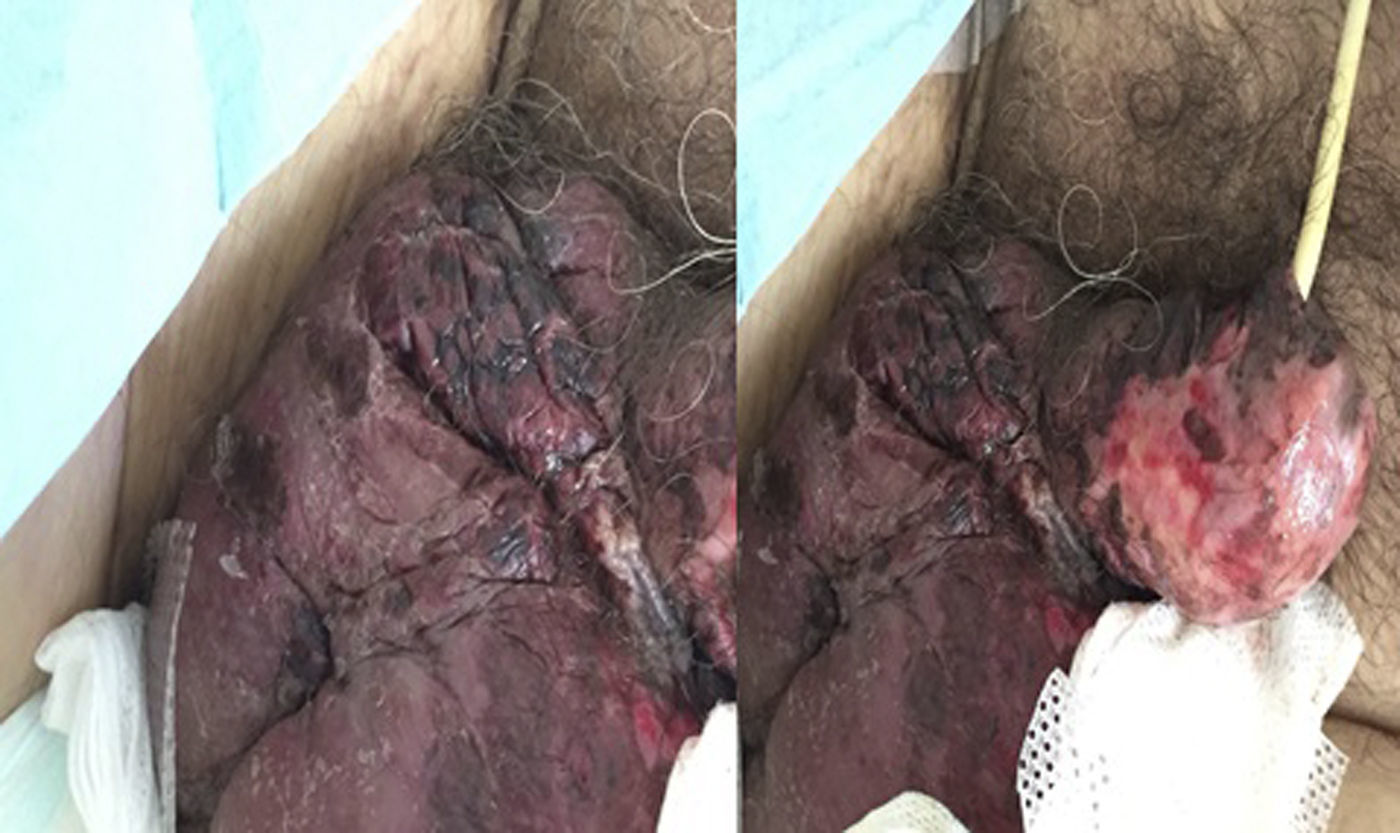
A 66-year-old male was admitted to our gastroenterology department with severe bilateral pneumonia. His medical history was relevant for liver cirrhosis secondary to alpha-1-antitrypsin deficiency and systemic hypertension. He had no other relevant comorbidities, and did not smoke or drink alcoholic beverages. The patient's clinical condition deteriorated with progressive liver failure. As he met criteria for hepatorenal syndrome, we decided to start treatment with terlipressin. Three days later, the patient started complaining of diffuse abdominal pain. His abdomen was significantly tender on palpation. Simultaneously, his genitalia were noted to be swollen and ulcerated, showing black spots of non-viable tissue (Fig. 1). As we suspected that ischemia due to terlipressin could be present, the drug was immediately discontinued. An urgent CT scan showed no evidence of acute abdomen or vascular thrombosis. Dermatologic and urologic consultation was requested, both supporting the diagnosis of genital ischemia. There was no evidence of spreading to deeper fascial planes. His creatine-kinase was not elevated. Unfortunately, the patient died on the following day from multi-organ dysfunction.
Terlipressin is a commonly prescribed drug in the setting of variceal bleeding and hepatorenal syndrome. Although ischemia is a known complication of terlipressin therapy, severe ischemic events have been reported in fewer than 5% of cases.1 The genitalia are highly vascularized by the pudendal arteries and therefore present an uncommon site of ischemia, with few reports available to date.2,3 Our patient presented a systemic infection but had no evidence of shock, hypovolemia or acidosis. In addition, there was no emphysema of the scrotum making Fournier's gangrene unlikely. The available literature suggests that drug withdrawal and correction of other underlying hemodynamic factors may improve this condition. The prognosis is highly dependent on the baseline disease.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Author contributionsAll authors contributed equally to the elaboration and review of the manuscript.
Conflicts of interestNone declared.