Pancreatic cancer is one of the digestive cancers with the poorest prognosis, so an early and correct diagnosis is of utmost importance. With the development of new therapeutic options an accurate staging is essential. Endoscopic ultrasonography (EUS) has a major role in all stages of the management of these patients.
EUS has a high accuracy in the diagnosis of pancreatic adenocarcinoma and the possibility to perform fine-needle aspiration/biopsy (FNA/FNB) increases the diagnostic yield of EUS. There is still no consensus on the several technical aspects of FNA, namely on the rapid on-site evaluation (ROSE), the diameter and type of needle, the number of passes and the use of stylet and suction. Contrast-enhanced EUS (CE-EUS) and EUS elastography (EUS-E) have been used in recent years as an adjunct to EUS-FNA. Given the higher sensitivity of these techniques a negative cytology by EUS-FNA should not exclude malignancy when CE-EUS and/or EUS-E are suggestive of pancreatic neoplasia. EUS remains one of the main methods in the staging of pancreatic adenocarcinoma, namely to further evaluate patients with non-metastatic disease that appears resectable on initial imaging.
EUS is crucial for an accurate preoperative evaluation of pancreatic cancer which is essential to choose the correct management strategy. The possibility to obtain samples from suspicious lesions or lymph nodes, by means of EUS-guided fine-needle aspiration as well as the use of contrast-enhanced and elastography, makes EUS an ideal modality for the diagnosis and staging of pancreatic cancer.
O adenocarcinoma do pâncreas é uma das neoplasias digestivas com pior prognóstico, sendo fundamental um diagnóstico correto e precoce. Com o desenvolvimento de novas opções terapêuticas é essencial um estadiamento preciso. A ecoendoscopia apresenta um papel relevante em todas as fases da abordagem destes doentes.
A acuidade da ecoendoscopia no diagnóstico de adenocarcinoma pancreático é elevada. A possibilidade de realização de punção aspirativa aumenta o potencial diagnóstico, não havendo ainda consenso relativamente a vários aspetos da técnica, nomeadamente em relação à presença de citopatologista durante o procedimento, tipo e diâmetro de agulha, número de passagens e utilização estilete e aspiração. Nos anos recentes tem-se assistido à utilização de ecoendoscopia com contraste (CE-EUS) ou elastografia (EUS-E) como adjuvante da ecoendoscopia. Estas técnicas apresentam elevada sensibilidade e uma citologia negativa não exclui malignidade se a CE-EUS e/ou EUS-E apresentarem características sugestivas de malignidade. A ecoendoscopia mantém-se um dos principais métodos no estadiamento do adenocarcinoma pancreático, em especial na presença de doença não metastática que aparenta ser ressecável noutras técnicas imagiológicas.
A ecoendoscopia é fundamental na avaliação pré-operatória do adenocarcinoma pancreático e na definição da correta estratégia de tratamento. A possibilidade de obtenção de amostras de lesões ou adenopatias suspeitas, através de punção aspirativa, assim como a utilização de contraste e elastografia, fazem da ecoendoscopia uma técnica ideal no diagnóstico e estadiamento.
Pancreatic neoplasia, particularly exocrine pancreatic cancer, is a common cause of cancer-related death and is second only to colorectal cancer as a cause of digestive cancer-related death.1 Due to its aggressive behavior and late presentation, this disease has a poor prognosis with a very low five-year survival rate. Surgical resection offers the only potential cure, but only 10–15% of patients are candidates for pancreatectomy. Because of these reasons it is of utmost importance to do an early and correct diagnosis as well as the most precise staging before providing therapeutic options.
This paper will discuss the role of endoscopic ultrasound in the diagnosis and staging of pancreatic cancer.
2How good is EUS guided FNA in the diagnosis of solid pancreatic lesions?EUS-FNA is technically successful in 90–95% of procedures, with high sensitivity and specificity for malignancy. This is confirmed by a recent meta-analysis that showed sensitivity of 85% and specificity of 98%.2 When patients with atypical or suspicious cytology were reclassified as positive for malignancy, the sensitivity increased to 91%, with a slight decrease in specificity. The negative predictive value of 64% reflects the important fact, that a negative result of EUS-FNA does not exclude malignancy with absolute certainty.
In spite of this excellent accuracy, there is some discussion if all pancreatic lesions should be sampled, particularly in patients who are good surgical candidates with lesions that appear resectable. This is mainly due to the fact that EUS-FNA can be hampered by the presence of stenosis or other anatomical factors; its accuracy appears to be diminished in the background of chronic pancreatitis3 and there is some concern with needle tract seeding, even if this last aspect has only been anecdotally reported.4 On the other hand, performing EUS-FNA to all patients may be advantageous since it allows ruling out other types of malignancy,5 assists with surgical planning and confirms the diagnosis in patients who want verification prior to surgery.
3ROSE, needle, suction and passesSeveral methodological features can contribute to the success of EUS guided FNA/FNB. These include the rapid on-site evaluation (ROSE), the type of needle, the number of passes and the use of stylet and suction.
The role of ROSE and its relevance in EUS-FNA has been described in a guideline published by the European Society of Gastrointestinal Endoscopy.4 Its use is considered controversial even if a recent meta-analysis6 and various studies have shown an improvement in adequacy rate.7,8 This is mainly due to the fact that ROSE only seems to improve the adequacy rate when it is below 90% and many recent studies have reported adequacy rates superior to 90% without the use of ROSE, indicating that, in high-volume centers, ROSE may not be indispensable. A valid alternative to the presence of a cytopathologist is the preparation of slides by a cytotechnician. This was retrospectively evaluated by Alsohaibani et al and the final diagnosis was higher in the group with on-site cytotechnologists preparing the slides (77% vs. 53%) and providing initial consideration about specimen adequacy.8
Unfortunately not all EUS-FNA performing units have access to ROSE. In such cases it is of utmost importance to optimize the FNA technique, namely the type of needle and the number of passes. The two most common needles used to obtain cytology specimens are the 22- and 25-gauge needles. Several studies, including 4 randomized controlled trials, have compared their cytological yield in the diagnosis of pancreatic malignancy.9–12 In general, these studies have not found significant differences between both needles, but suggest that 25 gauge could perform better in exceedingly fibrotic lesions and those located in the head or uncinate process. In a recent meta-analysis, that included 8 studies with 1292 patients, the sensitivity of the 25-gauge needle was higher than the 22-gauge needle (93% vs. 85%) with similar specificity.13
Previous studies, using the 22-gauge needle, showed that sensitivity improved with an increase in the number of passes.14 So, it was suggested that in absence of ROSE at least 7 passes should be performed in pancreatic lesions. However recent advances in the EUS-FNA technique and the later development of the 25-gauge needle should probably lead to a re-evaluation of this recommendation. In fact, a recent prospective study using the 25-gauge needle suggested that 4 passes may be sufficient to provide adequate cellularity in the absence of ROSE.15
It was previously believed that the use of a stylet during EUS-FNA prevents clogging of the needle lumen by GI wall tissue, which could limit the ability to aspirate cells from the target lesion. Hence, the use of the stylet is routine practice for some endosonographers, even though its insertion and removal is cumbersome and time consuming. Several studies, including 3 randomized controlled trials have addressed this subject.16–18 In none of these studies the use of stylet was associated with better specimen adequacy or improved diagnostic yield for malignancy. In the study by Sahai et al there was inclusive a better quality of the specimen and diminished bloodiness without the stylet.16 The stylet can also be used to express the aspirate and this was evaluated in a recent randomized controlled trial. There were no differences between reinsertion of the stylet or air flushing with regard to the number of diagnostic samples, overall accuracy, cellularity and air-drying artifact.19 Altogether, these data does not support the use of a stylet during EUS-FNA.
A more controversial topic is the use of suction during EUS-FNA since quality data from appropriate trials are scarce. A randomized controlled trial by Lee et al19 that focused on 81 patients with solid pancreatic lesions revealed a higher accuracy in the group where suction was used (85.2% vs. 75.9%) despite the increased bloodiness. This trial used both the 22- and 25-gauge needle which can be a confounding factor. The theoretical advantage of suction in pancreatic lesions is to improve sample cellularity since pancreatic adenocarcinoma tends to be a fibrotic not densely cellular lesion.
In certain pancreatic lesions, such as lymphoma, neuroendocrine tumors (NET) or autoimmune pancreatitis, it might be helpful to perform a histological analysis. The techniques and methods to do so are beyond the scope of this paper and those interested are referred to an excellent review by Panic et al.20 In a hand stroke, adequate histologic samples can be obtained using 19- and 22-gauge ProCore or regular needles. On the other hand, the use of the EUS-Trucut needle has been hampered by technical difficulties and low yield in the transduodenal route. Also, the 25-gauge needle does not seem to be adequate to collect core sample and the same applies to the more recent 25-gauge ProCore needle, as shown by a recent study in which a histologic core was found in only 32% of 50 patients with solid pancreatic lesions.21 Interestingly, combining histologic and cytological analysis may improve overall sensitivity.22
In summary, if one feels the need to improve the results of EUS-FNA in solid pancreatic lesions, the first attitude should probably be to contact the pathology department in order to arrange an in-room cytopathologist or technician. If that is not possible, a 25-gauge needle should be used, doing at least 4 passes with suction. The acquisition of a fragment for histological analysis should be considered, whether using a dedicated FNB needle or a conventional one. Lastly, if EUS-FNA fails, repeating the procedure should be considered, since it has been demonstrated that this will improve the diagnostic rate.
4Contrast-enhanced EUS and EUS-elastographyEUS provides high-resolution images of the pancreas and it is considered one of the most accurate methods for the diagnosis and staging of pancreatic neoplasia.23,24 Even with the addition of tissue sampling via FNA, some limitations exist in the differentiation of benign from malignant solid pancreatic lesions. EUS–FNA may be technically demanding and multiple punctures may be needed to obtain adequate cytological samples, which may be associated with few but not insignificant complications such as acute iatrogenic pancreatitis, bleeding, and infection.25,26 In addition, EUS-FNA provides false-negative results up to 20–40% for malignancy, especially in patients with underlying chronic pancreatitis.2,27 Recent innovations in EUS that intended to address these limitations include contrast-enhanced EUS (CE-EUS) and EUS elastography (EUS-E).28–31
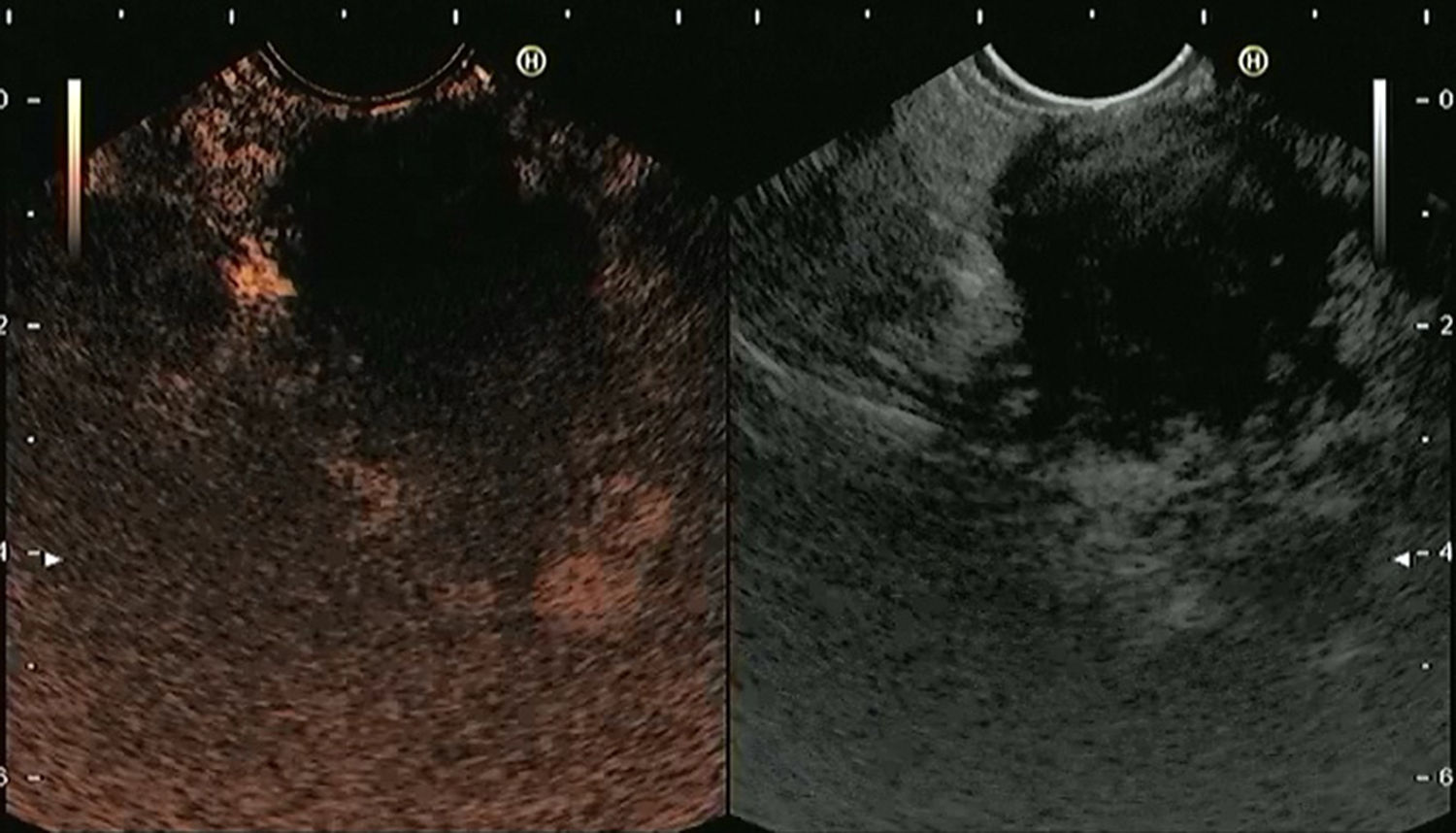
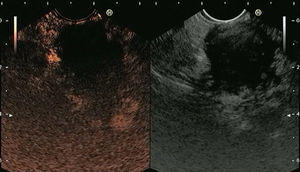
4.1Contrast-enhanced EUS (CE-EUS)CE-EUS allows real-time evaluation of the microvasculature of the lesion and the surrounding parenchyma through the use of an intravenous ultrasound contrast agent (UCA)31–33 (Fig. 1). Second-generation UCAs, such as Sonovue and Sonazoid, are composed of a microsphere containing an inert gas.32 Compared with first-generation UCAs they have higher stability allowing real-time vascular images for a prolonged time.32 CE-EUS can be performed by using color or power Doppler as a generic signal intensifier or, more appropriately, by using a dedicated contrast harmonic-enhanced EUS.34
Screen capture of video sequence of contrast-enhanced harmonic endoscopic ultrasonography (left panel). The video sequence also included a B-mode standard EUS image of the lesion of interest (right panel). This lesion has a hypo-enhancement pattern suggestive of pancreatic adenocarcinoma (this was confirmed by EUS-FNA).
The evaluation of CE-EUS images can be done qualitatively or quantitatively. In qualitative CE-EUS the enhancement of the lesion is compared with that of the surrounding parenchyma and classified as non-enhancement, hypo-enhancement, iso-enhancement and hyper-enhancement.35 This evaluation is easy to perform but it is very operator dependent. To increase interobserver agreement for CE-EUS recent studies used quantification methods to obtain objective results, such as uptake ratio index36 and time intensity curve (TIC) analysis.37,38
First reports have shown that solid pancreatic lesions may have 3 main enhancement patterns: hypo-, iso- and hyper-enhancement.14–16 Pancreatic adenocarcinoma most commonly shows a hypo-enhancement pattern.14,15 Pseudotumoral chronic pancreatitis is usually iso-enhanced or hyper-enhanced,38 while neuroendocrine tumor (NET) is commonly hyper-enhanced.39 Recently, three groups have prospectively evaluated the performance of qualitative CE-EUS in characterizing solid pancreatic lesions.39–41 Adenocarcinoma was diagnosed as a hypo-enhanced mass with high sensitivity (89–95%) and specificity (64–89%). A hyper-enhanced pattern of the mass on CE-EUS was 88–94% predictive of a lesion other than adenocarcinoma in the two first series, whereas a hyper-enhanced pattern diagnosed neuroendocrine tumor with a sensitivity and specificity of 79% and 99%, respectively, in the last series.
The performance of quantitative CE-EUS in the diagnosis of pancreatic adenocarcinoma has been evaluated in three studies.36–38 Seicean et al36 showed that the uptake ratio index was significantly lower for pancreatic adenocarcinoma than for pseudotumoral chronic pancreatitis, with a sensitivity of 80% and a specificity of 91%. Using time intensity curves (TICs), Matsubara et al37 revealed that the echo-intensity reduction rate from its peak at 1min was greatest in pancreatic adenocarcinoma, followed by chronic pancreatitis, autoimmune pancreatitis and NET. CE-EUS in combination with TIC increased the sensitivity, specificity and accuracy to 96%, 93%, and 95%, respectively. Gheonea et al38 confirmed statistically significant differences in TIC values when comparing pseudotumoral chronic pancreatitis and pancreatic adenocarcinoma.
In a recent meta-analysis,42 the pooled sensitivity of CE-EUS for the differential diagnosis of pancreatic adenocarcinoma was 94% and the specificity was 89%. The AUROC was 0.973. Besides characterization of pancreatic lesions in general, CE-EUS may be particularly useful for detection and characterization of small (<2cm)39 or solid pancreatic lesions that are associated with biliary stents or chronic pancreatitis.41 CE-EUS has also been shown to be useful for preoperative localization of NET's,43 differentiation of mural nodules in intraductal papillary mucinous neoplasms and detection of malignant transformations44,45 and for predicting and monitoring response of pancreatic cancer to chemotherapy.46,47
CE-EUS has however some limitations. As the component shell and excipients of the UCAs are derived from macromolecular substances, there is a risk of allergic reactions. In addition, UCAs should not be used in patients with unstable ischemic heart disease.48 The differential diagnosis between pancreatic adenocarcinoma and pseudotumoral chronic pancreatitis may also be particularly challenging since, similarly to pancreatic adenocarcinoma, pseudotumoral chronic pancreatitis may show hypo-enhancement at CE-EUS depending on the degree of inflammation and fibrosis.35,39–41
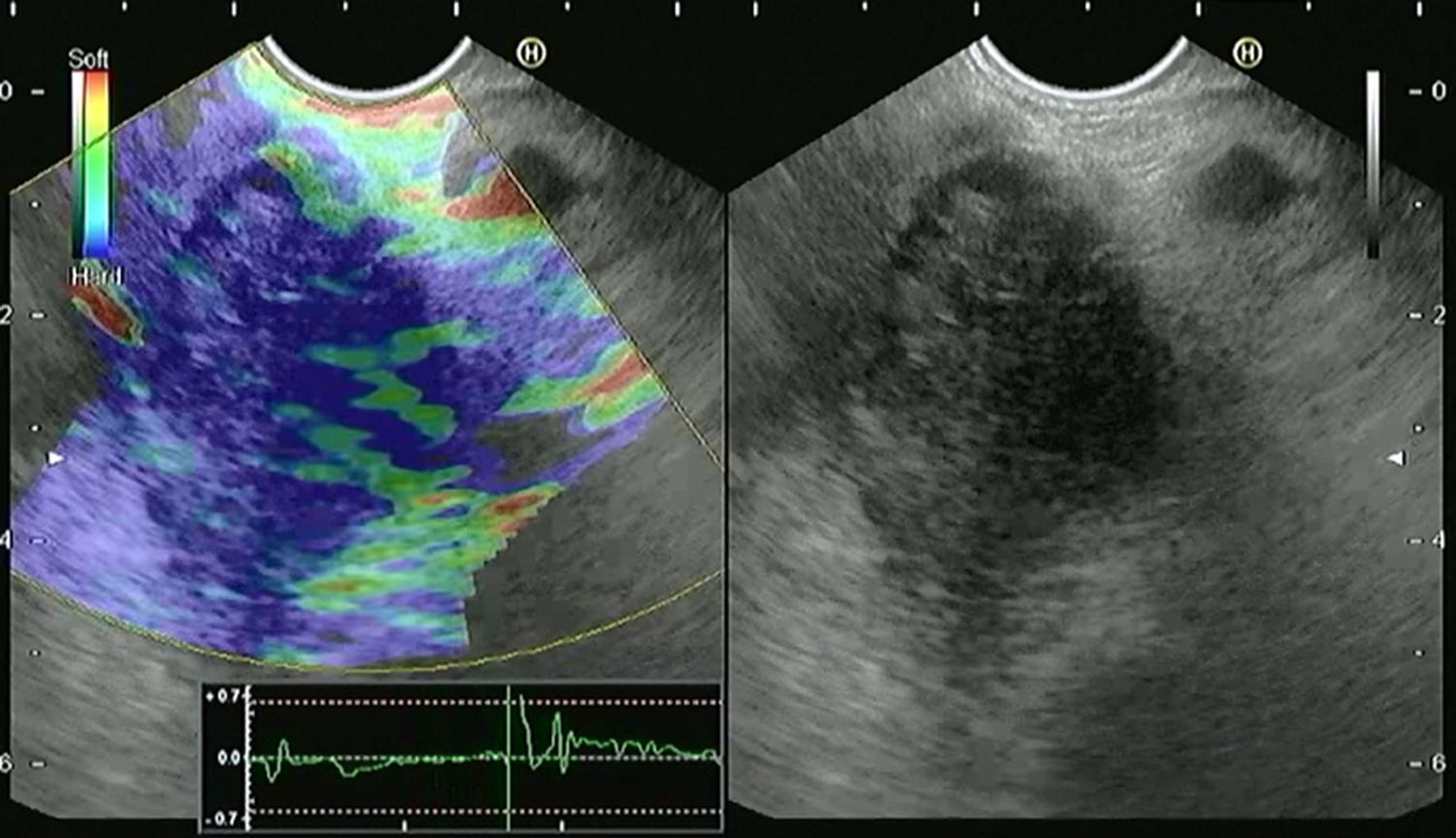
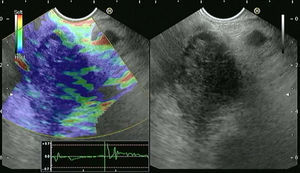
4.2EUS-elastography (EUS-E)The basis for elastography is the fact that many different pathologic processes, including inflammation, fibrosis, and cancer, induce alterations in tissue stiffness. EUS-E evaluates tissue stiffness through the application of slight compression to the targeted tissue and recording the resulting tissue displacement in the examined field.49 The images are obtained in real time, being overimposed as a transparent color overlay on the usual EUS gray-scale images (Fig. 2). Elasticity is depicted using a color map, wherein hard tissue is shown in dark blue, medium hard tissue in cyan, tissue with intermediate hardness in green, medium soft tissue in yellow and soft tissue in red. The region of interest (ROI) for the elastographic evaluation is manually selected. Optimally, the ROI includes the whole target lesion, as well as some surrounding tissues for reference. The first generation of EUS elastography allowed only qualitative evaluation. Today, the second generation also allows quantitative evaluation of tissue stiffness.50
Screen capture of video sequence of endoscopic ultrasound elastography (left panel). The video sequence also included a B-mode standard EUS image of the lesion of interest (right panel). This lesion has a heterogeneous blue-predominant pattern suggestive of pancreatic adenocarcinoma (this was confirmed by EUS-FNA).
Qualitative elastography involves the evaluation of the pattern of the elastogram such as the major color tone and the heterogeneity of the color tones.51 This method is highly operator dependent and so different image analysis techniques were developed to evaluate the characteristics of a lesion in a quantitative manner. These include the hue histogram and the strain ratio. The hue histogram is a graphical representation of the color distribution (hues) in a selected image field. Hue histograms are based on the qualitative EUS elastography data for a manually selected ROI within the standard elastography image.52 The histogram analysis can even be taken one step further, by training artificial neural networks to make the distinction between benign and malignant histograms.52 Strain ratio compares the average strain in two different areas of the ROI.50 Using the standard qualitative EUS elastographic image, the operator selects two no overlapping areas inside the ROI: The lesion (area A) and the reference zone (area B; usually adipose or connective tissues that have little to no inter-individual hardness variance). The B/A quotient obtained represents the SR.53
First qualitative EUS-E experiences were published by Giovannini et al who implemented a qualitative scoring system based on the lesions color pattern; hard, mostly blue lesions were classified as malignant.54 Sensitivity and specificity were, respectively, 100% and 67%. A refining of this system created a five-score classification that achieved overall accuracy of 89.2% in a multicenter study,55 with sensitivity and positive predictive value (PPV) being over 90%. Iglesias-Garcia et al have described four similar patterns: homogeneous green for normal pancreas; heterogeneous, green-predominant for inflammatory pancreatic masses; heterogeneous, blue-predominant for pancreatic malignant tumors; and homogeneous blue for pancreatic neuroendocrine malignant lesions. This classification brought an almost 5% increase of accuracy, up to 94.0%.56 More recently, studies have been published using quantitative EUS elastography. Saftoiu et al used hue histograms in two different studies27,51 obtaining good sensitivities (93.4% and 91.4%) but varying specificities (66.0% and 87.9%). Overall accuracies in these studies were only slightly lower than the ones obtained by Itokawa et al57 and Iglesias-Garcia et al53 using a strain ratio protocol. The same strain ratio protocol was also used by Dawwas et al in a large single-center prospective study58 achieving a sensitivity of 100.0%, poor specificity of 16.7% and an overall accuracy of 86.5%.
The subject of elastographic differential diagnosis was also covered in a recent meta-analysis.59 In this meta-analysis the pooled sensitivity, specificity, and diagnostic odds ratio of EUS-E distinguishing benign from malignant solid pancreatic masses were 95%, 67% and 42.28, respectively. The AUROC was 0.9046.
EUS-E has however some intrinsic limitations.53,56 Recent studies have indicated that elastography for the diagnosis of inflammatory masses may be particularly difficult in patients with advanced chronic pancreatitis since the inflammatory mass may produce a heterogeneous blue-predominant pattern, which is similar to that observed in pancreatic malignancies.27 Color pattern as a qualitative pattern analysis provides a subjective determination that is associated with intraobserver and interobserver variability.60 The quantitative method of hue histogram and strain ratio might be helpful, but there were still some limitations. Hue histogram and strain ratio could be hampered by the selection of the images.61,62 Although dynamic analysis of EUS-E for hue histogram could eliminate the selection bias, artifacts may be induced by the surrounding presence of very low or hard density and stiffness tissues.61 There is also a wide range of cut-offs for strain ratio within different studies, all of which were determined after data collection, and no study prospectively determined them.38
5Point of viewAlthough CE-EUS and EUS-E will hardly replace EUS-FNA, they can be efficient complementary tools to differentiate solid pancreatic lesions, mainly by reducing the number of false negatives of EUS-FNA. Given the higher sensitivity of CE-EUS and EUS-E a negative cytology by EUS-FNA should not be considered as a benign lesion when CE-EUS and/or EUS-E are suggestive of pancreatic adenocarcinoma. In these cases further cytological samples or surgery should be considered instead of follow-up. This has been evaluated in two studies. Napoleon et al40 observed that 4 out of 5 adenocarcinomas with false-negative EUS-FNA findings were hypo-enhanced at CE-EUS. Additionally, CE-EUS revealed that all ductal carcinomas with false-negative EUS-FNA results had hypo-enhancement in the study by Kitano et al.39 In addition, CE-EUS and EUS-E could guide FNA to choose an optimal puncture site to improve the diagnostic accuracy. This has been evaluated by Kitano et al63 who performed FNA guided by CE-EUS on 39 patients with pancreatic tumors. No viable cells were found in areas with no enhancement, whereas adequate samples were obtained from 80% of sites with a heterogeneous vascular pattern and in all cases from areas with a homogeneous pattern.
Based on the available data, we believe CE-EUS and EUS-E are ready for use in clinical practice as complementary tools for EUS-FNA. Nonetheless these techniques should be used only by endosonographers who have received proper training, ideally in reference centers.
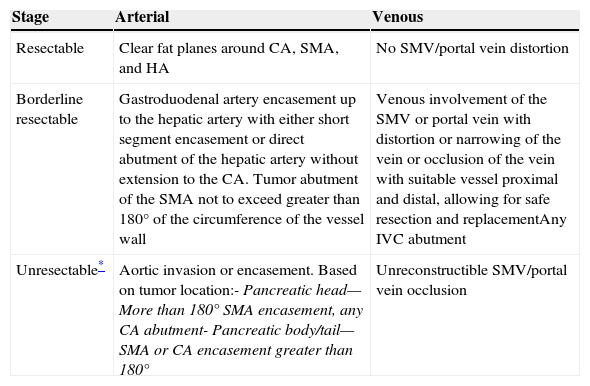
6EUS in the staging of pancreatic cancerTreatment of pancreatic cancer is an evolving field and an accurate staging becomes increasingly important. The staging of pancreatic cancer is based on the tumor-node-metastasis (TNM) system (Table 1). From a clinical point of view, the goal of preoperative staging is to identify 3 different stages of pancreatic cancer and its treatment: (1) resectable tumor that should be referred directly for curative surgery; (2) locally advanced/borderline tumor that can be considerer to neoadjuvant chemoradiation and (3) metastatic/unresectable tumor that should be directed to palliative chemotherapy. Current practice in expert centers has evolved to surgical excision of pancreatic adenocarcinoma regardless of extension to adjacent structures (such as transverse colon, stomach, spleen, adrenal gland and kidney), since these structures can be resected along with the primary tumor. On the other hand, the resectability status depends greatly on vascular invasion, and its criteria were recently reviewed (Table 2).64
TNM staging system for pancreatic adenocarcinoma.
| Primary tumor (T) |
| TX: Primary tumor cannot be assessed |
| T0: No evidence of primary tumor |
| Tis: Carcinoma in situ |
| T1: Tumor limited to the pancreas, ≤2cm in greatest dimension |
| T2: Tumor limited to the pancreas, >2cm in greatest dimension |
| T3: Tumor extends beyond the pancreas but without involvement of the celiac axis or the superior mesenteric artery |
| T4: Tumor involves the celiac axis or the superior mesenteric artery (unresectable primary tumor) |
| Regional lymph nodes (N) |
| NX: Regional lymph nodes cannot be assessed |
| N0: No regional lymph node metastasis |
| N1: Regional lymph node metastasis |
| Distant metastases (M) |
| M0: No distant metastasis |
| M1: Distant metastasis |
| Stage 0–Tis, N0, M0 |
| Stage IA–T1, N0, M0 |
| Stage IB–T2, N0, M0 |
| Stage IIA–T3, N0, M0 |
| Stage IIB–T1, N1, M0 or T2, N1, M0 or T3, N1, M0 |
| Stage III–T4, any N, M0 |
| Stage IV–any T, any N, M1 |
(AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010. p. 241).
Criteria for pancreatic adenocarcinoma staging.
| Stage | Arterial | Venous |
|---|---|---|
| Resectable | Clear fat planes around CA, SMA, and HA | No SMV/portal vein distortion |
| Borderline resectable | Gastroduodenal artery encasement up to the hepatic artery with either short segment encasement or direct abutment of the hepatic artery without extension to the CA. Tumor abutment of the SMA not to exceed greater than 180° of the circumference of the vessel wall | Venous involvement of the SMV or portal vein with distortion or narrowing of the vein or occlusion of the vein with suitable vessel proximal and distal, allowing for safe resection and replacementAny IVC abutment |
| Unresectable* | Aortic invasion or encasement. Based on tumor location:- Pancreatic head—More than 180° SMA encasement, any CA abutment- Pancreatic body/tail—SMA or CA encasement greater than 180° | Unreconstructible SMV/portal vein occlusion |
CA- celiac axis; HA- hepatic artery; IVC- inferior vena cava; SMA/SMV- superior mesenteric artery/vein.
The presence of distant metastasis, including metastases to lymph nodes beyond the field of resection, renders the patient unresectable irrespective of the type of vascular involvement.
(Al-Hawary et al Gastroenterology 2014; 146: 291–304, NCCN practice guidelines for Pancreatic Adenocarcinoma, version 1.2015; http://www.nccn.org).
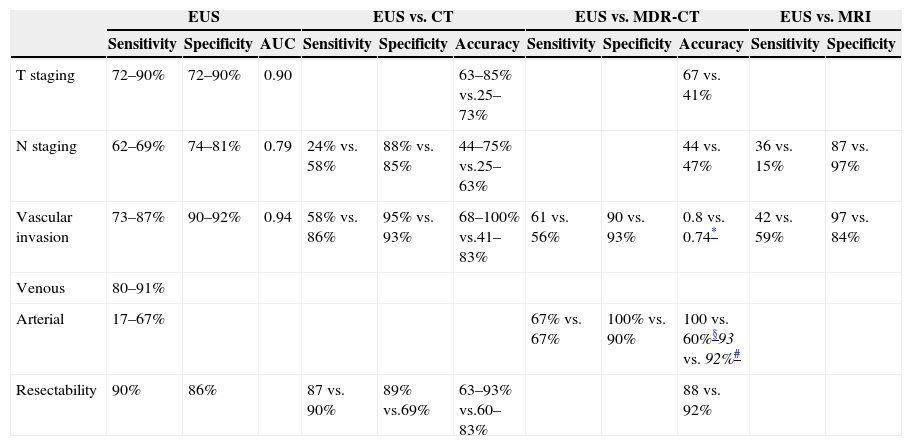
Multiple imaging modalities are available for staging pancreatic cancer, and there is no evidence-based consensus on the optimal preoperative imaging assessment of these patients. Several studies have been published assessing the accuracy of EUS for staging pancreatic cancer, and two recent meta-analyses evaluated the performance characteristics of EUS in this setting. In the first one, including 29 studies with a total of 1330 patients, the estimated pooled sensitivity and specificity was 69% and 81% for N staging; 85% and 91% for vascular invasion and 90% and 86% for resectability.65 In the second meta-analysis, including 20 studies with 726 patients, the estimated pooled sensitivity, specificity and AUROC were 72%, 90% and 0.90 for early and intermediate disease (T1 and T2); 90%, 72% and 0.90 for advanced disease (T3 and T4); 62%, 74% and 0.79 for N staging and 87%, 92% and 0.94 for vascular invasion.66 It seems that EUS has lower accuracy, mostly less sensitivity, in staging the N status. In this sense, the use of EUS-FNA in addition to diagnostic EUS has the potential to increase the accuracy of nodal staging up to 85%.67,68 At present, regarding EUS technologies, there is not enough evidence to show a difference in the accuracy between linear and radial probes.65,69 Keeping in mind the possible need to perform EUS-FNA as a diagnostic and staging adjuvant, the use of a linear probe could be more suitable.
6.2Resectability and vascular invasionAs previously stated, the evaluation of vascular invasion is determinant for assessing tumor resectability. Several EUS criteria have been proposed to predict vascular invasion, however the most reliable are70: (1) peripancreatic venous collaterals in an area of a mass that obliterates the normal anatomic location of a major vessel; (2) tumor within the vessel lumen and (3) abnormal vessel contour or irregular wall with loss of the vessel-parenchymal sonographic interface. Puli et al69 published a meta-analysis on the diagnostic accuracy of EUS for vascular invasion, in which the pooled sensibility was 73% and the specificity 90%. The accuracy of EUS also varies with the vessel being examined. As a general rule, EUS has a better performance for venous invasion (80–91%) compared to arterial invasion (17–67%).65 The better results are seen for portal vein and confluence, where the sensitivity increases up to 100%, and for the splenic artery and vein. In contrast, the sensitivity of EUS decreases for the evaluation of the celiac axis and the superior mesenteric artery and vein.71
6.3Comparison of EUS with computed tomography (CT)In a systematic review, Dewitt et al72 analyzed eleven well-designed studies comparing EUS and CT for preoperative staging of pancreatic cancer. Regarding the T stage accuracy, 4 of 5 studies concluded that EUS was superior to CT (63–85% vs. 25–73%). EUS was also superior to CT in 5 of 8 studies that assessed N staging accuracy (44–75% vs. 25–63%). Among the 4 studies that assessed resectability, 2 showed no difference between EUS and CT, and 1 favored each modality. For vascular invasion EUS was superior to CT in 6 of 8 studies (68–100% vs. 41–83%). The previously mentioned meta-analysis by Nawaz et al65 included 12 studies on the same subject. CT showed lower sensitivity than EUS for N staging (24% vs. 58%) and vascular invasion (58% vs. 86%); however, the specificities for N staging (88% vs. 85%) and vascular invasion (95% vs. 93%) were comparable. The sensitivity and specificity of CT in determining resectability (90% and 69%) was similar to that of EUS (87% and 89%).
Only a few studies comparing EUS with multidetector row CT (MDR-CT) for staging pancreatic cancer are available. In a report of 80 patients, EUS was superior to MDR-CT for T staging accuracy (67% vs. 41%) but equivalent for N staging accuracy (44% vs. 47%), detection of resectable tumors (88% vs. 92%) and unresectable tumors (68% vs. 64%).73 In a more recent study, the sensitivity, specificity and area under the curve for EUS vascular invasion were 61%, 90% and 0.80 compared to 56%, 93% and 0.74 for MDR-CT.74
6.4Comparison of EUS with magnetic resonance imaging (MRI)Likewise with MDR-CT, there are few studies comparing EUS and MRI. Soriano et al23 showed that for N staging MRI had a lower sensitivity (15%) but higher specificity (93%) when compared with EUS (36% and 87%, respectively) and CT (37% and 79%, respectively). Regarding vascular invasion MRI had sensitivity of 59% and specificity of 84% in comparison to EUS (42% and 97%, respectively) and CT (67% and 94%, respectively). In a recent prospective study the agreement between EUS and MRI in patients staging was 74%, with a fair correlation between both techniques (kappa 0.42).75
When comparing EUS with other imaging techniques, it is important to stress that EUS is a real-time operator-dependent technique with a slow learning curve that can affect its performance. However there are no data evaluating the impact of these variables in the staging of pancreatic cancer and it would be interesting to conduct a study on this specific subject. In conclusion, cross-sectional imaging techniques and EUS are not mutually exclusive and should be considered complementary for staging pancreatic cancer. Both EUS and CT/MDR-CT appear to be equivalent for nodal staging, overall vascular invasion, and assessment of tumor resectability (Table 3).
Global performance of different imaging methods for pancreatic adenocarcinoma.γ
| EUS | EUS vs. CT | EUS vs. MDR-CT | EUS vs. MRI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | AUC | Sensitivity | Specificity | Accuracy | Sensitivity | Specificity | Accuracy | Sensitivity | Specificity | |
| T staging | 72–90% | 72–90% | 0.90 | 63–85% vs.25–73% | 67 vs. 41% | ||||||
| N staging | 62–69% | 74–81% | 0.79 | 24% vs. 58% | 88% vs. 85% | 44–75% vs.25–63% | 44 vs. 47% | 36 vs. 15% | 87 vs. 97% | ||
| Vascular invasion | 73–87% | 90–92% | 0.94 | 58% vs. 86% | 95% vs. 93% | 68–100% vs.41–83% | 61 vs. 56% | 90 vs. 93% | 0.8 vs. 0.74* | 42 vs. 59% | 97 vs. 84% |
| Venous | 80–91% | ||||||||||
| Arterial | 17–67% | 67% vs. 67% | 100% vs. 90% | 100 vs. 60%§93 vs. 92%# | |||||||
| Resectability | 90% | 86% | 87 vs. 90% | 89% vs.69% | 63–93% vs.60–83% | 88 vs. 92% | |||||
EUS is crucial for an accurate preoperative evaluation of pancreatic cancer which is essential to choose the correct management strategy.23,24 The possibility to obtain samples, from suspicious lesions or lymph nodes, by means of EUS-FNA, makes this procedure an ideal diagnosing and staging modality for pancreatic cancer. EUS-FNA allows the diagnosis of solid pancreatic lesions other than ductal adenocarcinoma, staging of suspected or proven pancreatic cancer, and cytological/histological proof of unresectable pancreatic cancer.23,24 Nonetheless, EUS-FNA may provide false-negative results for malignancy, especially for the patients with underlying chronic pancreatitis.2,27 The diagnostic yield of EUS-FNA may be improved by ROSE,6 correct choice of the needle,13 the number of passes15 and repetition of the procedure. Another option to raise the diagnostic yield of EUS-FNA is the addition of CE-EUS and/or EUS-E. Given the higher sensitivity of CE-EUS and EUS-E, in cases of negative cytology by EUS-FNA but in which CE-EUS and/or EUS-E is/are suggestive of pancreatic adenocarcinoma, further cytological samples or surgery should be considered instead of follow-up.39,40 In addition, CE-EUS and EUS-E could guide FNA to choose an optimal puncture site to improve the diagnostic accuracy63.
For staging and assessment of resectability of pancreatic cancer, EUS is applied supplementary to CT.76 While the second should be the first choice in patients with suspected pancreatic cancer, EUS may provide better assessment of T staging and certain types of vascular invasion.77,78 Thus EUS plays a major role to further evaluate patients with non-metastatic disease that appears resectable on initial imaging.77,78
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.