A 78-year-old male presented to the emergency department (ER) with hematemesis. Two weeks earlier, he performed a gastroscopy for dyspepsia that showed an irregular convex deformity in greater curvature of the gastric body covered by congestive and ulcerated mucosa. Biopsies were diagnostic of a gastric gastrointestinal stromal tumor (GIST): fusocellular neoplasm with increased cellularity and marked pleomorphism, with immunohistochemical staining positive for CD117 and negative for smooth muscle actin, desmin and S100. CT scan confirmed an exophitic gastric tumor and showed multiple endoperitoneal locations. He started treatment at that time with imatinib-mesylate 400mg daily.
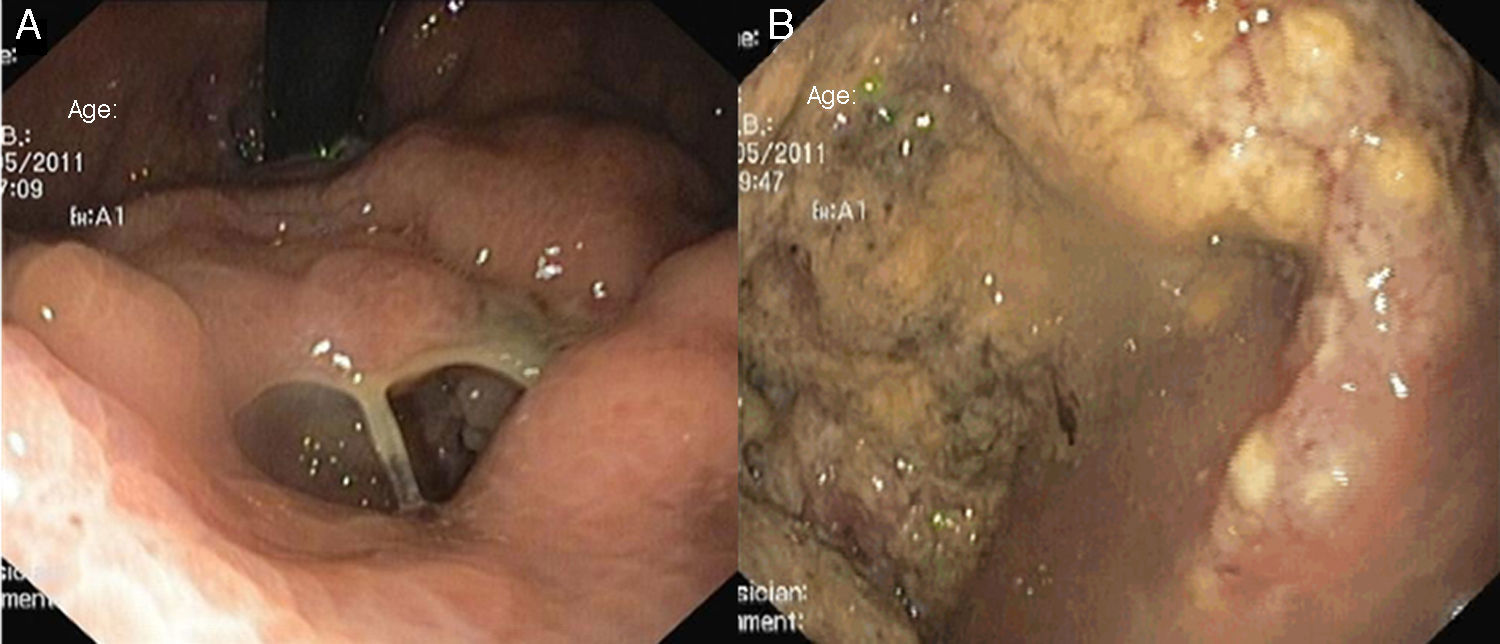
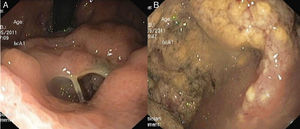
At the ER, laboratory tests revealed moderate anemia (Hb 9.7g/dL) with a white blood cell count of 21.200/mm3 (neutrophil count 19.400/mm3), CRP 72.2mg/L (normal value<5.0mg/L) and lactate dehydrogenase 380U/L (normal value<248U/L). Upper endoscopy again showed the abovementioned irregular deformity covered by irregular mucosa in the gastric body. It now had an orifice of about 15mm in diameter, which allowed the passage of the endoscope to a cavity with abundant exudate and necrotic debris (Fig. 1). No significant changes were noted in the remainder parts of the stomach, the esophagus and the duodenum. No blood was observed.
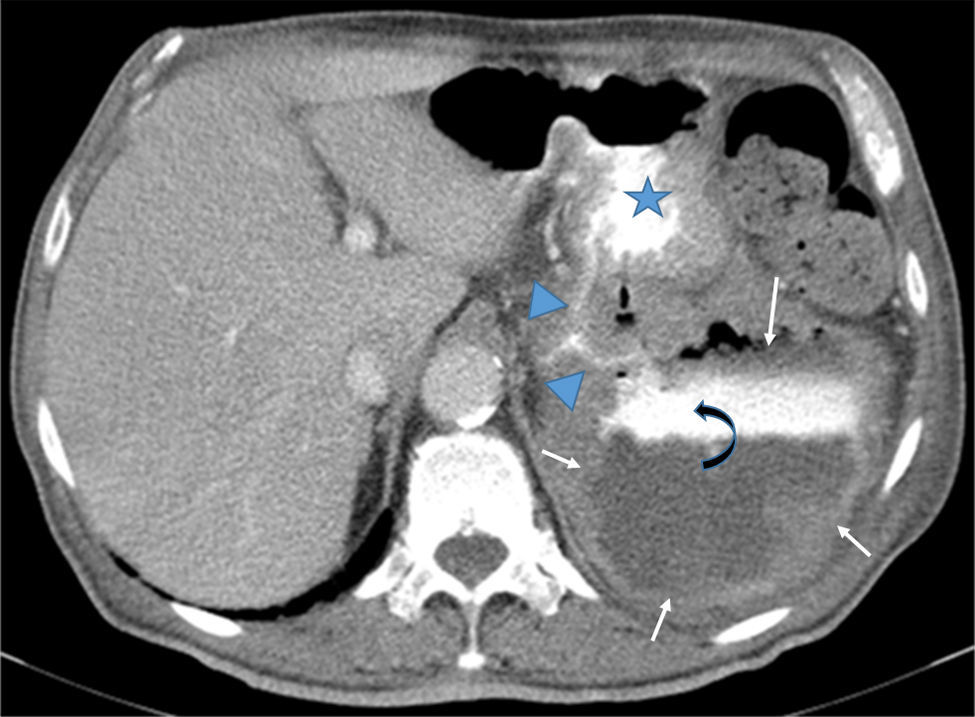
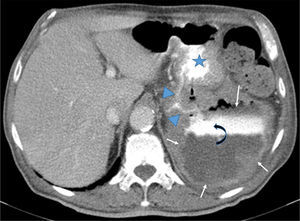
Contrast-enhanced CT scan showed a 9cm×12cm×10cm exophytic gastric tumor with central necrosis arising from posterior wall of the gastric body. It was possible to see contrast leaks from the stomach communicating with the cavities; there were neither fluid levels nor pneumoperitoneum (Fig. 2).
Axial CT after iodine injection and oral contrast. The image depicts a large exophitic tumor (arrows) arising from the posterior wall of the stomach (star). The tumor has central necrosis shown by a contrast-fluid level (curved-arrow). Oral contrast flowing from the stomach into the tumor cavity (arrowheads) is also noteworthy.
The patient was admitted for recovery and no recurrence of bleeding was noted. The multidisciplinar team (encompassing oncologists and surgeons) decided to stop imatinib and provide the best supportive care for the patient, who died one month later.
GISTs account for approximately 1–3% of all the gastrointestinal tract neoplasms with a malignant potential of 20–30%.1 Of all GISTs in the gastrointestinal tract, 50–60% occur in the stomach.2 Imatinib has become the standard treatment for metastatic GISTs. Exposure of GIST cells to imatinib, arrests cell proliferation, and causes apoptotic cell death. Because of the massive cytolytic effect of the drug, when treatment is given for GISTs located in the wall of the gastrointestinal tract not only gastrointestinal hemorrhage but also ulceration, cavitation, fistulization and perforation can occur due to massive necrosis of the tumour.3 The last complications are often first seen at imaging performed for assessment of tumor response to imatinib. However, gastroenterologists should be aware of such adverse events as these may be observed on the course of endoscopic procedures.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Conflicts of interestThe authors have no conflicts of interest to declare.