In the last years, a distinctive interest has been raised on large polypoid and non-polypoid colorectal tumors, and specially on flat neoplastic lesions ≥20mm tending to grow laterally, the so called laterally spreading tumors (LST). Real or virtual chromoendoscopy, endoscopic ultrasound or magnetic resonance should be considered for the estimation of submucosal invasion of these neoplasms. Lesions suitable for endoscopic resection are those confined to the mucosa or selected cases with submucosal invasion ≤1000μm. Polypectomy or endoscopic mucosal resection remain a first-line therapy for large colorectal neoplasms, whereas endoscopic submucosal dissection in high-volume centers or surgery should be considered for large LSTs for which en bloc resection is mandatory.
Nos últimos anos houve um crescente interesse pelas lesões colorretais polipoides e não polipoides de grande tamanho, especialmente pelas lesões planas neoplásicas ≥20mm que tendem a crescer lateralmente - as chamadas lesões de espraiamento lateral (LST). Para avaliar o acometimento submucoso dessas lesões, pode-se utilizar a cromoendoscopia real ou virtual, a ecoendoscopia e a ressonância magnética. A ressecção endoscópica está indicada em lesões restritas à mucosa ou em casos selecionados com invasão da submucosa ≤ 1000μm. A polipectomia e a ressecção endoscópica de mucosa permanecem um tratamento de primeira escolha para lesões colorretais grandes, enquanto que as LSTs cuja ressecção em bloco é mandatória devem ser submetidas à dissecção submucosa endoscópica em centros com grande experiência na técnica ou à ressecção cirúrgica.
Colorectal cancer (CRC) is a major cause of cancer-related morbidity and mortality.1 It affects mainly people older than 50 years and the detection and removal of precursor colorectal lesions has enabled a significant reduction in the incidence of cancer and in the CRC-related mortality of these cases.2 The characteristics of the removed lesions and its histopathology determine colonoscopy surveillance. A recent guideline3 indicates shorter intervals for advanced neoplasms (adenomas ≥10mm, villous histology or high-grade dysplasia, and cancer). In addition to these criteria, the presence of three or more adenomas and serrated polyps ≥10mm fulfill the requirements for the high-risk group. Thus, large lesions (≥2.0cm) are considered high-risk neoplasms, with potential for malignancy, submucosal invasion and lymphatic involvement, being the risk of harboring carcinoma as high as 20–50%.4 Lesions are called superficial when their features at endoscopy suggest that they are limited to the mucosa or submucosa. These lesions may be polypoid (sessile, pedunculated and subpedunculated) and non-polypoid (flat or depressed). Techniques for resection of these large lesions can use a diathermy loop for pedunculated or subpedunculated lesions, and the method of endoscopic mucosal resection or submucosal dissection for sessile and non-polypoid lesions.5,6
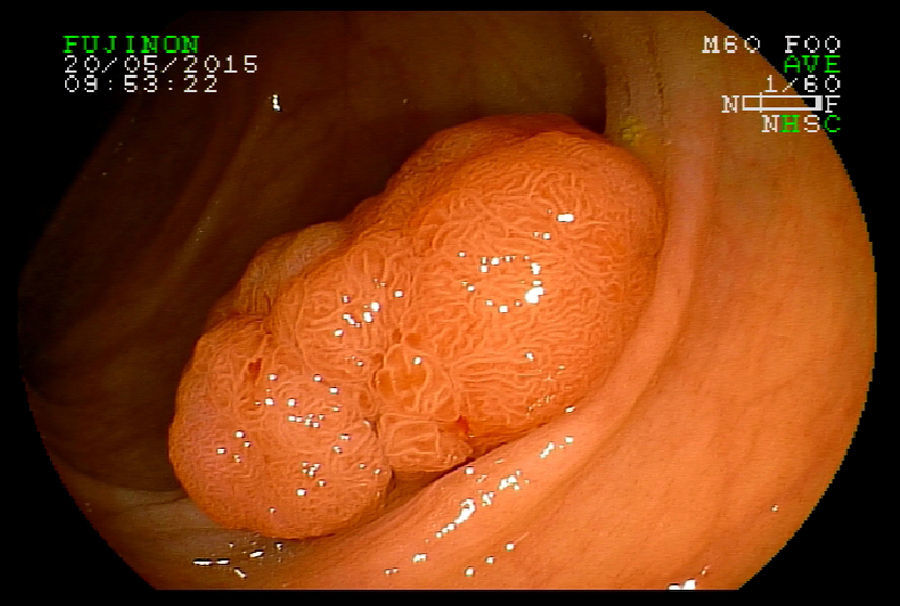
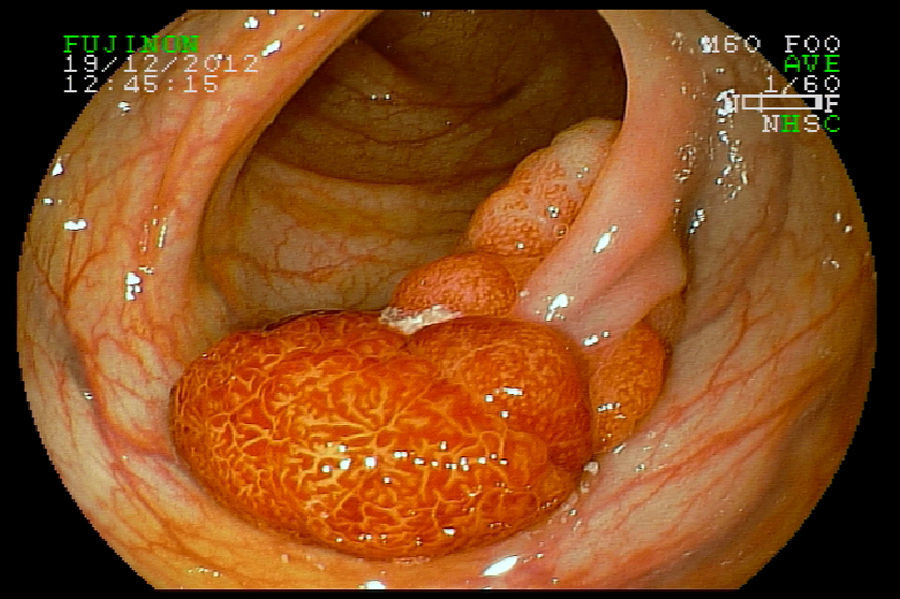
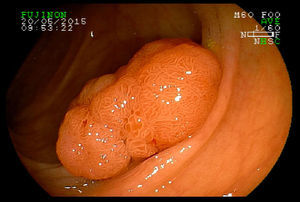
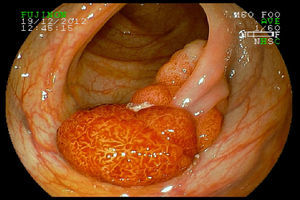
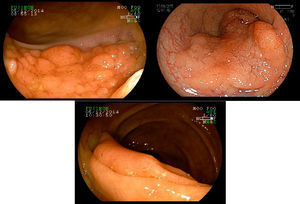
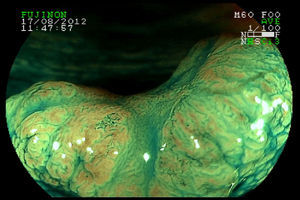
2Evaluation of large colorectal lesionsAccording to the Paris classification,7 lesions greater than 2.5mm in height are considered polypoid; they can be measured by positioning a closed biopsy forceps next to the lesion. They are more frequent in the left colon and are classified as type 0-Is (sessile), type 0-Ip (pedunculated) and type 0-Isp (subpedunculated) (Figs. 1–2).
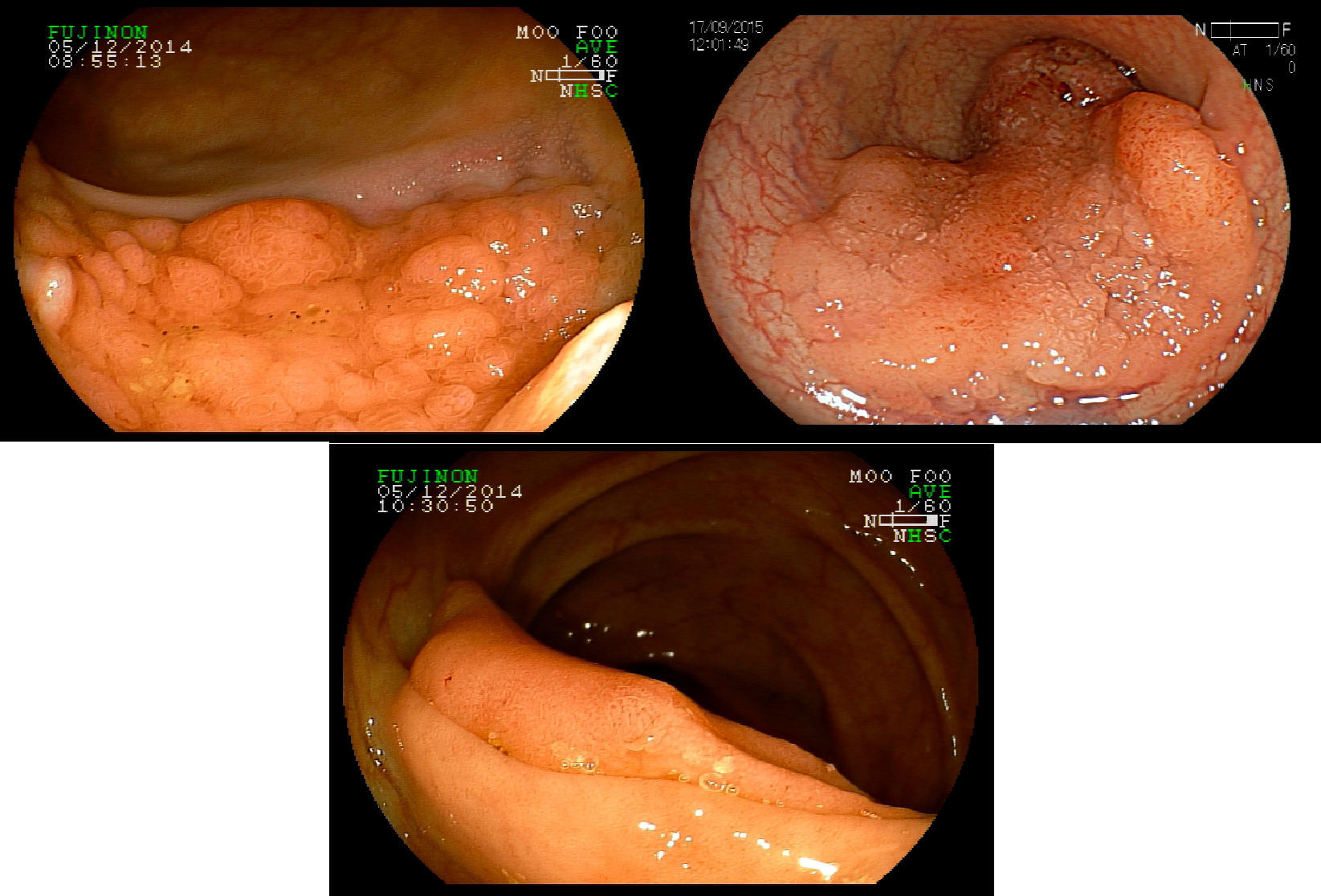
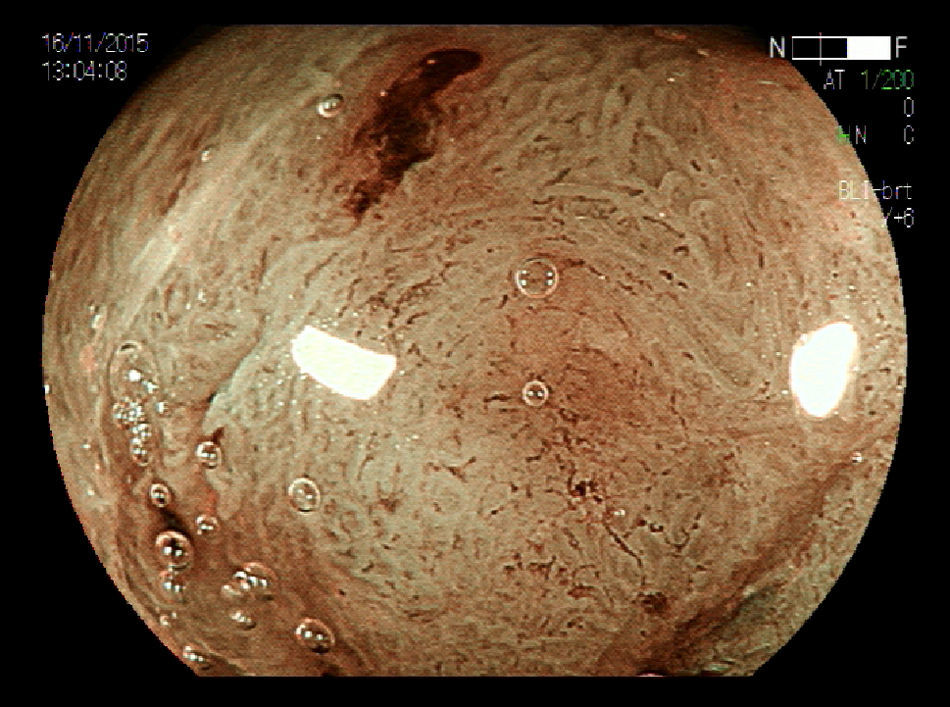
Non-polypoid lesions ≥20mm in diameter are defined as laterally spreading tumors (LSTs), which are characterized by a lateral and circumferential growth in the colonic wall, with deep invasion of the submucosa occurring only at later stages, and are more commonly diagnosed at the right colon. LSTs are classified as granular (LST-G) and non-granular (LST-NG) types, according to the presence or absence of a granular surface pattern. Kudo et al.8 proposed a sub-classification of LSTs, in which LST-Gs are classified as homogeneous and nodular mixed subtypes (LST-G-N), and LST-NGs are classified as flat elevated and pseudo depressed subtypes (LST-NG-PD)(Fig. 3A–C). The LST-G-N and the LST-NG-PD have a higher potential for malignancy.9 The rate of invasive carcinoma in LST-Gs is low, and most cases are considered adenomatous lesions, where the homogeneous subtype tends to be a tubular adenoma, and the nodular mixed subtype tends to have villous features.10
The British Society of Gastroenterology guidelines11 suggest that the term “non-pedunculated colorectal polyp” (NPCP) is the most appropriate term to define sessile and flat lesions, so the term “large NPCP” may be used to describe NPCP>2.0cm. This guideline considered the non-granular and granular nodular mixed subtype LST as having an increased risk for malignancy, as well as, pit patterns type V, capillary pattern Sano's type III and NICE (NBI International Colorectal Endoscopic) type III. This guideline suggests, as already pointed out by Japanese authors,12 that biopsies should be used with caution, since they can cause fibrosis in the biopsied area and thus, prevents endoscopic resection. When biopsies are necessary, they should be directed to the area exhibiting features indicative of cancer, avoiding flat areas and the lesion periphery.
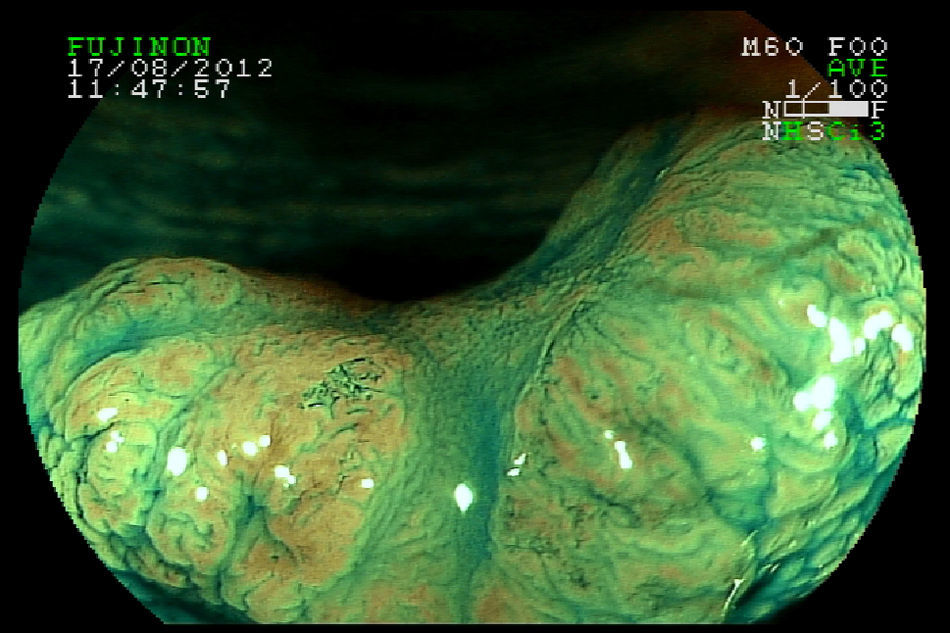
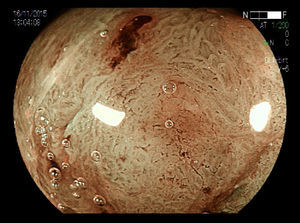
With respect to early CRC it is important to distinguish the presence or absence of deep submucosal invasion. Studies have shown that pit and capillary pattern analysis using image magnification is useful in diagnosing early invasive colorectal cancer13,14 (Figs. 4–5). These factors help in determining the therapeutic approach.15 Size is an important independent factor related to non-lifting sign. Ferrara F et al.16 showed this relationship in lesions≥25mm. Furthermore, according to guidelines of the Japan Gastroenterological Endoscopy Society (JGES),12 using magnifying endoscopy, known as optical biopsy, is more effective and highly accurate both for distinction between adenoma and adenocarcinoma and for evaluation of invasion depth, than carrying out a simple biopsy, which, in addition to not providing a qualitative diagnosis, may cause fibrosis, thus hindering endoscopic treatment.
High definition colonoscopy and chromoendoscopy are also widely used in the Western world. Averbach M et al. 17 had 79.7% accuracy, 88.8% sensitivity, 55% specificity, a VPP of 84.2% and a VPN of 64.7% in the differential diagnosis of neoplastic and non-neoplastic lesions. Yoshida et al. 18 studied the surface pattern of 151 polyps using the FICE system without magnification and reported an accuracy of 89.4%.
A recent study showed similar accuracy for measuring invasion depth of early CCR when comparing magnifying chromoendoscopy and EUS, in terms of submucosal invasion depth<1000μm (both with accuracy=71.2%) and deeper invasion (accuracy of 68.6% vs 70.9%, respectively).22 Other 2 prospective studies comparing these techniques demonstrated significant better figures for EUS.23,24
Endoscopic ultrasound (EUS) and Magnetic resonance imaging (MRI) are comparable in terms of accuracy and safety in the evaluation of wall penetration and perirectal nodal involvement and, consequently influence the management strategy.25,26 Both techniques present similar results for T and N staging of rectal cancer. EUS performs better than MRI in T1 cancers, whereas MRI has better results with T4 lesions. So, to study rectal early cancer EUS technique can help more to indicate an endoscopic treatment or a minimally invasive surgical procedure.25 Mukae et al.26 estimated the invasion depth of 714 cases of early CRC on EUS and showed an overall diagnostic accuracy of 89%. Their results were significantly higher for rectal tumors, for protruding tumors and LSTs, and Tis cancer.
2.1Risk evaluationStudies have shown a strong correlation between lesion size and its potential for malignancy,19–21 and larger lesions are considered to be at higher risk for submucosal invasion and lymph node involvement.27 A greater malignant potential for lesions >10mm (p<0.0001) was observed by Reinhart et al.,28 regardless of morphology. However, there have been series showing that small, non-polypoid lesions are more likely to contain carcinoma and a deeper infiltration of the submucosa, when compared to larger polypoid lesions.29–31 Kurisu et al.29 have investigated the development and progression of early CRC, and observed that non-polypoid lesions were significantly smaller than polypoid ones and presented with deeper invasion of the submucosa. Soetikno et al.31 have also found that non-polypoid lesions were more likely to contain carcinoma (OR=9.78) than polypoid ones, regardless of their size. Santos et al.32 showed that the presence of advanced histology was significantly more frequent in non-polypoid neoplasms than in polypoid lesions (p=0.007). The probability of non-polypoid lesions having advanced histology was twice higher (p=0.007), and when non-polypoid lesions were classified as flat or depressed type, presence of high-grade dysplasia or cancer was detected in 2% and 41.3% of the lesions, respectively, while being detected in 1.9% of polypoid lesions. However, when considering this risk for depressed lesions, advanced histology was 36-fold more likely to occur in this type of non-polypoid lesion. Matsuda et al.30 have shown a similar aggressiveness and malignant potential when comparing smaller and larger lesions.
Santos et al.32 found four (5.3%) adenocarcinomas among the 76 neoplasms ≥20mm, and 29 (38.2%) adenomas with high-grade dysplasia. Therefore, 43.4% presented with advanced histology. All adenocarcinomas were located in the left colon, but only one lesion had massively invaded the submucosa (1.3%). In the study by Ahlawat et al.,33 183 lesions of the colon and rectum ≥20mm, most of them sessile, were removed endoscopically, and the rate of invasive carcinoma was 10%.
Luigiano et al.34 have shown increased malignancy in sessile polypoid lesions when compared to non-polypoid, superficial lesions (21.6% vs 6%, p=0.0013). Another series has examined the risk of lymph node metastases in patients with invasive pedunculated polypoid type CRC. Among the patients that were treated surgically, the incidence of lymph node metastases was 3.5% (8/230); however, the incidence was 0% (0/101) in patients with invasion of the polyp head and 6.2% (8/129) when the invasion occurred at the polyp stalk.35 Caputi et al.19 have reported malignancy in 9.3% of lesions ≥20mm, with 3.3% of invasive carcinomas.
There was no statistical difference when comparing the site of the neoplasms with the occurrence of advanced histology, but all 15 polypoid tumors with advanced histology were located in the left colon, whereas 72.2% (13/18) of non-polypoid neoplasms were in the right colon.40 This was corroborated by Rondagh et al. 36, who found that proximal neoplasms with advanced histology were more likely to be non-polypoid (OR 4.68, p=0.006).
Chung et al.37 have shown a 6-fold higher incidence of metachronous advanced neoplasms for patients in the high-risk group, when compared to individuals without adenomas at the index colonoscopy, considering that the size ≥10mm is an independent predictor.
Martinez et al.38 have observed a 15.5% risk of advanced neoplasm during follow-up in the high-risk group and of 6.9% in the low risk group. The risk of invasive cancer was 1.2% in patients who had adenomas ≥20mm at the index colonoscopy and 1.3% in patients who presented lesions with high-grade dysplasia. Compared to patients with adenomas smaller than 20mm at the baseline colonoscopy, the adjusted probability for advanced neoplasm was 2.99.
3ManagementThe discontinuation or not of anti-thromboembolic therapy before endoscopic resection should be performed as recommended by published guidelines. The European Society of Gastrointestinal Endoscopy (ESGE) guideline39 recommends that the management of antiplatelet agents (APA) should be based on the thrombotic risk of the patient and the risk of bleeding of the procedure. To patients with low thrombotic risk (Coronary DES>12 months previously, bare metal coronary stents inserted>6 weeks previously without associated risk factors, stroke without cardiac failure>6weeks previously) in endoscopy with high bleeding risk (EUS-FNA of cysts, EMR and ESD) the recommendations are to stop aspirin 5 days prior to the procedure; in patients taking thienopyridine alone, it is recommended to substitute it for aspirin. Patients with high thrombotic risk (Coronary DES performed≤12months previously, bare metal coronary stents inserted≤6weeks previously or >6weeks with associated risk factors, stoke≤6 weeks previously) and with high bleeding risk, the procedure should be delayed and/or a cardiologist should be consulted to discuss temporary cessation of a thienopyridine. Aspirin should be maintained in all cases. The American Society for Gastrointestinal Endoscopy (ASGE) guideline40 recommends that clopidogrel or ticlopidine should be removed for about 7–10 days before an elective procedure in patients with a recently placed vascular stent or acute coronary syndrome. The APA may be reinitiated as soon as deemed safe with consideration of the patient's condition. Warfarin anticoagulation should be suspended in patients with a low risk of thromboembolic complications in the periendoscopic period, but the ASGE suggests continuing the anticoagulation in patients at higher risk of thromboembolic events, switching to unfractionated heparin and low molecular weight heparin before the endoscopic procedure.
4Endoscopic treatment modalities4.1PolypectomyIt is the technique indicated for pedunculated or subpedunculated lesions. The most common method of removing colon polyps is endoscopic resection, such as snare resection, among others, with or without fluid injection into the submucosal layer. Pedunculated polyps have the feeding artery running through the pedicle. Safety and effectiveness of endoscopic polypectomy of large colorectal polyps should be the main goals of this procedure, where diathermal snare is the most used accessory. The most common complication after polypectomy is, by far, hemorrhage, and bleeding is oftener after polypectomy for large polyps with a thick pedicle.
4.2Endoscopy mucosal resection (EMR)EMR is a minimally invasive, easy-to-learn, safe and effective technique used in the treatment of premalignant lesions and early carcinomas that have a low risk of lymph node metastasis. The most common technique is the ‘inject and cut’ EMR, originally described in 1973 by Deyhle et al.41. This technique involves the injection of saline solution into the submucosal layer under the lesion to be removed in order to lift the lesion and create a fluid cushion between the mucosa and the muscular wall, mitigating thermal effects on the organ wall, thus reducing the risk of perforation and bleeding and facilitating en bloc resection of lesions. Hyaluronic acid, glycerol, and hydroxypropylmethylcellulose (HPMC) are other injection options and appear to have a more durable cushioning effect. Unfortunately, these agents are expensive and are not readily available in most Endoscopy units, and are difficult to inject. A comparative study between a hypertonic solution and saline solution showed a lower volume of fluid injected into the submucosa in the former solution (p=0.033), fewer injections were needed to maintain the submucosal elevation, especially in lesions>40mm (p=0.039), and submucosal elevation time was longer (p=0.043).42
EMR is a less invasive alternative to surgical removal of adenomas, including large-size tumors,43–47 intramucosal carcinoma, and minimally invasive submucosal carcinoma.47–49 Furthermore, Tanaka et al.50 reported that well- or moderately differentiated carcinomas within 1500μm invasion are curatively treated by EMR, provided that no vascular involvement is observed. EMR has represented a major advance in therapy by allowing the resection of superficial lesions and also allowing the removal of large sessile lesions and laterally spreading tumors (LST). These larger lesions may be removed in pieces (piecemeal resection). This method has made endoscopic treatment safer, minimizing the risk of complications. To confirm recurrence (or residual neoplasm) follow-up colonoscopy should be done between 2 and 6 months after resection by the piecemeal technique.11,12
En bloc resection is the optimal treatment of colorectal neoplasms, especially adenocarcinomas, facilitating histopathological diagnosis and allowing lymphovascular invasion and invasion depth to be properly evaluated. Lesions up to 20mm in size can be easily removed en bloc by EMR. The choice between piecemeal EMR and endoscopic submucosal dissection (ESD) for large LSTs should be based on LST subtype and, when indicated, on magnification chromoendoscopy, as well as local expertise.12
Most LSTs are of the granular type and are more frequent in the right colon.51 Previous studies51,52 have identified large lesions, with pseudo depression and large nodules (≥10mm), as predictors of higher aggressiveness of LSTs, but most LSTs are adenomatous lesions, even when they reach large diameters, allowing endoscopic resection to be performed. Prior to selection of the resection technique, it is important to distinguish between adenoma and adenocarcinoma. Virtual chromoendoscopy or real-time chromoendoscopy (using indigo carmine) allow defining their pit and capillary patterns, influencing the choice of approach to LST management.
4.3Endoscopic submucosal dissection (ESD)In ESD technique, the solution is injected into the submucosa of a tumor through the injection needle. The circumference of the lesion is incised using a knife for ESD with electrical cutting current, and the submucosal layer is dissected. This technique can resect the lesion in one piece regardless of its size and shape. When it starts dissecting the submucosa and after circumferential incision of the lesion, the resection is finished using a snare, it is called hybrid ESD.
The traditional and most commonly used fluid for submucosal injection for endoscopic resection of large colorectal polyps is saline solution; however, it is difficult to maintain adequate mucosal elevation, due to its rapid absorption. A prolonged submucosal elevation provides an advantage for the resection of large and giant sessile lesions, because it is less often necessary to interrupt the procedure by injecting more submucosal fluid and maintain the elevation. This is achieved by hypertonic solutions, such as hypertonic saline solution and dextrose water >15%.53
ESD is indicated for lesions >20mm, lesions that are difficult to be removed en bloc by EMR, lesions with type Vi pit pattern (irregular arrangement – Kudo's Classification), carcinomas with shallow submucosal invasion, mucosal tumors with fibrosis, and recurrent or residual early carcinomas after endoscopic resection.9,12 A Japanese multicentre study54 demonstrated a significantly higher en bloc resection rate with ESD than with conventional endoscopic resection (94.5% vs 56.9%, p<0.01). Saito Y et al.55 had en bloc resection and curative rates (R0) of 90% and 87%, respectively. Toyonaga et al.56 observed that procedure time was faster in hybrid ESD (p<0.0001), but the en bloc resection rate was significantly higher in ESD than in hybrid ESD (p=0.0044). The complication rate in hybrid ESD tended to be higher, but without reaching statistical significance.
LST-G-N with large nodule and LST-NG-PD, which may have multifocal submucosal invasion, whose foci are often difficult to detect, should be removed preferably by en bloc resection, for which ESD is indicated, with reduced recurrence rates.9
The ESGE states that the majority of colonic and rectal superficial lesions can be effectively removed in a curative way by standard polypectomy and/or by EMR (strong recommendation, moderate quality evidence). ESD can be considered for removal of colonic and rectal lesions with high suspicion of limited submucosal invasion that is based on two main criteria of depressed morphology and irregular or nongranular surface pattern, particularly if the lesions are larger than 20mm; or ESD can be considered for colorectal lesions that otherwise cannot be optimally and radically removed by snare-based techniques (strong recommendation, moderate quality evidence).57
The ESD technique, however, is more complex than EMR and has been associated with a long learning curve; long procedure time and significantly more complications, especially perforation,58 and its use should be limited to specialized centers. So, when ESD expertise is not available, EMR piecemeal technique is a good option for the treatment of LSTs. Endoscopic resection should be tried and the final decision taken after pathologic evaluation.
5Surgical techniquesIn two metanalytic reviews, the disease-free and overall survival rates for stages I, II, and III colon cancer did not differ when comparing laparoscopic-assisted vs open colectomy. So, laparoscopically assisted colectomy for cancer is oncologically safe.59,60
Transanal endoscopic microsurgery (TEM) is a minimally invasive technique for excision of rectal tumors that avoids conventional pelvic resectional surgery along with its risks and side effects. Sajid MS et al.61 observed a higher risk of local recurrence (p<0.003) and overall recurrence (p<0.01) following this technique compared with radical surgery (RR). The risk of distant recurrence, overall survival and mortality were similar. TEM was associated with a shorter operation time and hospital stay and a reduced risk of postoperative complications (p<0.0001).
A systematic review has not shown difference between TEM and transanal excision in the postoperative complication rate. TEM had a higher rate of negative microscopic margins (p<0.001), had a reduced rate of specimen fragmentation (p<0.001) and lesion recurrence (p<0.001) compared with transanal excision.62
Transanal minimally invasive surgery (TAMIS) has emerged as an alternative to TEM for resection of benign polyps and early cancers of the rectum. This technique uses ordinary laparoscopic instruments to achieve high-quality local excision. In a recent study no difference was observed between the two techniques regarding the accuracy of dissection, but dissection and suturing were significantly quicker in the TEM group.63
Kawaguti et al.64 showed en bloc resection rates with free margins in 81.8% of patients in the ESD group and 84.6% of patients in the TEM group (p=0.40). No difference was observed regarding mean tumor size (p=0.13), local recurrence, mean procedure time (p=0.69) and mean hospital stay (p=0.81).
5.1RecurrenceRecurrence (or residual neoplasm) was defined as the presence of neoplastic tissue in the area of previous resection, as diagnosed by follow-up colonoscopy. Lesions that have an increased risk of incomplete resection or recurrence are lesions>40mm, those involving the ileocecal valve, the appendix, a diverticulum or the dentate line.11 The non-lifting sign after submucosal injection, prior failed attempt of resection or recurrence at the site of previous resection are also associated with disease recurrence.
Recurrence ranges in the literature from 1.2 to 55% according to a variety of risk factors.34,46,65–67 Most series have reported a higher recurrence rate associated with piecemeal resection as compared with en bloc resection.16,34,68 On the other hand, Lim et al.69 found no difference between the two techniques.
In our experience,15 it was observed a greater recurrence rate with piecemeal EMR (p<0.01), advanced neoplasms (p=0.01), and carcinomas (p=0.04). Piecemeal resection of malignancy was identified as an independent risk factor for incomplete resection (OR 3.36, p=0.013). Recurrence has also been associated with resection of more pieces of LST.70 A retrospective Japanese study71 demonstrated a significantly lower recurrence after ESD than after piecemeal EMR (2% vs 14%, p<0.0001), and a multicenter study54 reported a 98% 5yr. free-recurrence rate after ESD. Another study demonstrated a significant increase in the number of endoscopic procedures in lesions >40mm (p<0.001) and a greater need for surgical treatment (p<0.001).72 A prospective multicenter study in Japan73 showed that significant factors associated with recurrence after endoscopic resection for large colorectal neoplasia were piecemeal EMR, granular subtype LSTs, lesions ≥40mm, no pre-treatment magnification, and ≤10 years of experience in conventional endoscopic resection. This way, the thorough surveillance and the complete removal of colorectal neoplasms are of paramount importance. Robertson et al.74 have observed 11(19%) cases of CRC in colonoscopic surveillance, possibly associated with incomplete resection of the lesions.
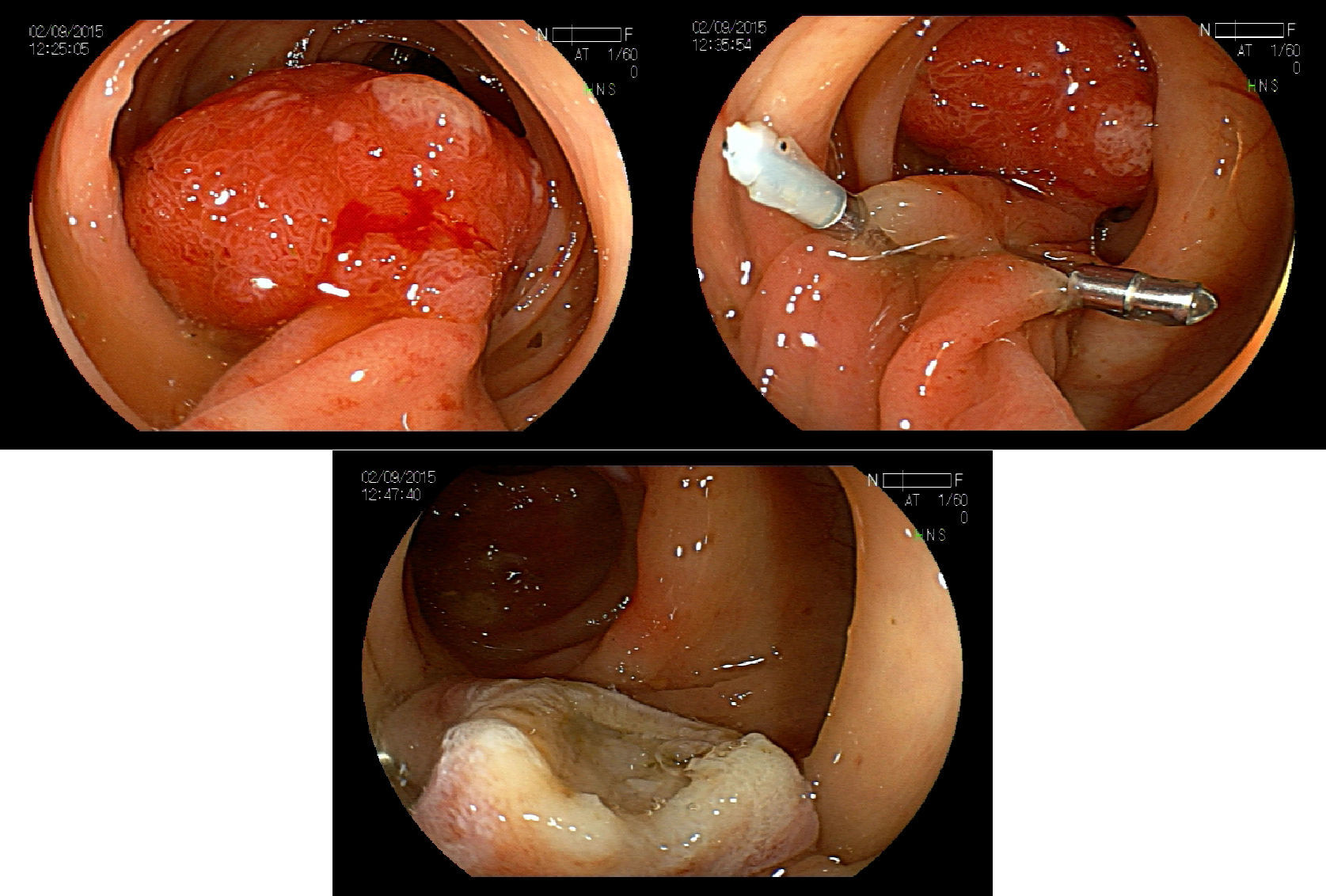
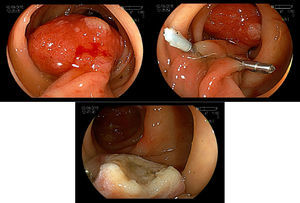
5.2Adverse events and managementImmediate bleeding can occur in up to 10% of the cases of polypectomy, and can also present as early or late bleeding.75,76 This complication could be treated endoscopically by injection therapy, endoloop, argon plasma coagulation and hemoclips. Adrenaline injection reduces post-polypectomy early bleeding, but not late bleeding. Endoloop or hemoclip could be used as single therapy or in combination (Fig. 6A–C). Late bleeding can occur even 15 days after polypectomy due to the ulcer caused by the thermal effect of the procedure.77 Buddingh et al.78 found that right colon lesions bled oftener after polypectomy, than left-sided ones (OR=4.67, p=0.001), and there was an increase in the bleeding risk of 13% for each mm increase in the polyp diameter (OR1.13, p<0.001).
In a randomized study evaluating two arms of 50 resected polyps each, there was less cases of post polypectomy hemorrhage in the group, which received adrenalin injection at the base or pedicle of the polyp (2% vs 16%).79
A metanalysis pointed out that early post polypectomy bleeding is significantly diminished, when a prophylactic therapy is adopted.80 This study also showed that combined therapy is more effective than monotherapy in the prophylaxis of post polypectomy bleeding. However there was no significant reduction in late bleeding irrespective of the chosen method, either alone or in combination.
Bleeding is considered the most common complication after ESD, occurring in 0.4–16% of the cases.45,81–83 Ferrara et al.16 concluded that bleeding was significantly related to malignancy. The use of preventive hemoclips after endoscopic resection is controversial. A randomized trial84 showed that preventive clipping did not decrease the delayed bleeding (0.98% vs 0.96%), while the Japan Gastroenterological Endoscopy Society suggests that clipping may be effective for patients with large lesions or for those undergoing antithrombotic therapy.12
Perforation has been reported to occur in 0–4% of cases.80,85–87 Caputi et al.19 reported in their series a 2.3% perforation rate after EMR (all polypoid carcinomas). Swan et al.88 described a target sign as a marker of muscularis propria resection and, therefore, potential perforation during EMR of colorectal lesions, with an incidence of 3.8% for en bloc resection and 1.6% for piecemeal resection (p=0.16). An increased risk of perforation occurs in right-sided lesions, because the wall of the right colon is thinner. Delayed perforation is rare. Most cases occur in the first 14h after resection and are oftener related to ESD.
A Japanese prospective multicenter study demonstrated that age under 60 years was a risk factor for bleeding, and en bloc resection and Vienna classification types 4–5 were risk factors for perforation.89
Low perforation rates have been reported for diagnostic and therapeutic colonoscopy, but serious complications with high rates of morbidity and mortality can occur. Perforations in diagnostic colonoscopy are larger than those in therapeutic colonoscopy, with surgery traditionally been indicated for the former and usually a conservative approach for the latter.90,91 Kim JS et al.92 had successful endoscopic closure in 81.3% of diagnostic colonoscopy-associated perforation patients. This suggests that immediate endoscopic closure with clips can be attempted for diagnostic perforations as well as therapeutic colonoscopy-associated perforations. The sigmoid colon is known as the most frequent perforation site. Perforation rates for ESD were significantly higher than those for polypectomy or EMR (p<0.01).93 Lesions>40mm (OR 3.90, p<0.001) and location at the ileocecal valve (OR 2.13, p<0.01) are recognized as strong risk factors for adverse events in endoscopic procedures.94 Polypectomies performed by unexperienced endoscopists were shown to have almost a three-fold increase in the risk of complications (OR 2.96, p=0.0008).95
5.3Early colorectal cancerAccording to the 2014 guidelines of the Japanese Society for Cancer of the Colon and Rectum,96 carcinomas treated by endoscopic resection are considered cured if they are T1 with a tumor-negative vertical margin, well or moderately differentiated, with a submucosal invasion depth <1000μm, absence of vascular invasion with tumor budding grade 1. T1 carcinomas should have additional surgical treatment if they present a tumor-positive vertical margin, vascular invasion, are poorly differentiated, have submucosal invasion depth >1000μm and the tumor budding is graded as 2 or 3.
6ConclusionsLarge colorectal lesions (≥20mm) tend to present with advanced histology, are more difficult to resect and are associated with more complications after endoscopic therapy. Although these caveats, endoscopic treatment is an effective therapy for large colorectal neoplasms, even the malignant ones, and may be done en bloc or by piecemeal resection, depending on the morphology of the lesion or local expertise, although lesions should preferably be resected en bloc. Proper lesion evaluation with magnification and chromoendoscopy (either virtual or real), EUS or MRI should be performed prior to removal depending on lesion site, morphology or availability of these methods. Surgical resection should be reserved for those cases with massive submucosal invasion or other unfavorable pathological features.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.