius (Hitachi Aloka Medical, Tokyo, Japan) and the EUP-L52 linear probe (3–7MHz, Hitachi Aloka Medical), on the right lobe of the liver through an intercostal space. Liver elasticity was evaluated through the LFI using Hitachi RTE software (Hitachi Aloka Medical), which performs a multiple regression analysis from nine ultrasound image features: the mean of relative strain value (MEAN), standard deviation of the relative strain value (SD), ratio of the blue area in the analyzed region (%AREA), complexity of the blue area (COMP), kurtosis of the strain histogram (KURT), skewness of the strain histogram (SKEW), entropy (ENT), inverse difference moment (IDM), and angular second moment (ASM). LFI is then automatically calculated according to the following formula:
LFI=−0.00897×MEAN−0.00502×SD+0.0232×%AREA+0.0253×COMP+0.775×SKEW−0.281×KURT+2.08×ENT+3.04×IDM+40.0×ASM−5.54.
RTE was performed by two independent operators (Bispo M and Marques S, with 3 years and 3 months of experience, respectively) blind to each other results and blind to liver biopsy staging. A total of three RTE histograms were collected from each patient and the LFI value was calculated for each histogram. For each patient, operators recorded the histogram acquisition, the mean LFI value and the duration of the complete RTE procedure.
2.3Liver biopsyPercutaneous ultrasound-assisted LB was performed in the same day of the RTE, using the same intercostal space chosen for RTE evaluation. A disposable 15 or 17-gauge Menghini needle was used (Hepafix, Braun). All biopsy specimens were fixed in formalin and embedded in paraffin and analyzed by an expert liver pathologist blind to the results of RTE. Hepatic fibrosis was evaluated and staged according to the METAVIR scoring system (F0–F4). The length and the number of portal spaces for each biopsy specimen were recorded.
2.4Statistical analysisDescriptive statistics were produced for demographic, anthropometric, laboratorial, imaging and histologic data.
The Student's t-test was used for comparative analysis between groups. Independent variables associated with unsuccessful histogram acquisition were determined by logistic regression analysis. The cut-off value of the abdominal wall thickness significantly associated with no histogram acquisition (in logistic regression analysis) was determined.
The intra-class correlation (ICC) was used to measure the LFI interobserver agreement.
The diagnostic performance of the LFI, AST/ALT ratio, APRI and FIB-4 for predicting advanced fibrosis (F≥3) was assessed using the area under receiver–operator characteristic curve (AUROC). Sensitivity, specificity, positive (PPV) and negative predictive values (NPV) were also calculated for the LFI, AST/ALT ratio, APRI and FIB-4. The optimal cut-off values for LFI, AST/ALT ratio, APRI and FIB-4 were chosen to maximize the sum of sensitivity and specificity for advanced fibrosis (F≥3).
Statistical analyses were performed using SPSS version 22.0 and results were considered statistically significant when the p value was <0.05.
3ResultsA total of 37 patients were enrolled, 25 men and 12 women and the mean age was 51 years old. The AST/ALT ratio, APRI and FIB-4 were calculated for 36 patients (one patient excluded because of no recent laboratory evaluation).
Thirty-seven RTE procedures were performed. Histogram acquisition was successfully achieved in 32 patients (86%). BMI and abdominal wall thickness were significantly higher in the group of patients with no histogram acquisition than in the group of patients with successful histogram acquisition (BMI: 32.1kg/m2vs 25.7kg/m2, respectively, p=0.001; abdominal wall thickness: 25.4mm vs 16.4mm, respectively, p<0.001). Although the waist circumference was higher in the group of patients in whom histogram acquisition could not be achieved, the difference was not statistically significant (101cm vs 91cm, respectively, p=0.132). For overweight and obese patients, the rate of unsuccessful histogram acquisition was 21% (vs 7%, p=0.178) and 43% (vs 6%, p=0.011), respectively, and for patients with increased waist circumference was 29% (vs 10%, p=0.206). By logistic regression, the cut off value for abdominal wall thickness significantly associated no histogram acquisition was 23mm. For patients with abdominal wall thickness ≥23mm, the rate of unsuccessful histogram acquisition was 57% (vs 3%, p<0.001). By logistic regression analysis, only abdominal wall thickness ≥23mm was associated with unsuccessful histogram acquisition (p=0.018; obesity: p=0.418; increased waist circumference: p=0.924).
The mean LFI value was recorded in all patients with histogram acquisition (n=32). The interobserver agreement for the LFI value was good (ICC=0.633, p=0.01).
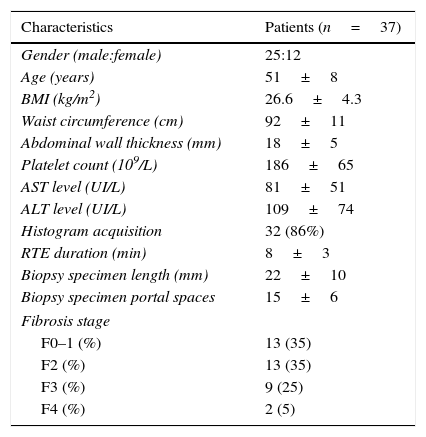
Thirty-seven liver biopsies were performed without any complication (mean biopsy length: 22mm; mean portal spaces number: 15). The distribution according to METAVIR score was: F0 in 1 patient (3%), F1 in 12 patients (32%), F2 in 13 (35%), F3 in 9 (25%) and F4 in 2 (5%). The complete characteristics of all 37 patients are listed in Table 1.
Patient demographics, anthropometrics and laboratory, imaging and histologic data.
| Characteristics | Patients (n=37) |
|---|---|
| Gender (male:female) | 25:12 |
| Age (years) | 51±8 |
| BMI (kg/m2) | 26.6±4.3 |
| Waist circumference (cm) | 92±11 |
| Abdominal wall thickness (mm) | 18±5 |
| Platelet count (109/L) | 186±65 |
| AST level (UI/L) | 81±51 |
| ALT level (UI/L) | 109±74 |
| Histogram acquisition | 32 (86%) |
| RTE duration (min) | 8±3 |
| Biopsy specimen length (mm) | 22±10 |
| Biopsy specimen portal spaces | 15±6 |
| Fibrosis stage | |
| F0–1 (%) | 13 (35) |
| F2 (%) | 13 (35) |
| F3 (%) | 9 (25) |
| F4 (%) | 2 (5) |
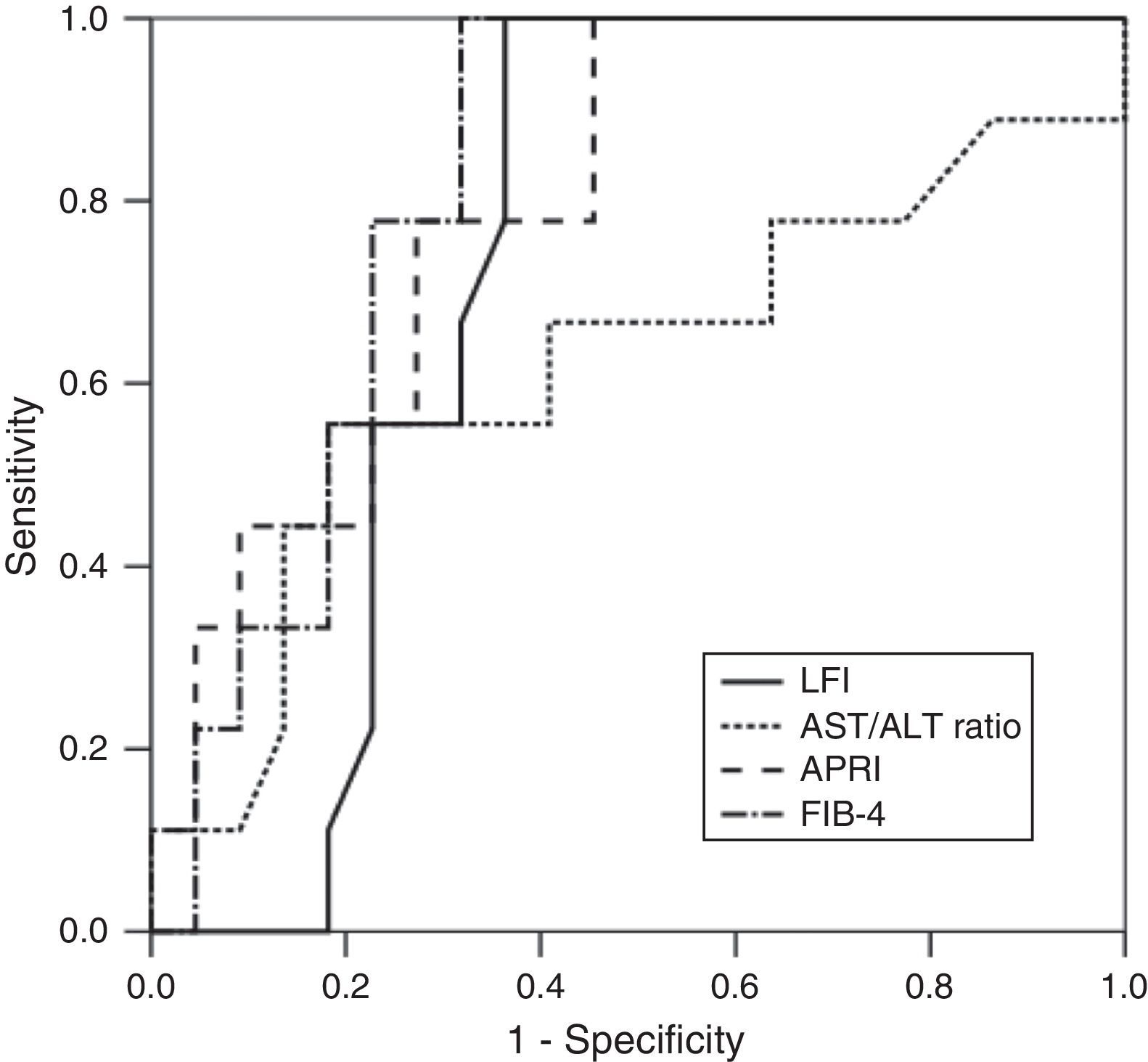
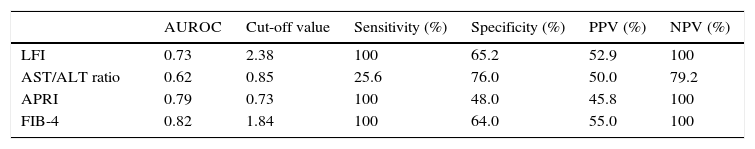
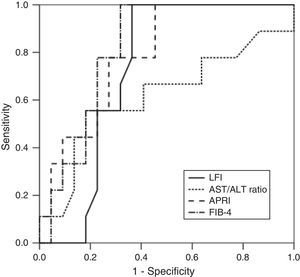
RTE (LFI) showed a very good diagnostic performance for predicting advanced fibrosis (F≥3) in chronic hepatitis C, with excellent sensitivity and NPV (both 100%). These results were comparable to those obtained from laboratory tests (AST/ALT ratio, APRI and FIB-4). The diagnostic performance of the LFI and laboratory tests for predicting advanced fibrosis (F≥3) is presented in Table 2 and Fig. 1.
Diagnostic performance of the LFI and laboratory tests for predicting advanced fibrosis (F≥3).
| AUROC | Cut-off value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|
| LFI | 0.73 | 2.38 | 100 | 65.2 | 52.9 | 100 |
| AST/ALT ratio | 0.62 | 0.85 | 25.6 | 76.0 | 50.0 | 79.2 |
| APRI | 0.79 | 0.73 | 100 | 48.0 | 45.8 | 100 |
| FIB-4 | 0.82 | 1.84 | 100 | 64.0 | 55.0 | 100 |
In recent years, there has been an increasing interest in non-invasive assessment of liver fibrosis in clinical practice, using laboratory tests and imaging techniques, as alternatives to LB. RTE is a non-invasive ultrasound method for the measurement of tissue elasticity with proven accuracy in evaluating liver fibrosis, especially in CHC. It is particularly useful to predict advanced fibrosis (F≥3), one of the main indications for antiviral therapy in patients with CHC.26–34 Very few studies have assessed the diagnostic performance of the LFI to evaluate liver fibrosis in CHC patients. Kim et al.,26 compared the diagnostic accuracy of the LFI, APRI and FIB-4 to predict advanced fibrosis (F≥3) and cirrhosis (F=4) in chronic viral hepatitis B and C. They concluded that only the LFI had a significant power to estimate advanced fibrosis (F≥3) and cirrhosis (F=4) (vs APRI and FIB-4). They also reported that the LFI was more accurate to predict advanced fibrosis (F≥3) in CHC than in chronic hepatitis B patients (AUROC 0.80 vs 0.64). Another study, from Tamaki et al.,27 revealed not only that the LFI was a very good method to predict advanced fibrosis (F≥3) in CHC, but also that its diagnostic accuracy was superior to laboratory tests (AUROC: LFI 0.84, platelet count 0.82, APRI 0.76, and FIB-4 0.80). Similar results were obtained by Ferraioli et al.,28 showing that the LFI was useful to predict advanced fibrosis (F≥3) in CHC (AUROC 0.80). The only portuguese study ever published to date concerning the use of RTE in CHC for liver fibrosis assessment was performed by Magalhães et al.37 This study included patients with chronic viral hepatitis B and C (5 patients with CHC and 10 patients with chronic hepatitis B) and showed that LFI had a moderate correlation with METAVIR histologic scoring system (Spearman's 0.56, p=0.03).
In our prospective study, RTE showed a very good diagnostic performance for predicting advanced fibrosis (F≥3) in patients with CHC. Using the optimal cut-off value of 2.38, the AUROC for the LFI was 0.73. These results were similar or better than those of the laboratory tests. In fact, LFI diagnostic performance was superior to AST/ALT ratio (AUROC 0.73 vs 0.62). Comparing to APRI (AUROC 0.79) and FIB-4 (AUROC 0.82), the LFI showed an identical diagnostic accuracy with excellent sensitivity and NPV (both 100%). The results obtained in our study were similar to those published in the literature.
Transient elastography, commonly known as Fibroscan, has been widely used to evaluate liver stiffness and is established in clinical practice to assess liver fibrosis. However, transient elastography cannot be performed in patients with narrow intercostal spaces, ascites or severe obesity and is influenced by the presence of liver inflammation and steatosis.24 Unlike TE, RTE is performed during conventional b-mode ultrasonography, therefore, allowing identification of a suitable location for liver fibrosis assessment. Still, RTE histogram acquisition may be limited by narrow intercostal spaces, poor ultrasound tissue penetration and poor cardiac and aortic movement.24 Poor ultrasound penetration is usually reported in patients with thick abdominal wall or obesity.29–32 According to the few studies that did not exclude patients with obesity or with a thick abdominal wall, the RTE histogram acquisition rate ranges from 73.5% to 84.9%.29,30 In our study, all patients were included, despite their BMI and abdominal wall thickness (51% overweigh and 19% obese patients and 19% patients with increased waist circumference). RTE histogram acquisition was achieved in 86% of patients. In the group of patients with unsuccessful histogram acquisition, the BMI and abdominal wall thickness were significantly higher than in the group of patients with successful histogram acquisition (p=0.001 and p<0.001, respectively). Also in patients with obesity or with abdominal wall thickness ≥23mm the rate of unsuccessful histogram acquisition was significantly reduced (p=0.011 and p<0.001). However, by logistic regression analysis, the only anthropometric feature associated with unsuccessful histogram acquisition was abdominal wall thickness ≥23mm (p=0.018). To our knowledge, no other study has evaluated the impact of patient anthropometric features on RTE histogram acquisition.
Our study had some limitations. The number of patients, particularly with advanced fibrosis, was small. Also, a second liver pathologist should have evaluated each LB to minimize bias related to interobserver variability. However, this prospective single centre study supports the promising results of RTE in the recent literature.
5ConclusionIn conclusion, RTE is a promising non-invasive, reproducible and easy to use technique to assess liver fibrosis in CHC. The LFI calculated by RTE shows a very good diagnostic performance to predict advanced fibrosis (F≥3) in CHC, with similar results to laboratory tests.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.