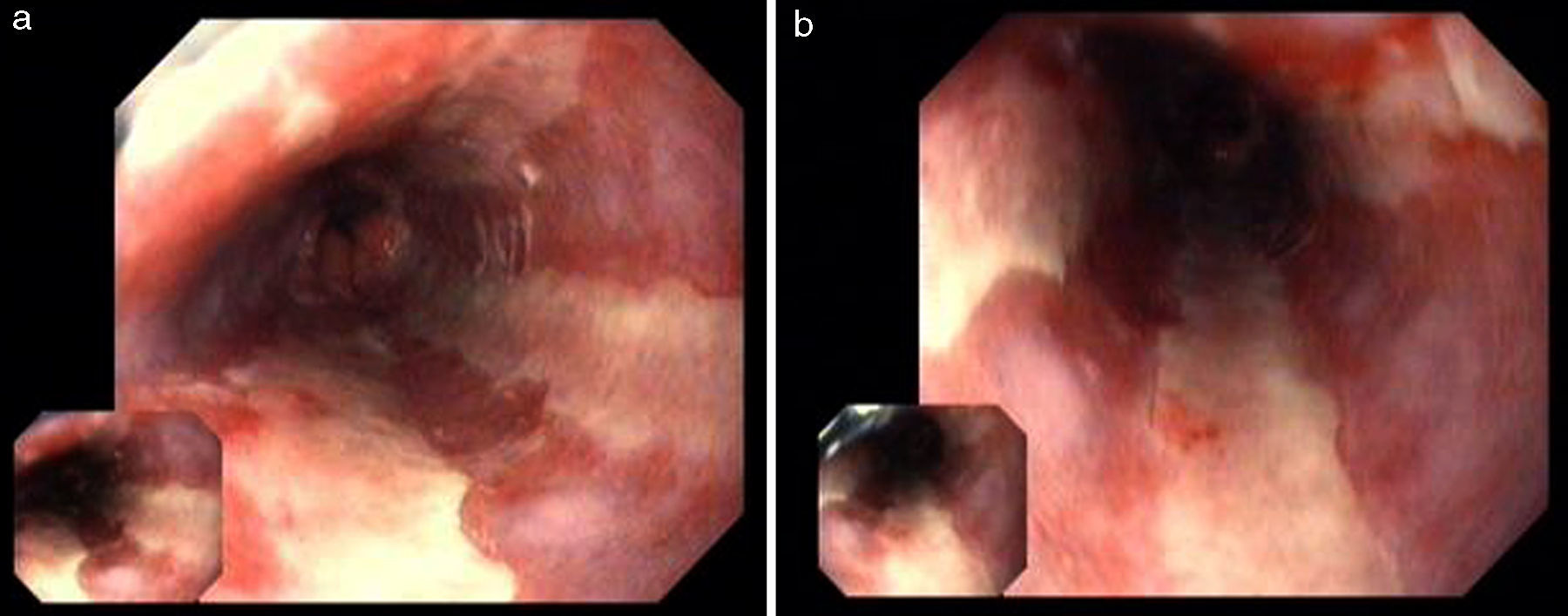
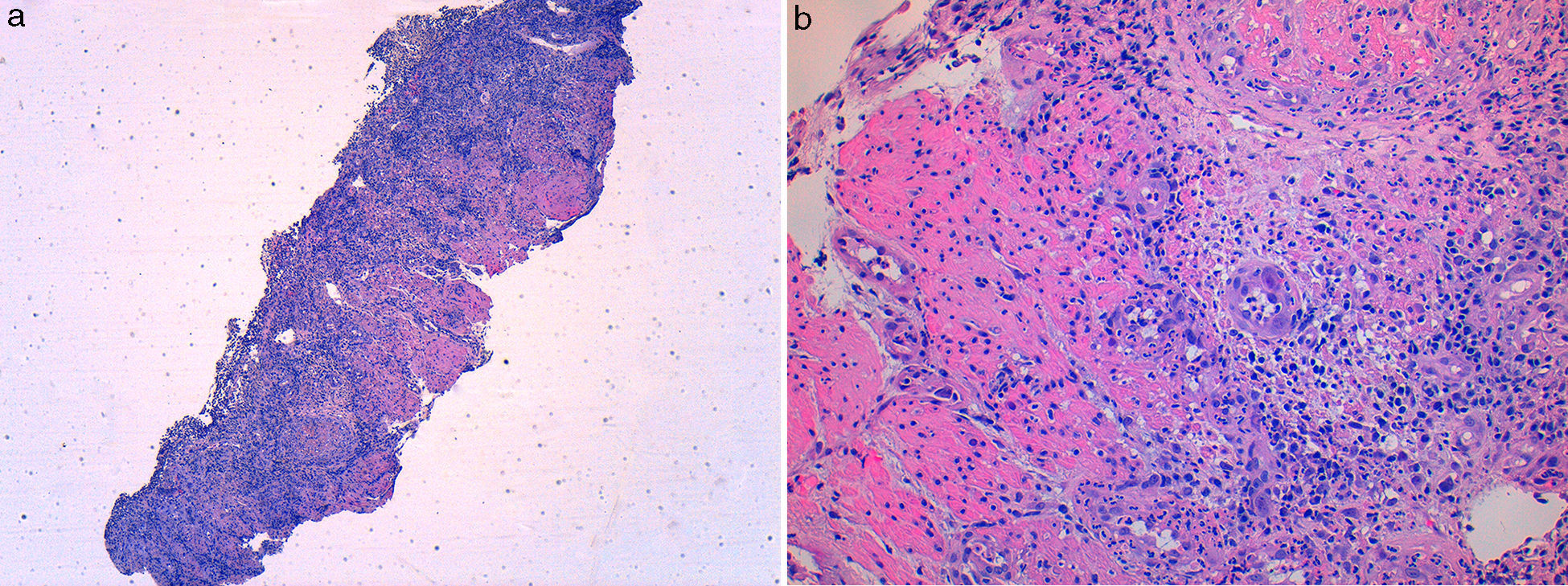
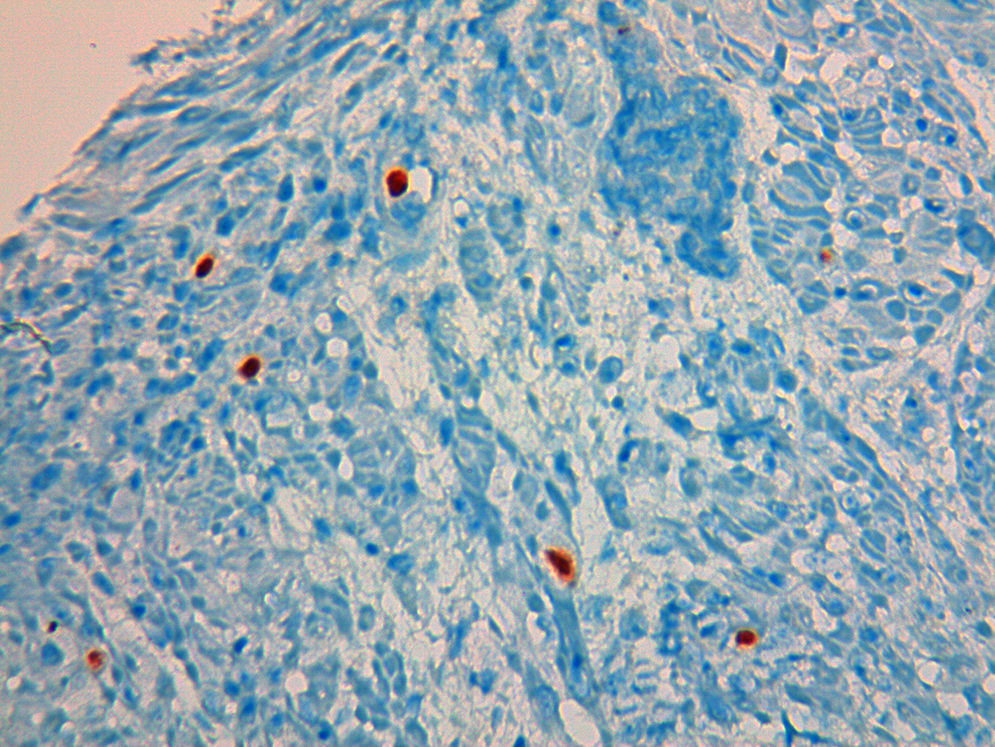
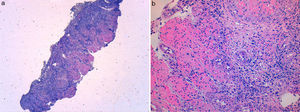
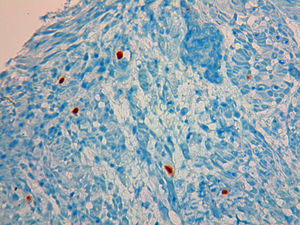
A 49 year-old male patient recurred to the emergency department complaining of anorexia, nausea, cough with mucopurulent sputum and dyspnea for 5 days. He had a history of poorly controlled type 1 diabetes mellitus with retinopathy, neuropathy and renal transplant (2 years before) for end-stage renal failure. The patient was taking immunosuppressive therapy with prednisolone (20mg/day), tacrolimus (2mg/day) and mycophenolic acid (720mg/day). A chest X-ray, blood and urine tests performed were consistent with pneumococcal pneumonia and diabetic ketoacidosis. Therefore he was admitted. In the 4th day after admission the patient complained of heartburn and dysphagia. An upper endoscopy was performed and revealed extensive longitudinal esophageal deep ulceration and whitish plaque in the medium and distal esophagus (Fig. 1). The histopathological exam of the esophageal biopsy showed ulcerated esophageal mucosa with viral inclusion-like structures in the epithelium, suggestive of virus-induced esophagitis (Fig. 2). Cytomegalovirus (CMV) immunohistochemical was positive and confirmed the diagnosis of CMV esophagitis (Fig. 3). Ganciclovir (5mg/kg every 12h) was administered intravenously for 14 days, and it was well-tolerated. After 2 weeks of intense therapy and immunosuppressive regimen adjustments, dysphagia improved and he was discharged with oral valganciclovir (900mg every 12h).
CMV is a major cause of morbidity and a preventable cause of mortality in immunocompromised patients, especially transplant recipients and those infected with human immunodeficiency virus.1–3 CMV esophagitis has mostly been described in patients with acquired immunodeficiency syndrome, however, also may occur as a complication of immunosuppressive therapy, or even in immunocompetent patients.3,4 The gastro-intestinal tract is the commonest organ to be involved in CMV infection and esophagitis is the second most common gastrointestinal manifestation, next to colitis.2
Patients with CMV esophagitis present with odynophagia or dysphagia. Symptoms are indistinguishable from those associated with Candida or HSV esophagitis.5 Usually, the endoscopic appearance is typified by large shallow ulcers, either solitary or multiple, located in the middle or distal part of the esophagus.5 Histopathologic examination analysis demonstrating active disease and immunohistochemistry are the most reliable diagnostic methods in cases of tissue-invasive disease.3
Potent antiviral agents, are essential in the treatment of patients with non-HIV causes of immunosuppression, such as transplantation. For nonsevere CMV disease, valganciclovir (900mg every 12h) or intravenous ganciclovir (5mg/kg every 12h) are recommended as first-line treatment in adults. Conversion from intravenous ganciclovir to valganciclovir may be performed without interrupting dosing.1 When optimal drug exposure is required, such in severe or life-threatening CMV disease, those with high viral load, and those with questionable gastrointestinal absorption, intravenous ganciclovir is recommended.1,2 Dose reduction of immunosuppressive therapy should be considered in cases of severe CMV disease, in nonresponding patients, in patients with high viral loads or leukopenia.1
CMV-associated gastrointestinal infections are generally resolved without clinical symptoms in immunocompetent patients, but the infection can be fatal in the immunocompromised.5 Early recognition of this clinical entity is of vital importance to implement appropriate therapy and avoid potentially fatal complications.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors wish to thank Dr. Rosa Coelho and Dr. João Magalhães, from the Gastroenterology and Pathology Department, for the support in their work.