A significant portion of chronic hepatitis B (HBV) infected patients is in the inactive carrier state, characterized by normal transaminase levels, little viral replication and minimal liver necroinflammatory activity. Diagnosis is made after at least one year of regular monitoring and requires lifelong follow-up to confirm that this state is maintained.
Studying the natural history of inactive carriers is currently hindered by the small number of studies on patients correctly diagnosed according to current guidelines.
When correctly defined, inactive carrier state carries a very good prognosis in the spectrum of chronic hepatitis B infection, with low rates of reactivation, hepatocellular carcinoma and progression of disease to cirrhosis. In addition, clearance of hepatitis B surface antigen is more common in inactive carriers compared to the general HBV infected population. Reactivation is more likely during the first years of follow-up and during immunosuppressive therapies. Prophylactic antiviral treatment should be initiated as soon as possible in this latter case.
Current guidelines do not routinely recommend liver biopsy in inactive carriers. However, some may have significant hepatic fibrosis at diagnosis and cannot therefore be classified as such; others may develop fibrosis during follow-up and consequentially have poorer prognosis. Despite some limitations, transient elastography appears an ideal approach for identifying such patients and for serial monitoring of liver changes in all inactive carriers.
Overall, more longitudinal studies on larger cohorts of true inactive carriers would be helpful for establishing with greater certainty the most appropriate management strategy in these patients.
Uma porção significativa dos doentes com infecção crónica pelo vírus da hepatite B (HBV) encontra-se num estado de portador inactivo, caracterizado por níveis normais de transamínases, baixa taxa de replicação vírica e actividade necroinflamatória hepática mínima. O diagnóstico é feito após pelo menos um ano de monitorização periódica e requer vigilância por tempo indeterminado para confirmar que se mantém.
O estudo da história natural dos portadores inacitvos é actualmente dificultado pelo número limitado de estudos em doentes correctamente diagnosticados de acordo com as guidelines actuais.
Quando corretamente definido, o estado de portador inativo associa-se a um excelente prognóstico dentro do espectro da infecção crónica por HBV, com baixas taxas de reactivação, carcinoma hepatocelular e progressão para cirrose. Além disso, a perda do antigénio de superfície da hepatite B é mais frequente em portadores inativos do que na generalidade da população infetada pelo VHB. A reativação é mais provável durante os primeiros anos de seguimento e após terapêuticas imunossupressoras: deve ser instituída profilaxia antivírica neste segundo caso.
As guidelines actuais não recomendam biópsia hepática por rotina em portadores inativos; no entanto, alguns deles podem ter fibrose hepática importante na altura do diagnóstico, não podendo ser classificados como tal; outros podem vir a desenvolvê-la, tendo consequentemente pior prognóstico. Apesar de algumas limitações, a elastografia hepática transitória afigura-se como um método ideal para identificar estes doentes e para a monitorização seriada de alterações hepáticas em todos os portadores inativos.
Novos estudos longitudinais em verdadeiros portadores inativos são necessários para estabelecer com maior certeza a melhor estratégia nestes doentes.
Infection with hepatitis B virus (HBV) affects approximately one third of the world's population during their lifetime.1 It is estimated that 240 million people worldwide have chronic HBV infection, defined by persistence in the serum of its surface antigen (HBsAg). It is especially prevalent in Sub-Saharan Africa and East Asia, where perinatal transmission accounts for the majority of infections. Transmission can occur through body fluids such as blood, semen or vaginal secretions; the major routes of infection are perinatal, sexual and blood-to-blood contact.2
Following acute exposure to HBV, the natural history of HBV infection goes sequentially through the following two phases:
- (1)
the immune-tolerant phase is characterized by hepatitis B early antigen (HBeAg) positivity (a serological marker of viral replication), high viral replication rate (elevated HBV DNA levels) and normal alanine aminotransferase (ALT) levels. There is little to no inflammation or fibrosis of the liver. This phase tends to be more prolonged in perinatally acquired infection.
- (2)
in the immune-reactive phase the host's immune system starts mounting a response against HBV: replication levels lower, with a parallel serum HBV DNA level drop, ALT levels rise and there is moderate to severe necroinflammatory activity in the liver. This phase ends with HBeAg loss and seroconversion to anti-HBe status, which is usually associated with viral suppression by the host's immune system.1 The likelihood of clearing the virus and curing infection during this phase, evidenced serologically by HBsAg clearance, depends largely on patient's age at the time of infection, so that chronicity occurs in 80–90% of infected newborns, in contrast with 30–50% if infection is acquired in early childhood and only <5% if infected in adulthood.2
After HBeAg seroclearance, two disease states, not necessarily static, are possible:
- (3)
Some patients remain in an inactive carrier (IC) state, defined by the European Association for the Study of the Liver (EASL) as fulfilling the following criteria: (1) HBeAg negativity; (2) anti-Hbe positivity; (3) persistently normal ALT (PNALT) levels (<40IU/mL, with measurements at least every 3–4 months during 1 year); (4) serum HBV DNA levels <2000IU/mL.
- (4)
Others evolve to HBeAg-negative chronic hepatitis B (CHB), usually associated with precore or basal core promoter mutant HBV, in which viral replication and hepatic inflammation persist despite seroconversion to anti-HBe status. It is characterized by fluctuating ALT and serum HBV DNA levels that can drop below IC cut-off levels, despite persistent viral replication and hepatic inflammation.
Differentiating between IC and CHB status is of paramount importance in clinical practice, since it has serious implications in follow-up, management and prognosis.1
2Definition of inactive chronic HBV carriers2.1HBA DNA and ALT cut-off levelsAt the Management of Hepatitis B workshop in 2000, an arbitrary level of 20,000IU/mL was adopted as the serum HBV DNA cut-off level distinguishing active and inactive chronic hepatitis.3 After it was demonstrated that this cut-off would misclassify 45 and 30% of active CHB based on one or three serial HBV DNA measurements, respectively,4 major guidelines adopted a lower serum HBV DNA cut-off value of 2000IU/mL.1,5 Still, the EASL acknowledges that there can be inactive HBV carriers with DNA levels between 2000 and 20,000IU/mL.
Debate on the value that best discriminates between active and inactive infection is ongoing. Chen et al. followed 64 inactive carriers who maintained PNALT during a mean follow-up period of 17.6 years and measured HBV DNA levels periodically (minimum of 5 measurements); 68% had at least one HBV DNA level >2000IU/mL (excluding those obtained during the first year of follow-up).6
Because HBV DNA levels in HBe-negative active hepatitis can fluctuate from undetectable to >2,000,000IU/mL5 and some inactive carriers occasionally have HBV DNA levels between 2000 and 20,000IU/mL, a single HBV DNA level between 2000 and 20,000IU/mL appears to be a “gray area” which can correspond to both active CHB or inactive carriers. It is thus important for the clinician to be aware of the importance of serial HBV DNA measurements and life-long follow-up to confirm that inactive carrier state is maintained.
Another IC state diagnostic criterion has recently been revised: lower ALT upper limit of normal (ULN) levels of 30IU/L for men and 19IU/L for women were proposed based on its 95th percentile in healthy Italian blood donor candidates.5 Indeed, there appear to exist differences in viremia, risk of reactivation and liver histological lesions between patients with low-normal (<0.5×ULN) and high-normal ALT (0.5–1×ULN), as a review by Andreani recently summarized.7 However, most of these results come from studies on chronic HBV populations with heterogeneous serological profiles and HBV DNA levels. These observations may therefore not extend to inactive carriers, especially since several prospective studies on HBe-negative carriers with PNALT have failed to demonstrate significant differences in clinical outcomes between patients with higher-normal and lower-normal ALT levels.8,9 It remains to be determined whether ALT levels near the ULN warrant management changes for inactive carriers.
2.2HBsAg quantificationThe HBV marker HBsAg has been the foundation of HBV infection diagnosis since its discovery over 40 years ago; however, its significance in clinical practice has mostly been limited to qualitative status. Recently, interest has sparked regarding its quantification as an important diagnostic, prognostic and predictive tool.10
HBsAg is the glycosylated protein of the HBV virion envelope. The DNA template for HBV replication exists in hepatocytes’ nuclei as covalently closed circular DNA (cccDNA) and segments of it can become integrated in the host's hepatocyte genome. Unlike mature virions, HBsAg can also be produced from integrated HBV DNA. It is produced in excess of mature virions and consequently exists in circulation both associated with virions and as subviral particles.
HBsAg levels tend to decline as disease progresses to HBe-negativity and inactivity but they re-elevate if there is reactivation to active hepatitis.11 Several cross-sectional12–14 and longitudinal15–17 studies have also demonstrated a significant difference in HBsAg levels between inactive carriers versus active HBe-negative chronic hepatitis, revealing its potential usefulness in discriminating between them. Using a HBsAg cut-off level of 650IU/mL, Brunetto et al. found a diagnostic accuracy of 88% for diagnosing inactive carriers in a population of 209 Italian treatment-naïve HBe-negative genotype D infected patients; sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were 82, 90, 75 and 93%, respectively.16
In addition, HBsAg appears to have prognostic significance for both HBsAg seroclearance and disease reactivation. Lower baseline HBsAg levels and yearly HBsAg decline are independently associated with subsequent HBsAg loss.16 In Taiwanese HBe-negative treatment-naïve patients, baseline HBsAg <50IU/mL could predict HBsAg loss during a median follow-up of 8 years with 82% sensitivity and 67% specificity.18 In Korean genotype C-infected inactive carriers, baseline HBsAg>850IU/mL and HBV DNA >850IU/mL could predict reactivation with 85% accuracy, 65% sensitivity, 93% specificity, 80% PPV and 86% NPV.15
Interestingly, while HBsAg correlates strongly with serum HBV DNA in global chronic hepatitis B populations,13,19 correlation is only weak in HBe-negative patients.12,15,20,21 It appears HBsAg levels do not fall in the same proportion as HBV DNA levels as disease progresses. Accordingly, the serum HBV DNA/HBsAg ratio appears to be significantly higher in active CHB versus inactive HBV carriers.12,14,19,20
Indeed, it is the combination of serum HBsAg and HBV DNA levels that shows most promise as a single-point measurement for differentiating active CHB versus inactive carriers. A single-point measurement of serum HBsAg (cut-off <1000IU/mL) and HBV DNA (cut-off ≤2000IU/mL) in the abovementioned Italian population achieved even better discriminatory power between these two stages of chronic HBV infection (94% diagnostic accuracy, 91% sensitivity, 95% specificity, 88% PPV, 97% NPV).16 However, Park et al. tested these cut-offs in 104 Korean treatment-naïve genotype C HBe-negative patients and obtained poorer values: 62% diagnostic accuracy, 55% sensitivity, 77% specificity, 85% PPV and 42% NPV.15 Furthermore, in 228 heterogeneous HBV-infected European patients, active hepatitis prevalence was substantial (28%) even when HBsAg levels were <100IU/mL,22 demonstrating the need for further studies in larger cohorts of HBV infected patients with different characteristics before a clinically useful cut-off level is achieved.
2.3Liver histologyInactive carriers have by definition minimal to no necroinflammatory activity in the liver. However, because liver biopsy is seldom recommended in these patients, this is rarely confirmed histologically. Serial liver biopsies are even less frequently performed, making follow-up of liver histologic changes in inactive carrier populations difficult to evaluate.1
A recent review raised the concern that in approximately 10% of inactive HBV carriers diagnosed based on ALT and HBV DNA levels alone, significant liver disease might be missed,23 based on liver biopsies conducted in Indian and French inactive carriers.
Indeed, a 2012 systematic review of 6 studies with liver histology data from inactive carriers (which included the two above-mentioned studies) concluded that mild liver necroinflammatory activity and moderate fibrosis were present in 10 and 8% of patients, respectively, when PNALT definition was appropriate, but only 1.4 and 0.7%, respectively, when viremia levels <2000IU/mL were also a diagnostic criterion. In contrast, studies with poor definitions of PNALT, viremia levels >2000IU/mL and/or significant baseline liver disease found a higher proportion of moderate to severe liver changes.24
Liver biopsy is the gold-standard for evaluation of disease activity and liver fibrosis in HBV infection; however it is invasive and observer-dependent and carries a small risk of serious complications,1 all of which make its use in asymptomatic patients with modest clinical alterations open to question.
Transient elastography is a recent simple and non-invasive method for fibrosis assessment based on liver stiffness measured with an ultrasonographic method. It has been shown to be accurate and reproducible in hepatitis B25 and might supplant liver biopsy in assessing fibrosis in these patients.
Liver stiffness is significantly lower in true inactive carriers (defined using previous cut-off of 20,000IU/mL) than in HBe-negative active hepatitis patients and was similar to that of healthy controls in three European studies.26–28 In inactive carriers and untreated HBe-negative CHB patients, its diagnostic accuracy for the presence of Ishak fibrosis score ≥3 was 90.1% (sensitivity, 93.9%; specificity, 88.5%; PPV, 76.7%; NPV, 97.3%) and 94.2% for diagnosing cirrhosis (sensitivity, 86.5%; specificity, 96.3%; PPV, 86.5%; NPV, 96.3%).26 The cut-offs used were those with greater areas under ROC curve (AUROCs): 7.5 and 11.8pKa for diagnosing Ishak fibrosis score ≥3 and cirrhosis, respectively. These are similar to those proposed in a 2012 systematic review of heterogeneous chronic hepatitis B patients (7.9 and 11.7pKa for fibrosis METAVIR score ≥2 and cirrhosis, respectively). The same review points out that transient elastography in HBV infection may yield false results under certain circumstances. On the one hand, liver stiffness readings increase with active hepatic inflammation and false positive results are possible if there is ALT elevation. On the other, false negatives may result from the ultrasound beam missing fibrotic bands, especially considering the macronodular nature of cirrhosis in HBV infection.25
However, there is another important aspect other than the staging of fibrosis that could reinforce the need for performing liver biopsies in these patients: the determination of the amount of HBV infected hepatocytes, which currently relies on a liver tissue sample. Nonetheless, HBsAg quantification displays some promising value in this regard. HBsAg levels have been shown to correlate positively with cccDNA (an accurate marker of the burden of infected liver cells) in HBe-positive CHB patients21,29 and have displayed utility in predicting and monitoring response to therapy.10,11,30 However, studies so far have found this correlation to be weaker in HBe-negative HBV infected patients.21,29,31 Further studies in HBe-negative populations could shed some light on HBsAg and cccDNA kinetics during later phases of HBV infection.
3Natural history of HBV inactive carriersAmong HBV-infected patients, the inactive carrier state generally has a very good prognosis; however, reactivation of active hepatitis, cirrhosis and hepatocellular carcinoma (HCC) are rare but nevertheless possible complications.
3.1Spontaneous reactivationDisease progression to HBe-negative active hepatitis with or without reversion to HBeAg positivity is a possible outcome for inactive carriers; this may happen spontaneously, or following immunosuppressant therapy or disease.
Spontaneous relapse of hepatitis and reversion to HBe-positivity have been extensively studied in prospective studies of HBe-negative patients. A recent review by Villa et al. has highlighted that discordant relapse rates appear to stem from different study designs and populations. In studies of non-endemic populations, incidence of reactivation ranged from 0.03 to 0.8 per 100 person-years, whereas this incidence was somewhat higher in endemic areas (1.4–2.8 per 100 person-years). Relapse rate also seemed to vary according to sampling method: when patients were studied after recent seroconversion to HBe-negativity during follow-up, incidence of reactivation ranged from 0.8 to 2.8 per 100 person-years, whereas those who were already HBe-negative at enrollment had reactivation rates of 0.08 to 1.4 per 100 person-years. The authors conclude that longer time from seroconversion may account for this lower incidence of reactivation.23
Accordingly, in asymptomatic HBe-negative Taiwanese patients, cumulative incidences of relapse were 10.2, 17.4, 19.3 and 20.2% at 5, 10, 15 and 20 years, respectively. The increases in cumulative incidence tended to be progressively lower as time went on, with approximately half of relapses occurring during the first 5 years of follow-up.32 Both these data suggest that risk of spontaneous reactivation tends to decrease with time from seroconversion.
However, studying the rate of spontaneous relapse in true inactive carriers proves more difficult because only a few longitudinal studies correctly diagnose them with regular ALT and HBV DNA measurements during one year before beginning follow-up.
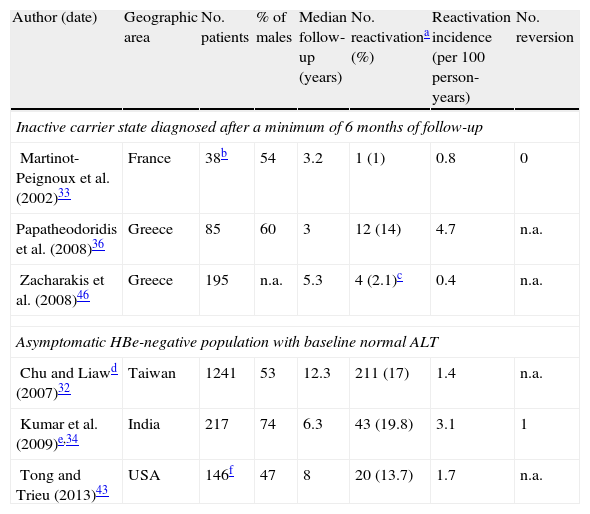
Incidence rates from three prospective studies in which inactive carrier state were defined after at least 6 months of persistently normal ALT are summarized in Table 1, as well as data from three additional studies on asymptomatic HBe-negative patients with normal ALT.
Incidence of reactivation of hepatitis B in Hbe-negative patients.
| Author (date) | Geographic area | No. patients | % of males | Median follow-up (years) | No. reactivationa (%) | Reactivation incidence (per 100 person-years) | No. reversion |
| Inactive carrier state diagnosed after a minimum of 6 months of follow-up | |||||||
| Martinot-Peignoux et al. (2002)33 | France | 38b | 54 | 3.2 | 1 (1) | 0.8 | 0 |
| Papatheodoridis et al. (2008)36 | Greece | 85 | 60 | 3 | 12 (14) | 4.7 | n.a. |
| Zacharakis et al. (2008)46 | Greece | 195 | n.a. | 5.3 | 4 (2.1)c | 0.4 | n.a. |
| Asymptomatic HBe-negative population with baseline normal ALT | |||||||
| Chu and Liawd (2007)32 | Taiwan | 1241 | 53 | 12.3 | 211 (17) | 1.4 | n.a. |
| Kumar et al. (2009)e,34 | India | 217 | 74 | 6.3 | 43 (19.8) | 3.1 | 1 |
| Tong and Trieu (2013)43 | USA | 146f | 47 | 8 | 20 (13.7) | 1.7 | n.a. |
ALT, alanine aminotransferase; ULN, upper limit of normal.
Reactivation defined as ALT elevation >2×ULN and/or serum HBV DNA≥20,000IU/L unless otherwise specified.
Reactivation defined as ALT elevation >2×ULN and detectable HBV DNA by hybridization assay (sensitivity=140,000copies/mL).
The two factors most consistently associated with risk of relapse in HBe-negative patients are male gender32–35 and older age at HBe seroconversion.32,34,35 Other patient characteristics that seem to confer greater risk of reactivation are baseline ALT and HBV DNA levels,36 maximum ALT during follow-up, genotype C35 and endemic geographic area.23 It is possible that HBV primo-infection at a very young age accounts for the higher reactivation rate in endemic populations, where transmission occurs most commonly perinatally and in early childhood.2
A study on true inactive carriers found no statistically significant difference in the age and sex between true inactive carriers who remained so and those who reactivated; however, the latter had significantly higher baseline serum ALT and HBV DNA.36
3.2Non-spontaneous reactivationReactivation of active hepatitis in inactive HBV carriers (including HBsAg-negative anti-HBs-positive patients) has been observed during or after cytotoxic treatments for malignancies. It is most often asymptomatic, but a hepatitis flare with hepatic decompensation and even death can occur in 5–40% of cases.37 It appears to be more common when corticosteroids, anthracyclines or rituximab are part of the treatment regimen.37,38 Reactivation has also been described in association with imunossupressive drugs used in rheumatic and autoimmune diseases, including corticosteroids, methotrexate and anti-TNFα drugs.39
Major guidelines therefore recommend prophylactic tenofovir or entecavir for all HBsAg carriers during any cytotoxic or imunossupressive treatment and for 6–12 months thereafter.1,5 For HBV-infected patients who have cleared HBsAg and have detectable serum HBV DNA (occult HBV infection), the EASL recommends similar prophylaxis; on the other hand, if HBV DNA is undetectable, either prophylaxis with lamivudine or strict follow-up with ALT and HBV DNA levels every 1–3 months are appropriate approaches.
The natural history of inactive carriers is also influenced by human immunodeficiency virus (HIV) co-infection. In general, untreated HIV infection is associated with higher HBV replication, higher reactivation rate and reduced liver necroinflammatory activity in comparison with HBV infection alone, probably due to CD4+ cell depletion and immune system impairment. Initiation of highly active antiretroviral therapy may help immune control of viral replication, but it is also associated with hepatitis reactivation due to the immune reconstitution syndrome.40 Still, inactive carrier state alone does not mandate any changes in HIV infection treatment according to the European AIDS Clinical Society guidelines.41
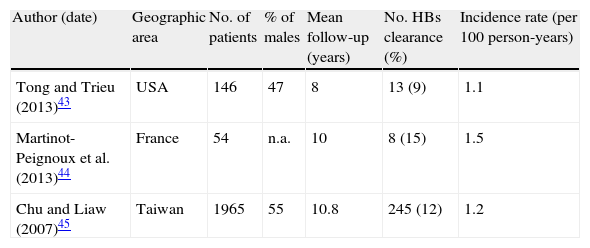
3.3Spontaneous clearanceThe annual rate of HBsAg clearance among heterogeneous HBsAg carriers is 0.5%.5 This is considered the best possible outcome of HBV infection; however, residual viral replication can persist and HBV DNA is detectable in the liver even decades later.42
Table 2 summarizes incidences of HBsAg loss from three longitudinal studies in HBe-negative patients with baseline normal ALT.43–45 Only one of them discriminated results from true inactive carriers classified after one year of PNALT and the higher HBsAg seroclearance rate in this population possibly reflects that.44
Incidence of HBsAg loss in HBe-negative HBsAg carriers with normal baseline ALT.
| Author (date) | Geographic area | No. of patients | % of males | Mean follow-up (years) | No. HBs clearance (%) | Incidence rate (per 100 person-years) |
| Tong and Trieu (2013)43 | USA | 146 | 47 | 8 | 13 (9) | 1.1 |
| Martinot-Peignoux et al. (2013)44 | France | 54 | n.a. | 10 | 8 (15) | 1.5 |
| Chu and Liaw (2007)45 | Taiwan | 1965 | 55 | 10.8 | 245 (12) | 1.2 |
HBsAg, hepatitis B surface antigen; ALT, alanine aminotransferase.
Yuen et al. have extensively elucidated the natural history of disease after HBsAg clearance in a longitudinal study of 298 heterogeneous HBV-infected patients followed for a median of 3 years after HBsAg loss (96% of it spontaneous). All became HBe-negative before clearing HBsAg and 52% of them developed anti-HBsAg. In a subset of 212 patients who had at least 4 ALT measurements, 82% maintained PNALT during the rest of follow-up; in those who did not (18%), alternative causes for transaminase elevation other than active HBV hepatitis were present in all but 6 (2.8%) of patients (fatty liver, hepatocellular carcinoma or intake of potentially hepatotoxic traditional herbal medicines). The number of patients with detectable serum HBV DNA diminished with time from seroclearance; however, integrated HBV DNA remained detectable in all 29 liver biopsies performed and cccDNA in 23. There were 5 clinical complications of cirrhosis (all with HBsAg clearance after 47 years of age; 3 had transient elastography readings compatible with cirrhosis within 1 year of seroclearance) and 7 patients developed HCC (all were men and lost HBsAg aged >50 years; 6 had cirrhosis on ultrasound within 1 year of seroclearance).42
It seems HBsAg loss is associated with improved prognosis particularly when no cirrhosis has yet developed and with younger age at seroclearance.
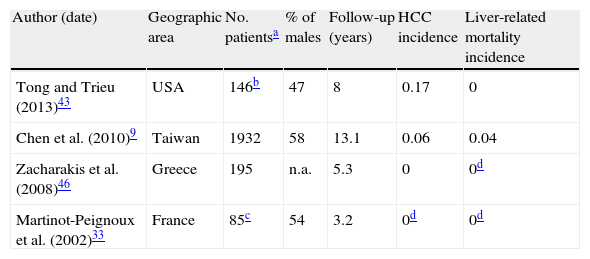
3.4Complications and survivalStudying the incidence of liver complications in inactive carriers is likewise hindered by the small number of prospective studies which correctly define this state. In two longitudinal studies of strictly defined inactive carriers followed for a median of 3.2 and 5.3 years, none developed cirrhosis or HCC (Table 3).33,46 Another study on 145 true inactive carriers with a longer follow-up (mean of 8 years) found two instances of HCC and no cases of liver-related death.43
Liver-related outcomes in inactive carriers (incidence per 100 person-years).
| Author (date) | Geographic area | No. patientsa | % of males | Follow-up (years) | HCC incidence | Liver-related mortality incidence |
| Tong and Trieu (2013)43 | USA | 146b | 47 | 8 | 0.17 | 0 |
| Chen et al. (2010)9 | Taiwan | 1932 | 58 | 13.1 | 0.06 | 0.04 |
| Zacharakis et al. (2008)46 | Greece | 195 | n.a. | 5.3 | 0 | 0d |
| Martinot-Peignoux et al. (2002)33 | France | 85c | 54 | 3.2 | 0d | 0d |
HBsAg, hepatitis B surface antigen; ALT, alanine aminotransferase; HCC, hepatocellular carcinoma.
In a larger scale sample of 1932 Taiwanese inactive carriers, annual rates of HCC and liver-related mortality were 0.06 and 0.04%, respectively. Serum HBV DNA level was the most important predictor of both outcomes. Interestingly, its prognostic value for HCC was greater in HBe-negative patients with normal ALT and no cirrhosis when compared with the whole HBsAg-positive cohort; this greater association was not found for prediction of cirrhosis.47 Another study on the same population found older age and alcohol consumption to be predictors of HCC and liver-related death in inactive carriers; on the contrary, no statistically significant difference in outcomes was demonstrated for gender, smoking habits and high-normal vs. low-normal ALT levels in inactive carriers.9
4Management of inactive carriersDue to its general excellent prognosis, antiviral treatment is not generally recommended for HBV patients in the inactive carrier state.
Once true inactive carrier state is confirmed, regular lifelong monitoring with ALT levels every 6–12 months and periodic HBV DNA measurements is advised.1 Because liver biopsy is not routinely advised in inactive carriers, hepatic fibrosis remains uninvestigated during the follow-up of most of these patients. To this end, transient elastography appears an advantageous fibrosis assessment tool that can easily be used for baseline and even serial monitoring of liver fibrotic changes.
Decision to treat patients with ALT>twice the upper limit of normal (ULN) and HBV DNA >20,000IU/mL is unanimous1,5 but it is less firm when ALT is minimally elevated (<2ULN) and/or HBV DNA levels are between 20,000 and 2000IU/mL. Patients with normal ALT and HBV DNA levels between 2000 and 20,000IU/mL (the aforementioned HBV DNA level “gray area”) should be followed more closely with ALT determinations every 3 months for at least three years, because as previously discussed, relapse to active hepatitis appears more likely during the beginning of follow-up.23,32 Indeed, in a study of 217 HBe-negative asymptomatic patients with normal baseline ALT, time to reactivation (defined as ALT and HBV DNA elevation) after beginning of follow-up was analyzed: the 10th percentile was 3.4 months, which suggests that analytical assessment of inactive carriers every 3 months can detect approximately 90% of relapses.34 When there are only small ALT or HBV DNA elevations (ALT<2×ULN or HBV DNA <20,000IU/mL), guidelines also suggest liver biopsy5 or transient elastography1 to assess severity of disease before decision to treat is made.
Though a rare complication, HCC is a possible life-threatening event; however, guidelines only recommend screening in high-risk patients.1,5
5DiscussionHBsAg inactive carriers represent the majority of hepatitis B virus carriers who have seroconverted to anti-HBe,5 making them an especially important subpopulation of chronic HBV patients, both due to their significant number but also because of the particularities in their management. Specifically, they are not candidates for any of the current hepatitis B therapies available.
The clinician's main goals when faced with an inactive carrier must be (A) confirming inactive carrier status, (B) monitoring for reactivation and (C) screening for liver complications.5 We will comment on each of these items separately.
5.1Confirming inactive carrier statusDebate continues regarding the most recent update of lowering the HBV DNA cut-off level to 2000IU/mL for diagnosing inactive carriers. One study counter-argues this cut-off alteration with the evidence that 23% of inactive carriers diagnosed after one year of PNALT had at least one HBV DNA measurement between 2000 and 20,000IU/mL during a median follow-up of 3 years.36 The new cut-off level would therefore leave one-fifth of these probable inactive carriers robbed of this classification while still not fulfilling the criteria for chronic active hepatitis. However, we believe that this particular subset of patients has an important risk of active disease and according to guidelines warrants different management from true inactive carriers, deserving therefore clinical distinction. Guideline recommendations for these patients vary between tighter follow-up (with ALT determination every 3 months and HBV DNA measurements every 6–12 months for 3 years) and liver fibrosis assessment (with a non-invasive method such as transient elastography or even liver biopsy).1,5 As such, a cut-off level of 2000IU/mL seems to us more useful clinically: even though it is more specific for inactive carriers, it is less likely to misdiagnose patients with active disease who might benefit from antiviral treatment.
In recent years, HBsAg quantification has been gaining prominence as a tempting substitute for the yearlong follow-up necessary to diagnose inactive carriers. As a standalone single-point test, an HBsAg level <1000IU/mL demonstrated good discriminatory power for diagnosing inactive carrier state in a European population; diagnostic power was even stronger when associated with a same time-point HBV DNA level <2000IU/mL.16 Even though these cut-off levels remain to be tested and validated in different hepatitis B patient cohorts, quantitative HBsAg seems a very promising diagnostic marker.
HBsAg levels are generally correlated with viremia, but in low replicative states such as inactive carrier state, they remain proportionally higher to those of serum HBV DNA,21,29,31 probably reflecting HBsAg secretion from integrated HBV DNA and/or reduced host immune control over HBsAg production versus viral replication. It provides additional information about the patient's immune control over the disease and possibly about the extent of affected hepatocytes: HBsAg loss represents optimal viral suppression and lower HBsAg levels as well as higher rate of HBsAg decline during the course of the disease appear to be predictive of this outcome.16,18
As for the assessment of liver fibrosis in this subset of HBV-infected patients, it seems to us that despite being the gold-standard, liver biopsy does not constitute an optimal first-line exam in this population, mainly because it is invasive, observer-dependent and carries a small risk of serious complications, making its use in asymptomatic patients with low risk of severe liver disease very questionable.
When available, liver transient elastography represents a more convenient screening test, both for health-care providers and patients, in diagnosing inactive carriers and even serially monitoring their degree of fibrosis. Liver biopsy should be reserved for patients with unexplained ALT elevations of >2×ULN, isolated viremia of >20,000IU/mL or abnormal liver stiffness measurements.
5.2Monitoring for reactivationThere are few prospective studies of inactive carriers defined according to the most recent guidelines. Still, prognosis and outcomes are decidedly favorable in asymptomatic HBsAg carriers with normal ALT levels and low viremia.
Reactivation rates in true inactive carriers are a very rare outcome: incidences were 0.8 and 0.4 per 100 person-years in two studies from France and Greece, respectively.33,36 Another Greek study revealed a surprising reactivation rate of 4.7 per 100 person-years.46 Even though no significant differences in study design or population sampling method were reported, it is possible that in this study undisclosed alcohol consumption or unidentified advanced liver disease at baseline (no initial liver biopsies were performed) may account for this discrepancy.
Most longitudinal studies investigating reactivation in inactive carriers fail to mention HBeAg reversion rates; in those who do, these are negligible (0–0.07 per 100 person-years).33,34
Monitoring for relapse of active hepatitis can be accomplished with regular ALT and HBV DNA measurements. As mentioned previously, HBV DNA elevations between 2000 and 20,000IU/mL do not necessarily correspond to disease activity: these patients benefit from more frequent follow-up to clarify this. In isolated viremia in this range with persistently normal ALT levels, liver fibrosis stage should be investigated with transient elastography, since active liver inflammation is an unlikely confounding factor. Conversely, isolated ALT elevations <2ULN may benefit more from liver biopsy to exclude other causes of raised transaminase levels, such as alcohol abuse, hepatic steatosis or HCC.
5.3Liver complicationsCirrhosis and hepatocellular carcinoma are rare but possible outcomes even after years of inactive disease and even after HBsAg loss.9,43 Higher HBV DNA levels (within inactive carrier range), older age and alcohol consumption were found to be risk factors for both complications in this subpopulation. It is interesting to note that HBV DNA has a proportionally greater predictor effect for HCC in inactive carriers compared to HBV chronic hepatitis in general, demonstrating the relevance of a viral-dependent, rather than fibrosis-dependent, oncogenic pathway in these patients.9,47
In conclusion, we consider hepatitis B inactive carrier state a relatively benign condition in the HBV infection spectrum, but only if accurately diagnosed. Transient elastograhy and the promising HBsAg quantification in serum are the two most recent major advances that have contributed to more accurate diagnosis of this state and simplified its follow up, obviating the need for biopsy in many circumstances. After correct diagnosis, lifelong monitoring is warranted for early detection of possible reactivation and hepatic complications. The goal in clinical practice is to recognize all situations which mandate a change in management (be it with tighter follow-up, further investigations or treatment), so it can be implemented as soon as possible in order to minimize liver damage and negative clinical outcomes.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestNo potential conflict of interest relevant to this article was reported.